Beyond Biology: Uncovering Structural and Sociocultural Predictors of Breast Cancer Incidence Worldwide
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Analysis
2.3.1. Correlation Analysis with FDR Adjustment
2.3.2. Incorporation of R and R-Squared (R2) Values
2.3.3. Mathematical Modeling
2.3.4. Receiver Operating Characteristic (ROC)
2.4. Ethical Considerations
2.5. Limitations and Assumptions
3. Results
3.1. Correlation Analysis
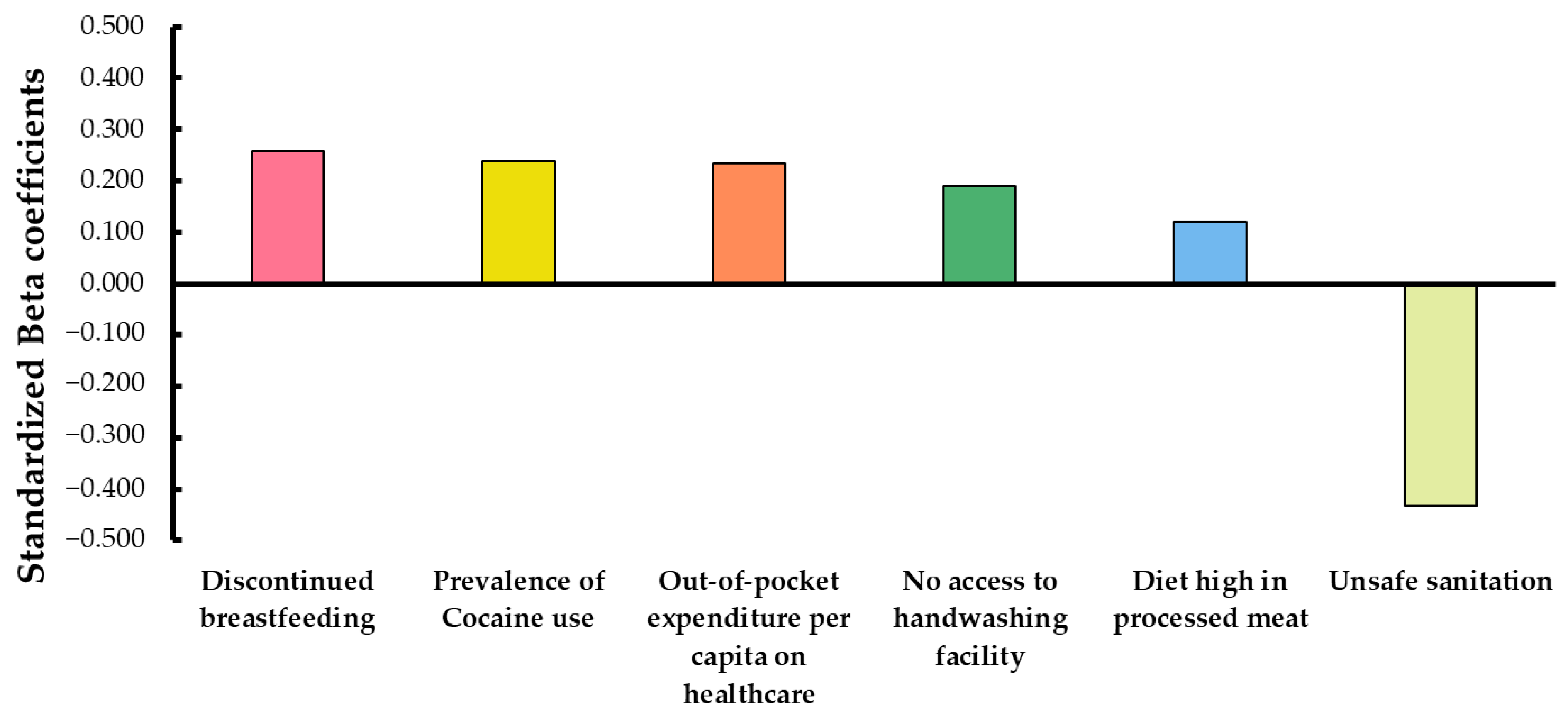
3.2. Multiple Regression Analysis
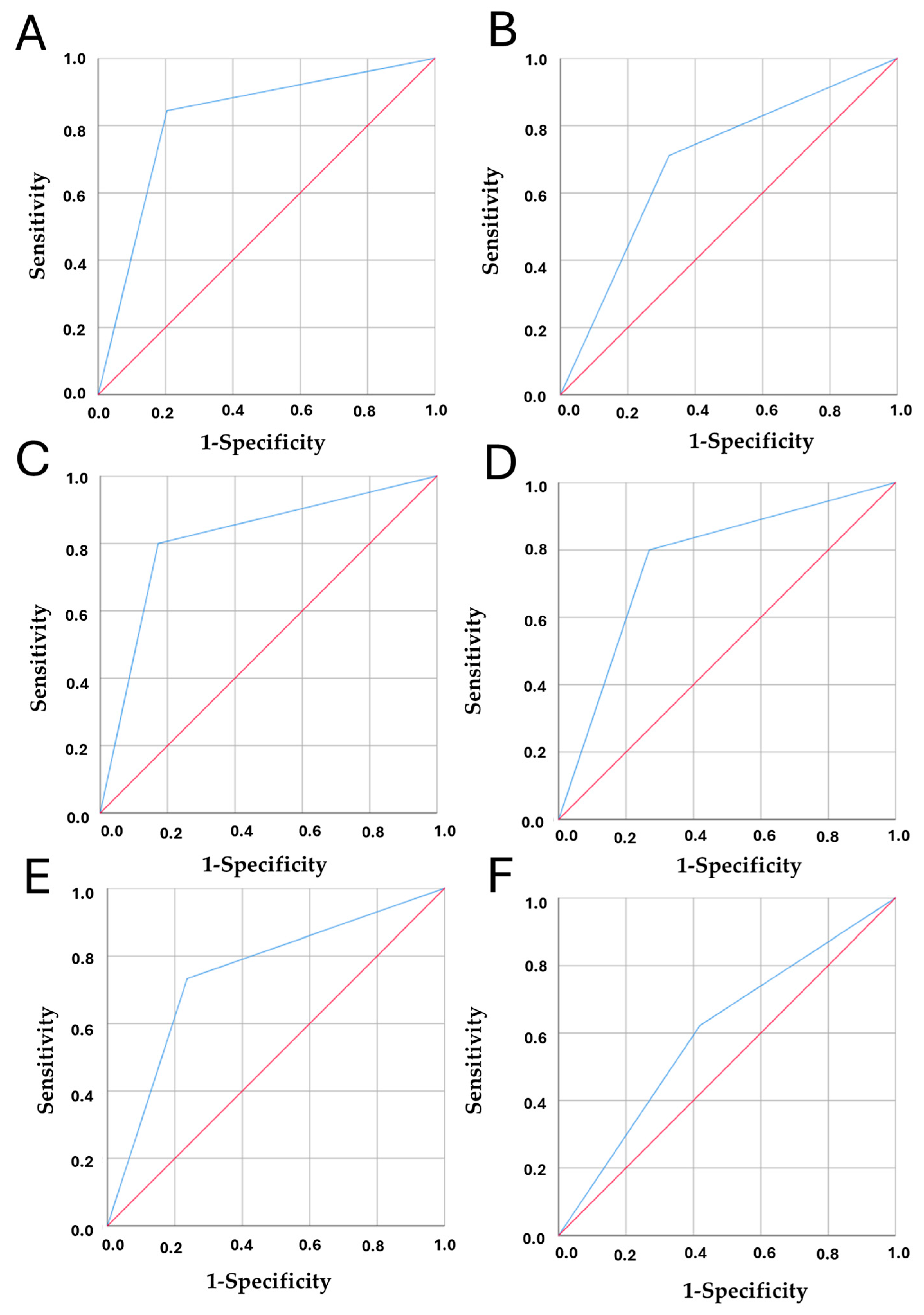
3.3. Model Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Gehlert, S.; Hudson, D.; Sacks, T. A Critical Theoretical Approach to Cancer Disparities: Breast Cancer and the Social Determinants of Health. Front. Public Health 2021, 9, 674736. [Google Scholar] [CrossRef]
- Coughlin, S.S. Epidemiology of Breast Cancer in Women. Adv. Exp. Med. Biol. 2019, 1152, 9–29. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer Is a Preventable Disease That Requires Major Lifestyle Changes. Pharm. Res. 2008, 25, 2097. [Google Scholar] [CrossRef] [PubMed]
- Clapp, R.W.; Howe, G.K.; Jacobs, M.M. Environmental and Occupational Causes of Cancer: A Call to Act on What We Know. Biomed. Pharmacother. 2007, 61, 631–639. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R. The Causes of Cancer: Quantitative Estimates of Avoidable Risks of Cancer in the United States Today. J. Natl. Cancer Inst. 1981, 66, 1191–1308. [Google Scholar] [CrossRef]
