Removal of a Recurrent Calvarial Hemangioma Followed by Autologous Iliac Crest Bone Reconstruction: A Case-Based Experience
Simple Summary
Abstract
1. Introduction
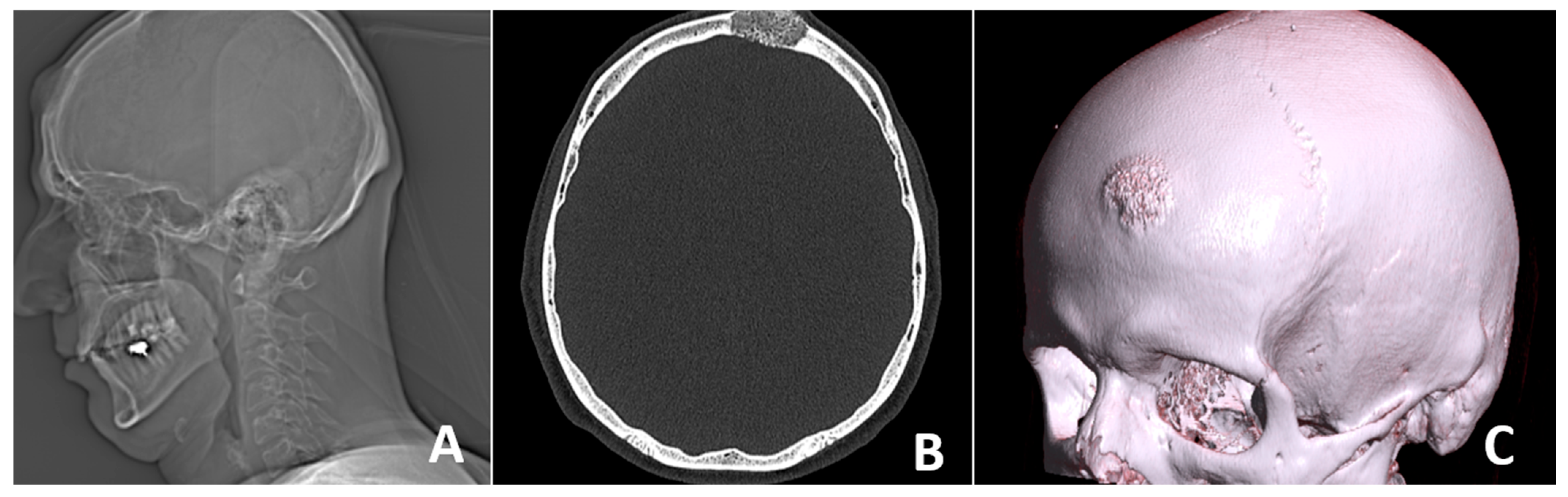
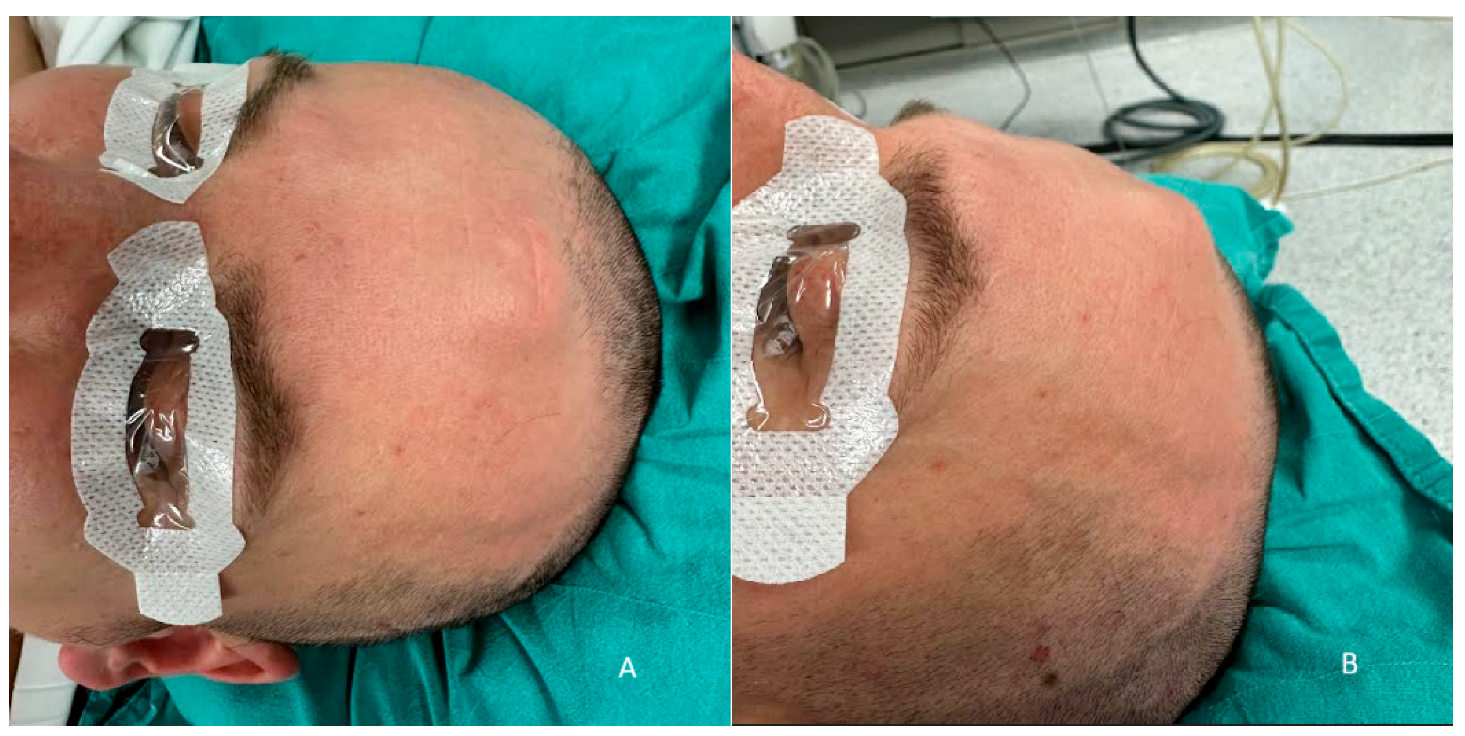