- Olakowski, M.; Bułdak, Ł. Modifiable and Non-Modifiable Risk Factors for the Development of Non-Hereditary Pancreatic Cancer. Medicina (B Aires) 2022, 58, 978. [Google Scholar] [CrossRef]
- Nindrea, R.D.; Aryandono, T.; Lazuardi, L. Breast Cancer Risk from Modifiable and Non-Modifiable Risk Factors among Women in Southeast Asia: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2017, 18, 3201–3206. [Google Scholar] [CrossRef]
- Pérez-Romero, S.; Gascón-Cánovas, J.J.; Salmerón-Martínez, D.; Parra-Hidalgo, P.; Monteagudo-Piqueras, O. Características Sociodemográficas y Variabilidad Geográfica Relacionada Con La Satisfacción Del Paciente En Atención Primaria. Rev. De Calid. Asist. 2016, 31, 300–308. [Google Scholar] [CrossRef]
- UNDP, H.D.R. (2021-22) Our World in Data. 2020–2022. Available online: https://ourworldindata.org/grapher/expected-years-of-schooling (accessed on 7 October 2024).
- World Health Organization via World Bank Our World in Data. 2022. Available online: https://ourworldindata.org/grapher/out-of-pocket-expenditure-per-capita-on-healthcare?tab=table (accessed on 7 October 2024).
- Institute for Health Metrics and Evaluation Global Burden of Disease (GBD) Study Compare. Available online: https://vizhub.healthdata.org/gbd-compare/# (accessed on 7 October 2024).
- United Nations Office on Drugs and Crime. Drug Use & Treatment: Drug Use Prevalence. UNODC Data Portal. Available online: https://dataunodc.un.org/dp-drug-use-prevalence (accessed on 20 August 2022).