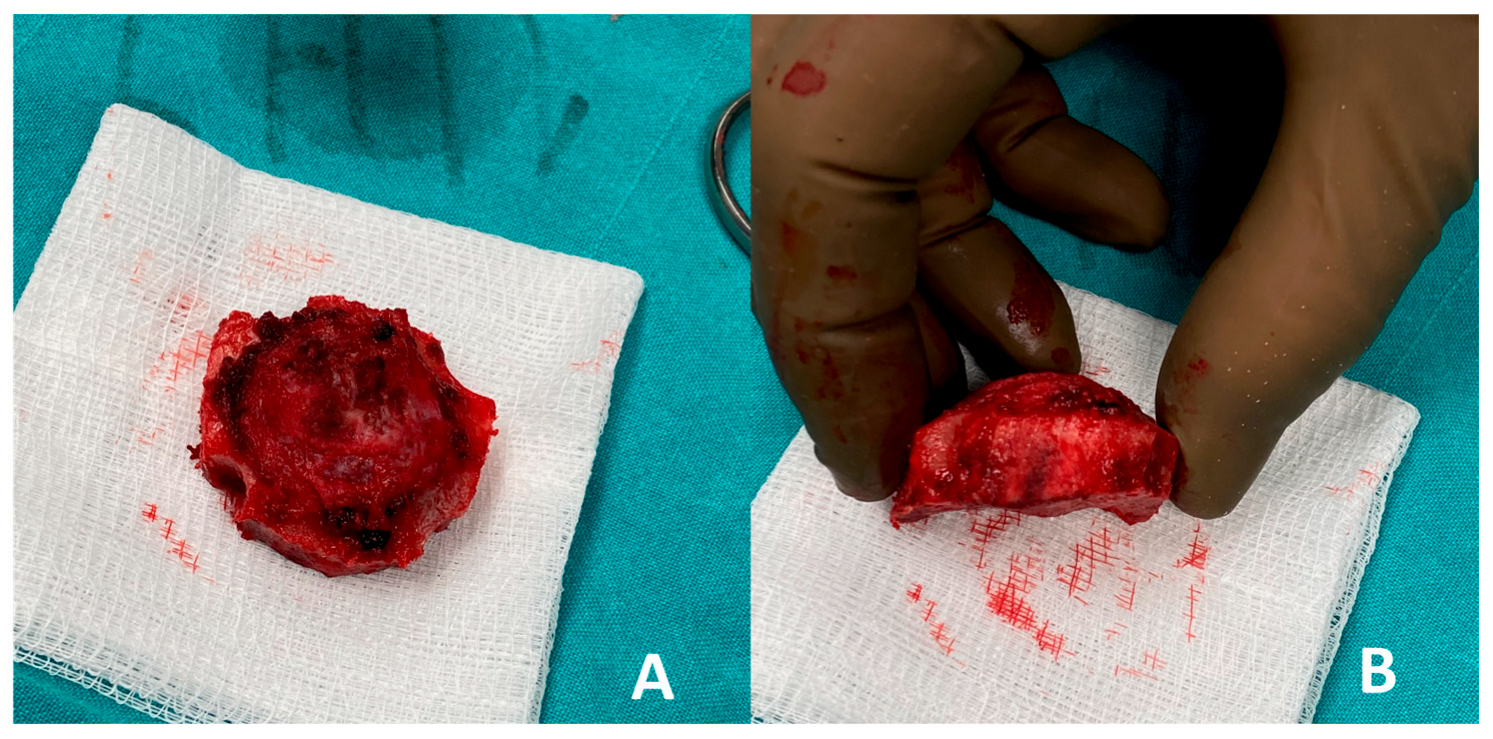
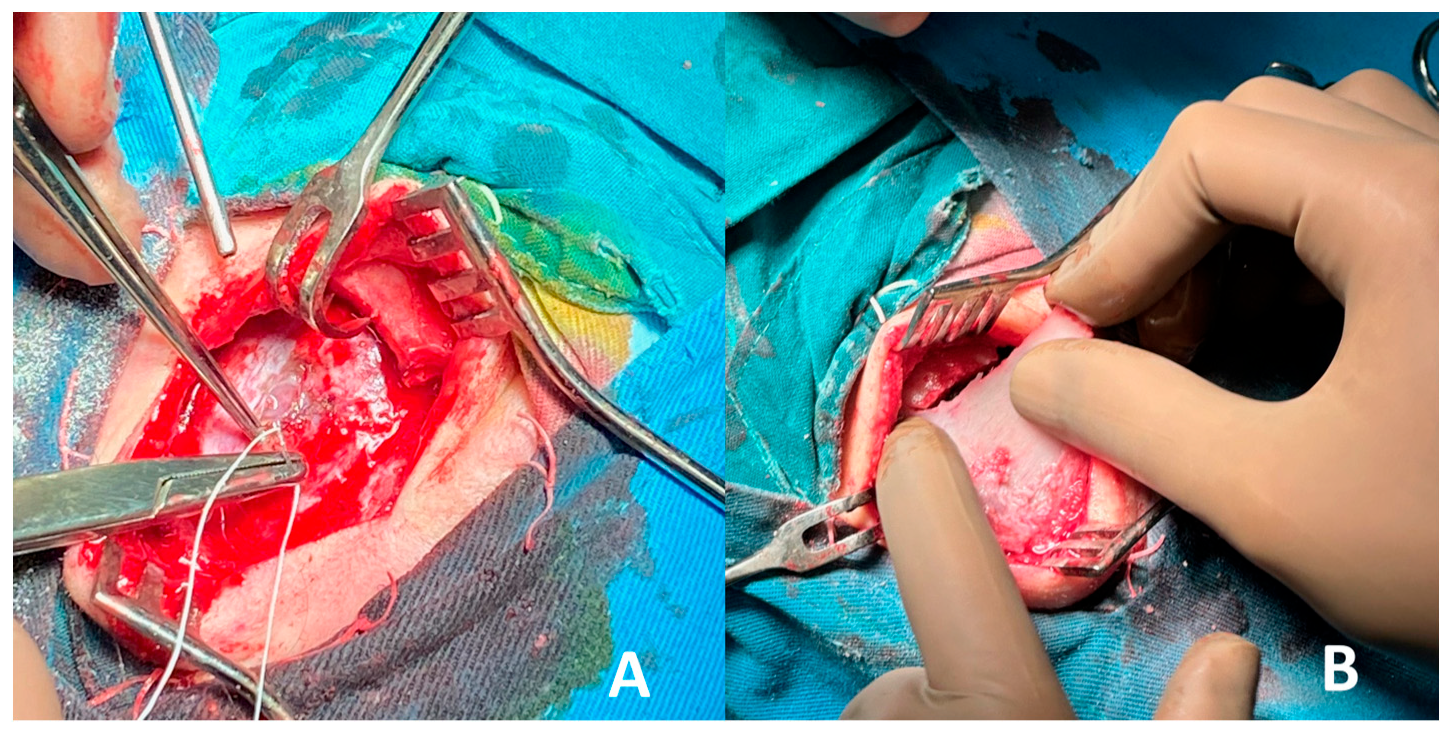
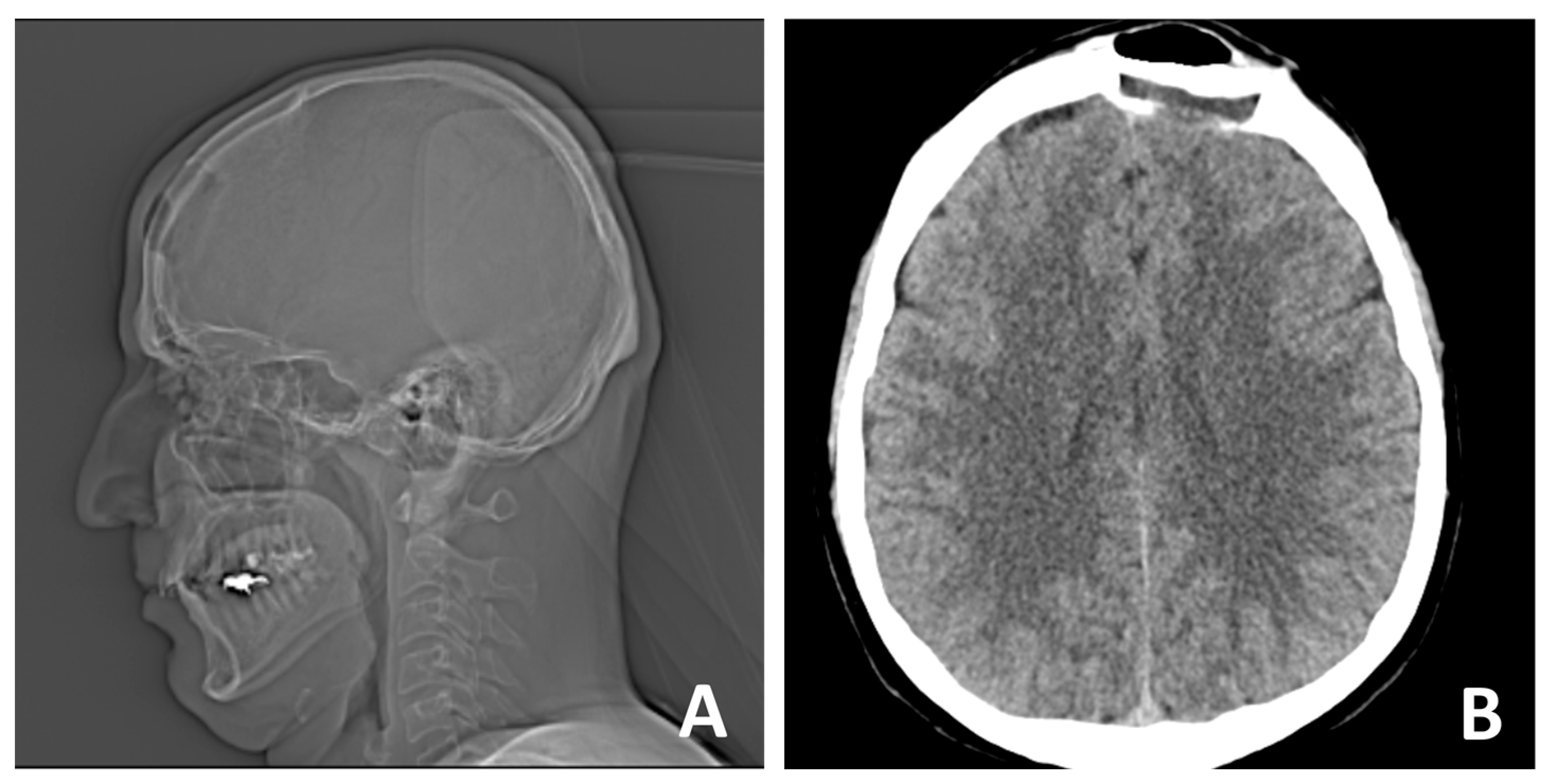
2. Case Report
3. Results and Discussion
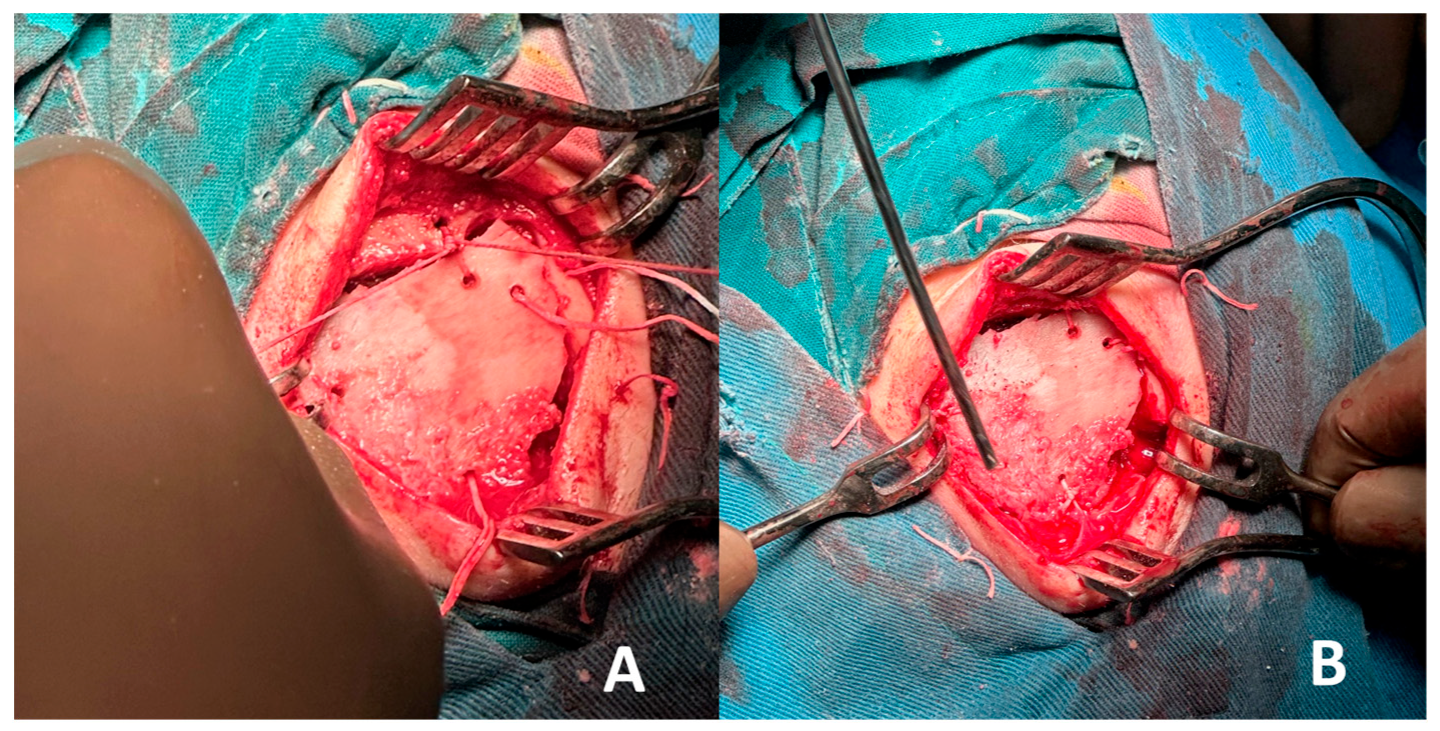
- Autologous iliac crest bone grafts offer robust cortical and cancellous components suitable for reconstructing moderate to large cranial defects and remain the gold standard for calvarial reconstruction due to their osteoconductive, osteoinductive, and osteogenic properties. Despite potential donor-site morbidity, including pain, gait disturbance, hematoma, or sensory disturbance, the iliac crest remains a favored source due to its accessibility and structural quality [28,29,30]. In our case, the bone graft was successfully harvested and contoured to match the frontal defect and was secured with vicryl suture, achieving a stable reconstruction and satisfactory aesthetic outcome. Especially in the frontal region, calvarial grafts and iliac crest grafts yield excellent contour match and longevity.