- Ritchie, H.; Roser, M. Alcohol Consumption. Our World in Data 2022. Available online: https://ourworldindata.org/alcohol-consumption (accessed on 25 October 2022).
- WHO. Global Health Observatory Data Repository. Available online: https://data.who.int/indicators/i/C6262EC/BEFA58B (accessed on 7 October 2024).
- UN Food and Agriculture Organization (FAO)—Processed by Our World in Data Food Supply (g per Capita per Day)” [Dataset]. UN Food and Agriculture Organization (FAO) [Original Data]. Our World Data 2022, 1, 1.
- Food and Agriculture Organization of the United Nations—Processed by Our World in Data. Vegetable Consumption per Capita, 2016 to 2020. 2020. Available online: https://ourworldindata.org/grapher/vegetable-consumption-per-capita?tab=table&time=2016.latest&country=PER~Middle+Africa+%28FAO%29~SSD~BHS~CMR (accessed on 7 October 2024).
- UN Food and Agriculture Organization (FAO)—Processed by Our World in Data Global Food Data Explorer: Per Capita Kilocalorie Supply from All Meat per Day, 1961 to 2020. Available online: https://www.fao.org/faostat/en/#data/FBS (accessed on 7 October 2024).
- UN Food and Agriculture Organization (FAO). Global Food Data Explorer: Per Capita Kilocalorie Supply from Eggs per Day, 1961 to 2020. Per Capita Kilocalorie Supply from Milk per Day, 1961 to 2020. Available online: https://ourworldindata.org/explorers/global-food?tab=table&facet=none&hideControls=true&Food=Eggs&Metric=Food+available+for+consumption&Per+Capita=true&Unit=Kilocalories+per+day&country=OWID_WRL~OWID_SAM~OWID_NAM~OWID_EUR~OWID_AFR~OWID_ASI (accessed on 7 October 2024).
- Food and Agriculture Organization of the United Nations (FAO)—Processed by Our World in Data. Global Food Data Explorer. Available online: https://ourworldindata.org/explorers/global-food (accessed on 7 October 2024).
- Farfán Gutiérrez, M.; Pérez-Salicrup, D.R.; Flamenco-Sandoval, A.; Nicasio-Arzeta, S.; Mas, J.-F.; Ramírez Ramírez, I. Modeling Anthropic Factors as Drivers of Wildfire Occurrence at the Monarch Butterfly Biosphere. Madera Y Bosques 2018, 24, 2431591. [Google Scholar] [CrossRef]
- Li, H.; Song, X.; Liang, Y.; Bai, X.; Liu-Huo, W.-S.; Tang, C.; Chen, W.; Zhao, L. Global, Regional, and National Burden of Disease Study of Atrial Fibrillation/Flutter, 1990–2019: Results from a Global Burden of Disease Study, 2019. BMC Public Health 2022, 22, 2015. [Google Scholar] [CrossRef] [PubMed]
- Hintermeier, M.; Gold, A.W.; Erdmann, S.; Perplies, C.; Bozorgmehr, K.; Biddle, L. From Research into Practice: Converting Epidemiological Data into Relevant Information for Planning of Regional Health Services for Refugees in Germany. Int. J. Environ. Res. Public Health 2022, 19, 8049. [Google Scholar] [CrossRef]
- Dudley, W.N.; Benuzillo, J.G.; Carrico, M.S. SPSS and SAS Programming for the Testing of Mediation Models. Nurs. Res. 2004, 53, 59–62. [Google Scholar] [CrossRef]
- Chen, S.Y.; Feng, Z.; Yi, X. A General Introduction to Adjustment for Multiple Comparisons. J. Thorac. Dis. 2017, 9, 1725. [Google Scholar] [CrossRef]
- Haynes, W. Method. In Encyclopedia of Systems Biology; Springer: New York, NY, USA, 2013; p. 78. [Google Scholar] [CrossRef]
- Rosner, B. Fundamentals of Biostatistics, 7th ed.; Cengage Learning, Inc: Boston, MA, USA, 2010; Volume 1. [Google Scholar]
- Porgo, T.V.; Norris, S.L.; Salanti, G.; Johnson, L.F.; Simpson, J.A.; Low, N.; Egger, M.; Althaus, C.L. The Use of Mathematical Modeling Studies for Evidence Synthesis and Guideline Development: A Glossary. Res. Synth. Methods 2019, 10, 125–133. [Google Scholar] [CrossRef]
- Kamarudin, A.N.; Cox, T.; Kolamunnage-Dona, R. Time-Dependent ROC Curve Analysis in Medical Research: Current Methods and Applications. BMC Med. Res. Methodol. 2017, 17, 53. [Google Scholar] [CrossRef]
- Mendoza-Hernandez, M.; Hernandez-Fuentes, G.; Sanchez-Ramirez, C.; Rojas-Larios, F.; Guzman-Esquivel, J.; Rodriguez-Sanchez, I.; Martinez-Fierro, M.; Cardenas-Rojas, M.; De-Leon-Zaragoza, L.; Trujillo-Hernandez, B.; et al. Time-dependent ROC Curve Analysis to Determine the Predictive Capacity of Seven Clinical Scales for Mortality in Patients with COVID-19: Study of a Hospital Cohort with Very High Mortality. Biomed. Rep. 2024, 20, 100. [Google Scholar] [CrossRef]