- Intraosseous cavernous hemangiomas of the skull often involve the diploic space. Although rare, cases treated with split-calvarial grafts after resection have shown good long-term outcomes, including stability and minimal recurrence over follow-up (~5 years) [29]. Such lesions affecting the diploe warrant en bloc removal with margins to prevent recurrences, followed by primary bone reconstruction [31]. Non-vascularized iliac crest bone grafts have also been used for frontal sinus obliteration, with minimal morbidity and no significant resorption at one and five-year follow-up. Vascularized iliac crest flaps (e.g., DCIA) have also been described in frontal cranioplasty, combining a muscle cuff and skin paddle for dead-space obliteration, offering rapid integration and sustained volume over time [29,32]. When local calvarium cannot be harvested or is inadequate, iliac crest grafts offer robust corticocancellous bone with sufficient volume and curvature to restore frontal contour. The iliac crest provides a broad surface suitable for large frontal defects and can be contoured to match convexity without multi-segment constructs [33,34].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed Tomography |
| DCIA | Deep Circumflex Iliac Artery |
| NOE | Naso-ethmoid-orbital |
| PSI | Patient Specific Implant |
| PMMA | poly-methyl-meta-acrylate |
| PEEK | polyetheretherketone |
References
- Ginsberg, L.E. Imaging of perineural tumor spread in head and neck cancer. Semin. Ultrasound CT MRI 1999, 20, 175–186. [Google Scholar]
- Garfinkle, J.; Melançon, D.; Cortes, M.; Tampieri, D. Imaging pattern of calvarial lesions in adults. Skelet. Radiol. 2011, 40, 1261–1273. [Google Scholar]
- Shimanuki, M.N.; Nishiyama, T.; Hosoya, M.; Wakabayashi, T.; Ozawa, H.; Oishi, N. Imaging of temporal bone mass lesions: A pictorial review. Diagnostics 2023, 13, 2665. [Google Scholar] [CrossRef] [PubMed]
- Gordhan, A.; Soliman, J.; Malpani, A.; Peg, E., III. Symptomatic calvarial cavernous hemangioma: Presurgical confirmation by scintigraphy. J. Radiol. Case Rep. 2009, 3, 25. [Google Scholar] [CrossRef]
- Koulouris, G.; Rao, P. Multiple congenital cranial hemangiomas. Skelet. Radiol. 2005, 34, 485–489. [Google Scholar] [CrossRef]
- Alexandre, A.M.; Romi, A.; Gaudino, S.; Gessi, M.; Frassanito, P.; Camilli, A.; Luca, S.; Pedicelli, A. Giant Congenital Hemangioma of the Skull: Prenatal Diagnosis and Multimodal Endovascular and Surgical Management. Medicina 2024, 60, 145. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Bhosle, R.; Patel, S.; Raju, D.; Cincu, R.; Moscote-Salazar, L.R.; Gupta, A.; Agrawal, A. Calvarial hemangiomas: Series of 6 cases and review of literature. World Neurosurg. X 2024, 23, 100297. [Google Scholar] [PubMed]
- Maiorano, E.; Spena, G.; Sovardi, F.; Dehgani-Mobaraki, P.; Pagella, F.; Montalbetti, A.; Peppucci, E.; Grasso, C.; Zoia, C. Extremely rare pathologies of the craniovertebral junction region: A case series and review of the literature. Surgeries 2023, 4, 420–433. [Google Scholar] [CrossRef]
- Khanam, H.; Lipper, M.H.; Wolff, C.L.; Lopes, M.B.S. Calvarial hemangiomas: Report of two cases and review of the literature. Surg. Neurol. 2001, 55, 63–67. [Google Scholar] [CrossRef]
- Hong, B.; Hermann, E.J.; Klein, R.; Krauss, J.K.; Nakamura, M. Surgical resection of osteolytic calvarial lesions: Clinicopathological features. Clin. Neurol. Neurosurg. 2010, 112, 865–869. [Google Scholar] [CrossRef]