- Arredondo Montero, J.; Martín-Calvo, N. Diagnostic Performance Studies: Interpretation of ROC Analysis and Cut-Offs. Cirugía Española (Engl. Ed.) 2023, 101, 865–867. [Google Scholar] [CrossRef]
- Stevens, G.A.; Alkema, L.; Black, R.E.; Boerma, J.T.; Collins, G.S.; Ezzati, M.; Grove, J.T.; Hogan, D.R.; Hogan, M.C.; Horton, R.; et al. Guidelines for Accurate and Transparent Health Estimates Reporting: The GATHER Statement. PLoS Med. 2016, 13, e1002056. [Google Scholar] [CrossRef]
- Hashem, S.; Habashy, S.; Elakel, W.; Raouf, S.; Esmat, G.; Eladawy, M.; Elhefnawi, M. A Simple Multi-Linear Regression Model for Predicting Fibrosis Scores in Chronic Egyptian Hepatitis C Virus Patients. Int. J. Bio-Technol. Res. 2014, 4, 37–46. [Google Scholar]
- Çorbacıoğlu, Ş.K.; Aksel, G. Receiver Operating Characteristic Curve Analysis in Diagnostic Accuracy Studies: A Guide to Interpreting the Area under the Curve Value. Turk. J. Emerg. Med. 2023, 23, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.H.; O’Malley, A.J.; Mauri, L. Receiver-Operating Characteristic Analysis for Evaluating Diagnostic Tests and Predictive Models. Circulation 2007, 115, 654–657. [Google Scholar] [CrossRef]
- de Paiva, J.P.S.; Magalhães, M.A.F.M.; Leal, T.C.; da Silva, L.F.; da Silva, L.G.; do Carmo, R.F.; de Souza, C.D.F. Time Trend, Social Vulnerability, and Identification of Risk Areas for Tuberculosis in Brazil: An Ecological Study. PLoS ONE 2022, 17, e0247894. [Google Scholar] [CrossRef]
- Coelho Tavares da Silva, S.; Tavares da Silva, P.H.; Antão de Medeiros, R.; Barbosa do Nascimento, V. Litigation in Access to Universal Health Coverage for Children and Adolescents in Brazil. Front. Public Health 2024, 12, 1402648. [Google Scholar] [CrossRef]
- Teixeira, O.F.B.; Xavier, S.P.L.; Félix, N.D.d.C.; Silva, J.W.M.d.; Abreu, R.M.S.X.d.; Miranda, K.C.L. Repercusiones de La Pandemia de COVID-19 Para Las Personas Con Autismo y Sus Familias: Revisión de Alcance. Rev Lat Am Enferm. 2022, 30, e3729. [Google Scholar] [CrossRef]
- Health Research Authority. Governance Arrangements for Research Ethics Committees: 2020 Edition; Health Research Authority: London, UK, 2021. [Google Scholar]
- Chandramohan, D.; Singh, P.; Garapati, H.N.; Konda, R.; Chandramohan, D.; Jena, N.; Bali, A.; Simhadri, P.K. Cardiac Implantable Electronic Device Infections in Patients with Renal Insufficiency: A Systematic Review and Meta-Analysis. Diseases 2024, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- The University of Newcastle, A. Human Research Ethics. Available online: https://www.newcastle.edu.au/research/support/services/human-research-ethics/before-you-begin/research-that-requires-ethics-approval (accessed on 14 October 2024).
- FAO. FAOLEX Act No. 593 Relative to Ethical Medical Research. Available online: https://leap.unep.org/en/countries/dk/national-legislation/act-no-593-relative-ethical-medical-research (accessed on 14 October 2024).
- Fallowfield, L.; Jenkins, V. Psychosocial/Survivorship Issues in Breast Cancer: Are We Doing Better? J. Natl. Cancer Inst. 2014, 107, dju335. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S. Social Determinants of Breast Cancer Risk, Stage, and Survival. Breast Cancer Res. Treat. 2019, 177, 537–548. [Google Scholar] [CrossRef]
- Emilee, G.; Ussher, J.M.; Perz, J. Sexuality after Breast Cancer: A Review. Maturitas 2010, 66, 397–407. [Google Scholar] [CrossRef]
- Mitchell, J.; Lannin, D.R.; Mathews, H.F.; Swanson, M.S. Religious Beliefs and Breast Cancer Screening. J. Women’s Health 2002, 11, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Flores, N.J.; Mathew, M.J.; Fortson, L.S.; Abernethy, A.D.; Ashing, K.T. The Influence of Culture, Social, and Religious Support on Well-Being in Breast Cancer Survivorship. Cureus 2021, 13, e14158. [Google Scholar] [CrossRef]
- Kuzhan, A.; Adli, M. The Effect of Socio-Economic-Cultural Factors on Breast Cancer. J. Breast Health 2015, 11, 17–21. [Google Scholar] [CrossRef]
- Breast Cancer Risk Assessment Tool: Online Calculator—NCI. Available online: https://bcrisktool.cancer.gov/ (accessed on 16 July 2025).
- Kim, H.Y.; Mullaert, J.; Tondreau, A.; Park, B.; Rouzier, R. Development of a Model to Predict the Age at Breast Cancer Diagnosis in a Global Population. Sci. Rep. 2024, 14, 13845. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wu, P.; He, J.; Zhang, G.; Zhou, W.; Chen, Q. Machine Learning Algorithms Predict Breast Cancer Incidence Risk: A Data-Driven Retrospective Study Based on Biochemical Biomarkers. BMC Cancer 2025, 25, 1061. [Google Scholar] [CrossRef]
- Obeng-Gyasi, S.; Obeng-Gyasi, B.; Tarver, W. Breast Cancer Disparities and the Impact of Geography. Surg. Oncol. Clin. N. Am. 2022, 31, 81–90. [Google Scholar] [CrossRef]
- Wu, A.H.; Wu, J.; Tseng, C.; Stram, D.O.; Shariff-Marco, S.; Larson, T.; Goldberg, D.; Fruin, S.; Jiao, A.; Inamdar, P.P.; et al. Air Pollution and Breast Cancer Incidence in the Multiethnic Cohort Study. J. Clin. Oncol. 2025, 43, 273–284. [Google Scholar] [CrossRef]
- Monroe-Lord, L.; Harrison, E.; Ardakani, A.; Duan, X.; Spechler, L.; Jeffery, T.D.; Jackson, P. Changes in Food Consumption Trends among American Adults since the COVID-19 Pandemic. Nutrients 2023, 15, 1769. [Google Scholar] [CrossRef]
- Chung, M.G.; Li, Y.; Liu, J. Global Red and Processed Meat Trade and Non-Communicable Diseases. BMJ Glob. Health 2021, 6, e006394. [Google Scholar] [CrossRef]
- Palme, M.; Simeonova, E. Does Women’s Education Affect Breast Cancer Risk and Survival? Evidence from a Population Based Social Experiment in Education. J. Health Econ. 2015, 42, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Moshoeshoe, R.; Holmes, M.D.; Subramanian, S.V.; De Neve, J.-W. Effect of Girls’ Education on Cancer Awareness and Screening in a Natural Experiment in Lesotho. Nat. Commun. 2025, 16, 3737. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.A.; Khouchani, M.; Renne, E.P. Sociocultural Barriers Related to Late-Stage Presentation of Breast Cancer in Morocco. J. Cancer Educ. 2019, 34, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Rokhzadi, K.; Khani, S.; Khaleghpanah, K.; Daneshmehr, H.; Haji-Allahverdipoor, K. A Critical Analysis of the Potential of Formal Education Programs in Breast Cancer Management in Iran: A Qualitative Content Analysis. Innov. Pract. Breast Health 2024, 5–6, 100023. [Google Scholar] [CrossRef]
- Jiang, R.; Wang, X.; Sun, Z.; Wu, S.; Chen, S.; Cai, H. Association of Education Level with the Risk of Female Breast Cancer: A Prospective Cohort Study. BMC Womens Health 2023, 23, 91. [Google Scholar] [CrossRef]
- Pizzato, M.; McCormack, V.; Dossus, L.; Al-Alem, U.; Delpierre, C.; Lamy, S.; Macciotta, A.; Ricceri, F.; Mellemkjær, L.; Tjønneland, A.; et al. Education Level and Risk of Breast Cancer by Tumor Subtype in the EPIC Cohort. Int. J. Cancer 2025, 157, 672–686. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Qin, L.-Q. Education Level and Breast Cancer Incidence: A Meta-Analysis of Cohort Studies. Menopause 2020, 27, 113–118. [Google Scholar] [CrossRef]
- Stordal, B. Breastfeeding Reduces the Risk of Breast Cancer: A Call for Action in High-Income Countries with Low Rates of Breastfeeding. Cancer Med. 2023, 12, 4616–4625. [Google Scholar] [CrossRef]
- Wallace, R.B.; Sherman, B.M.; Bean, J.A. A Case-Control Study of Breast Cancer and Psychotropic Drug Use. Oncology 1982, 39, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Dahlman, D.; Magnusson, H.; Li, X.; Sundquist, J.; Sundquist, K. Drug Use Disorder and Risk of Incident and Fatal Breast Cancer: A Nationwide Epidemiological Study. Breast Cancer Res. Treat. 2021, 186, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Medgyesi, D.N.; Trabert, B.; Sampson, J.; Weyer, P.J.; Prizment, A.; Fisher, J.A.; Beane Freeman, L.E.; Ward, M.H.; Jones, R.R. Drinking Water Disinfection Byproducts, Ingested Nitrate, and Risk of Endometrial Cancer in Postmenopausal Women. Environ. Health Perspect. 2022, 130, 057012. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Laird-Fick, H.S.; Wali, R.K.; Roy, H.K. Surveillance for Gastrointestinal Malignancies. World J. Gastroenterol. WJG 2012, 18, 4507. [Google Scholar] [CrossRef]
- Filip, R.; Gheorghita Puscaselu, R.; Anchidin-Norocel, L.; Dimian, M.; Savage, W.K. Global Challenges to Public Health Care Systems during the COVID-19 Pandemic: A Review of Pandemic Measures and Problems. J. Pers. Med. 2022, 12, 1295. [Google Scholar] [CrossRef]
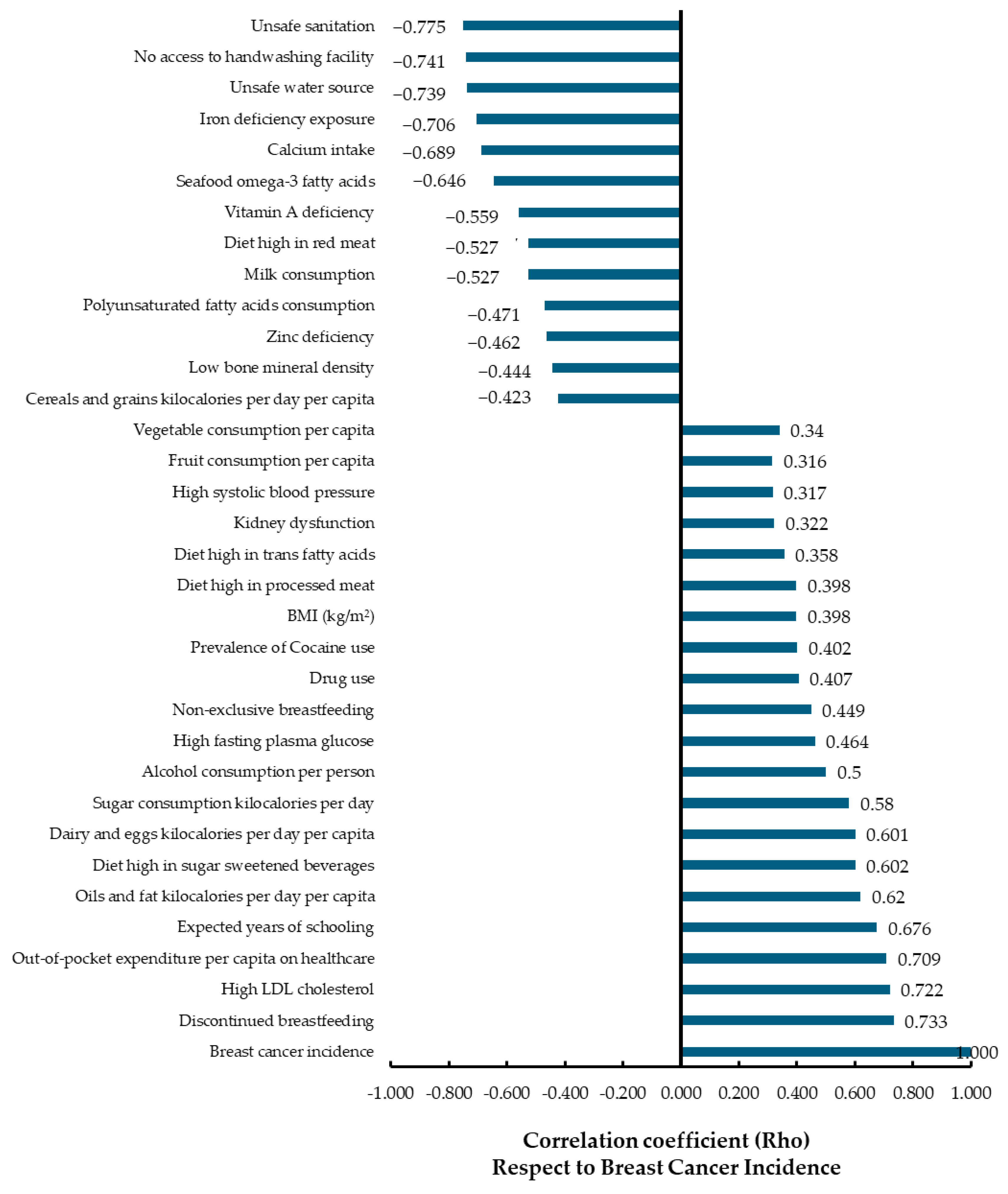

| Type of Variable | Variable | Description | Data Source |
|---|---|---|---|
| Epidemiology | Breast cancer incidence | Estimated age-standardized incidence rates in 2020, breast cancer, females, all ages | Global Cancer Observatory (GCO), hosted by the International Agency for Research on Cancer (IARC), World Health Organization (WHO) [3] |
| Social and Health | Expected years of schooling | The number of years a child of school entrance age can expect to receive if the current age specific enrolment rates persist throughout the child’s years of schooling. 2021 | United Nations Development Programme (UNDP)—Human Development Report, processed by Our World in Data [13] |
| Out-of-pocket expenditure per capita on healthcare | Estimates the average health expenditure through out-of-pocket payments per capita, indicates how much every person pays out of pocket on average in USD PPP at the point of use. High out of pocket payments are associated with catastrophic and impoverishing household spending. 2019 | World Health Organization (WHO) via World Bank, processed by Our World in Data [14] | |
| Drug use | The drug use risk factor includes the risk of suicide in prevalent cases of opioid, amphetamine, and cocaine use disorders, as well as the cumulative incidence of bloodborne infections due to current and past injection drug use. | Institute for Health Metrics and Evaluation (IHME) and Global Burden of Disease Study (GBD) 2019 [15] | |
| Unsafe water source | Women of all ages exposed to unsafe water at its primary source, 2019. (Rate exposure per 100) | ||
| Unsafe sanitation | Females exposed to unsafe sanitation based on the primary toilet type used, 2019. (Rate of exposure per 100) | ||
| No access to handwashing facility | Female exposure to no access to handwashing facility with available soap and water, 2019. (Rate per 100) | ||
| Prevalence of Cocaine use | Annual Prevalence (percentage) of the use of cocaine, by region and globally. Cocaine includes cocaine salt, “crack” cocaine and other types such as coca paste, cocaine base, basuco, paco and merla. Data period could include years 2015–2021 | United Nations Office on Drugs and Crime (UNODC) [16] | |
| Nutritional | Alcohol consumption per person | Alcohol consumption per person, 2018. Consumption of alcohol is measured in liters of pure alcohol per person aged 15 or older, per year. | Our World in Data, using data from World Health Organization (WHO) [17] |
| BMI (kg/m2) | Mean BMI (kg/m2) (age-standardized estimate) Female 2019 | IHME, GBD 2019, and WHO [18] | |
| High fasting plasma glucose | Female exposure to high fasting plasma glucose among all age groups in 2019, values represented by the rate exposure per 100 individuals. | IHME (The Institute for Health Metrics and Evaluation) with Global Burden of Disease (GBD) study 2019 [15] | |
| High LDL cholesterol | Female exposure to high LDL cholesterol levels across all age groups in 2019 is presented as the rate of exposure per 100 individuals. | ||
| High systolic blood pressure | Female exposure to high systolic blood pressure across all age groups in 2019, values represented by the rate of exposure per 100 individuals. | ||
| Low bone mineral density | Female exposure to low bone mineral density among all age groups in 2019, presented as rate of exposure per 100 individuals. | ||
| Kidney dysfunction | Female exposure to kidney dysfunction across all age groups in 2019 presented as the rate of exposure per 100 individuals. is defined as estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 or albumin to creatinine ratio (ACR) ≥30 mg/g. The theoretical minimum risk exposure level value is ACR <30 mg/g and eGFR ≥60 mL/min/1.73 m2. | Institute for Health Metrics and Evaluation (IHME) with Global Burden of Disease (GBD) study 2019 [15] | |
| Iron deficiency exposure | Female exposure to iron deficiency among all age groups in 2019 presented as the rate of exposure per 100 individuals. Defined as iron deficiency exposure was operationalized as the modeled population mean hemoglobin for a given location, year, age, and sex. | ||
| Zinc deficiency | Female exposure to zinc deficiency across all age groups in 2019 presented as the rate of exposure per 100 individuals. | ||
| Vitamin A deficiency | Female exposure to vitamin A deficiency across all age groups in 2019 presented as the rate of exposure per 100 individuals. | ||
| Fruit consumption per capita | Fruit consumption per capita, 2020. Average fruit consumption per person, measured in kilograms per year (kg/person/year). | Our World in Data, using data from World Health Organization (WHO) [19,20] | |
| Vegetable consumption per capita | Vegetable consumption per capita, 2020. Average per capita vegetable consumption, measured in kilograms per person per year (kg/person/year). | ||
| Cereals and grains kilocalories per day per capita | Average daily kilocalories consumption by cereals and grains (2020): “This data represents the daily per capita supply of calories categorized by food group, specifically cereals and grains, for all age groups in the year 2020. | Food and Agriculture Organization of the United Nations (FAO) and Our World in Data [21] | |
| Sugar consumption kilocalories per day | This data represents the daily per capita supply of calories from sugar, measured in kilocalories, for the year 2020. | Our World in Data, using data from World Health Organization (WHO) [22,23] | |
| Dairy and eggs kilocalories per day per capita | Represents the daily per capita supply of calories categorized by food group, specifically dairy (milk) and eggs, for all age groups in the year 2020. | ||
| Oils and fat kilocalories per day per capita | This data represents the average daily per capita supply of dietary fat, measured in grams per person per day, for the year 2020. | ||
| Diet high in red meat | Female exposure to a diet high in red meat across all age groups in 2019 is presented as the rate of exposure per 100 individuals. Defined as intake above an average of 0 g per day (95% UI 0–200) of unprocessed red meat. Unprocessed red meat includes pork and bovine meats such as beef, lamb, and goat, but excludes all processed meats, poultry, fish, and eggs. | ||
| Diet high in processed meat | Female exposure to a diet high in processed meat across all age groups in 2019 is presented as the rate of Summary Exposure Value (SEV) per 100 individuals. Diet high in processed meat is defined as any intake (in grams per day) of meat preserved by smoking, curing, salting, or addition of chemical preservatives. | Institute for Health Metrics and Evaluation (IHME) and Global Burden of Disease Study (GBD) [19,23] | |
| Seafood omega-3 fatty acids consumption | Defined as average daily consumption (in milligrams per day) of less than 470–660 milligrams of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) from seafood sources. | ||
| Polyunsaturated fatty acids consumption | Defined as average daily consumption (in % daily energy) of less than 9–10% total energy intake from omega-6, specifically linoleic acid, γ-linolenic acid, eicosadienoic acid, dihomo-γ-linolenic acid, and arachidonic acid. | ||
| Diet high in trans fatty acids | Female exposure to a diet high in trans fatty acids across all age groups in 2019 is presented as the rate of exposure per 100 individuals. Defined as intake greater than 0–1·1% daily energy of trans fat from all sources, mainly partially hydrogenated vegetable oils and ruminant products. | ||
| Diet high in sugar-sweetened beverages | Female exposure to a diet high in sugar-sweetened beverages across all age groups in 2019 is presented as the rate of exposure per 100 individuals. Defined as any intake (in grams per day) of beverages with ≥50 kcal per 226·8 g serving, including carbonated beverages, sodas, energy drinks, and fruit drinks, but excluding 100% fruit and vegetable juices. | ||
| Milk consumption | Defined as average daily consumption in grams per day from all dairy milk sources, including non-fat, low-fat, and full-fat, and excluding soy milk and other plant derivatives. The optimal intake for females is defined as 500–610 g per day | ||
| Calcium intake | Calcium intake is defined as average daily consumption of dietary calcium in grams per day from all sources, including milk, yogurt, and cheese. | ||
| Non-exclusive breastfeeding | Female exposure to non-exclusive breastfeeding in 2019 is presented as rate per 100 individuals. | ||
| Discontinued breastfeeding | Female exposure to discontinued breastfeeding in 2019 is presented as the rate of exposure per 100 individuals. (refers to the process in which a mother stops breastfeeding her child or less than 6 months of breastfeeding). |
| Model | R | R2 | Adjusted R2 | Standard Error of Estimate | Change in R Square | Contribution Per Variable (Coefficients) | |
|---|---|---|---|---|---|---|---|
| 1 | 0.754 | 0.538 | 0.565 | 15.075 | 0.568 | Constant | 15.407 |
| Discontinued breastfeeding | 2.522 | ||||||
| 2 | 0.796 | 0.634 | 0.629 | 13.931 | 0.065 | Constant | 14.407 |
| Discontinued breastfeeding | 2.226 | ||||||
| Prevalence of Cocaine use | 7.811 | ||||||
| 3 | 0.826 | 0.682 | 0.675 | 13.029 | 0.048 | Constant | 32.170 |
| Discontinued breastfeeding | 1.488 | ||||||
| Prevalence of Cocaine use | 7.330 | ||||||
| Unsafe sanitation | −0.251 | ||||||
| 4 | 0.843 | 0.710 | 0.702 | 12.477 | 0.028 | Constant | 31.338 |
| Discontinued breastfeeding | 1.013 | ||||||
| Prevalence of Cocaine use | 7.179 | ||||||
| Unsafe sanitation | −0.202 | ||||||
| Out-of-pocket expenditure per capita on healthcare | 0.017 | ||||||
| 5 | 0.850 | 0.722 | 0.713 | 12.257 | 0.012 | Constant | 28.833 |
| Discontinued breastfeeding | 1.036 | ||||||
| Prevalence of Cocaine use | 7.795 | ||||||
| Unsafe sanitation | −0.337 | ||||||
| Out-of-pocket expenditure per capita on healthcare | 0.018 | ||||||
| No access to handwashing facility | 0.160 | ||||||
| 6 | 0.855 | 0.731 | 0.721 | 12.085 | 0.010 | Constant | 29.396 |
| Discontinued breastfeeding | 0.893 | ||||||
| Prevalence of Cocaine use | 7.273 | ||||||
| Unsafe sanitation | −0.369 | ||||||
| Out-of-pocket expenditure per capita on healthcare | 0.013 | ||||||
| No access to handwashing facility | 0.161 | ||||||
| Diet high in processed meat | 0.123 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Martinez, J.; Hernández-Fuentes, G.A.; Delgado-Enciso, J.; Alcalá-Pérez, M.A.; Jiménez-Calvo, I.; Sánchez-Ramírez, C.A.; Rojas-Larios, F.; Rodriguez-Hernandez, A.; Ramírez-Flores, M.; Guzmán-Esquivel, J.; et al. Beyond Biology: Uncovering Structural and Sociocultural Predictors of Breast Cancer Incidence Worldwide. Curr. Oncol. 2025, 32, 553. https://doi.org/10.3390/curroncol32100553
Diaz-Martinez J, Hernández-Fuentes GA, Delgado-Enciso J, Alcalá-Pérez MA, Jiménez-Calvo I, Sánchez-Ramírez CA, Rojas-Larios F, Rodriguez-Hernandez A, Ramírez-Flores M, Guzmán-Esquivel J, et al. Beyond Biology: Uncovering Structural and Sociocultural Predictors of Breast Cancer Incidence Worldwide. Current Oncology. 2025; 32(10):553. https://doi.org/10.3390/curroncol32100553
Chicago/Turabian StyleDiaz-Martinez, Janet, Gustavo A. Hernández-Fuentes, Josuel Delgado-Enciso, Mario A. Alcalá-Pérez, Isaac Jiménez-Calvo, Carmen A. Sánchez-Ramírez, Fabian Rojas-Larios, Alejandrina Rodriguez-Hernandez, Mario Ramírez-Flores, José Guzmán-Esquivel, and et al. 2025. "Beyond Biology: Uncovering Structural and Sociocultural Predictors of Breast Cancer Incidence Worldwide" Current Oncology 32, no. 10: 553. https://doi.org/10.3390/curroncol32100553
APA StyleDiaz-Martinez, J., Hernández-Fuentes, G. A., Delgado-Enciso, J., Alcalá-Pérez, M. A., Jiménez-Calvo, I., Sánchez-Ramírez, C. A., Rojas-Larios, F., Rodriguez-Hernandez, A., Ramírez-Flores, M., Guzmán-Esquivel, J., Sánchez-Meza, K., Espíritu-Mojarro, A. C., Montesinos-López, O. A., & Delgado-Enciso, I. (2025). Beyond Biology: Uncovering Structural and Sociocultural Predictors of Breast Cancer Incidence Worldwide. Current Oncology, 32(10), 553. https://doi.org/10.3390/curroncol32100553