- Wecht, D.A.; Sawaya, R. Lesions of the calvaria: Surgical experience with 42 patients. Ann. Surg. Oncol. 1997, 4, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Nasi-Kordhishti, I.; Hempel, J.M.; Ebner, F.H.; Tatagiba, M. Calvarial lesions: Overview of imaging features and neurosurgical management. Neurosurg. Rev. 2021, 44, 3459–3469. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Choi, C.H. A case of calvarial hemangioma in cranioplasty site. J. Korean Neurosurg. Soc. 2009, 46, 484. [Google Scholar] [CrossRef]
- Artico, M.; Ferrante, L.; Pastore, F.S.; Ramundo, E.O.; Cantarelli, D.; Scopelliti, D.; Iannetti, G. Bone autografting of the calvaria and craniofacial skeleton: Historical background, surgical results in a series of 15 patients, and review of the literature. Surg. Neurol. 2003, 60, 71–79. [Google Scholar] [PubMed]
- Bhardwaj, S.; Pendem, S.; Selvarasu, K.; Krishnan, M. A Novel Method of Frontal Bone Reconstruction Using Patient-Specific Implants and Costochondral Grafts: A Case Report. Cureus 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.; Estévez, O.; Centella, C.; Sanjuan-Sanjuan, A.; Sánchez-Frías, M.E.; Alamillos, F.J. Surgical Navigation and CAD-CAM-Designed PEEK Prosthesis for the Surgical Treatment of Facial Intraosseous Vascular Anomalies. J. Clin. Med. 2024, 13, 4602. [Google Scholar] [CrossRef]
- Umana, G.E.; Ranganathan, S.; Marrone, S.; Naimo, J.; Giunta, M.; Spitaleri, A.; Fricia, M.; Ferini, G.; Scalia, G. Optimizing Strategies in Patients Affected by Tumors Infiltrating the Skull: A Single Center Experience. Brain Sci. 2025, 15, 420. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, A.F.; Rossi, G.; Calabrò, T.; Altimari, G.; Rimondi, E.; Ruggieri, P. The role of embolization for hemangiomas. Musculoskelet. Surg. 2012, 96, 125–135. [Google Scholar] [CrossRef]
- Tyagi, D.K.; Balasubramaniam, S.; Sawant, H.V. Giant primary ossified cavernous hemangioma of the skull in an adult: A rare calvarial tumor. J. Neurosci. Rural. Pract. 2011, 2, 174. [Google Scholar] [CrossRef]
- Yoshimura, J.; Tsukamoto, Y.; Sano, M.; Hasegawa, H.; Nishino, K.; Saito, A.; Fukuda, M.; Okamoto, K.; Fujii, Y. Successful removal of a huge hypervascular tentorial cavernous angioma after preoperative endovascular embolization: Case report. J. Neurosurg. Pediatr. 2014, 14, 43–47. [Google Scholar] [CrossRef]
- Shen, Z.; Mizumoto, M.; Oshiro, Y.; Jin, Y.; Wang, J.; Zhang, S.; Liu, C.; Wang, Z.; Li, Y.; Wang, W.; et al. Effective local control of a giant calvarial hemangioma in a child by proton beam therapy: A case report and literature review. Exp. Ther. Med. 2024, 29, 14. [Google Scholar] [CrossRef] [PubMed]
- Rootman, D.B.; Rootman, J.; Gregory, S.; Feldman, K.A.; Ma, R. Stereotactic fractionated radiotherapy for cavernous venous malformations (hemangioma) of the orbit. Ophthalmic Plast. Reconstr. Surg. 2012, 28, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Helmy, M.; Liao, Y.; Zhang, Y.; He, K. The treatment outcomes of radiotherapy and surgical treatment for patients with cavernous sinus hemangioma: A meta-analysis. World Neurosurg. 2023, 178, e345. [Google Scholar] [CrossRef]
- Brichacek, M.; Naeem, A.; Filler, G.; Hammond, R.; Yazdani, A.; Ranger, A. Congenital calvarial hemangioma. J. Craniofacial Surg. 2018, 29, 1625–1628. [Google Scholar] [CrossRef] [PubMed]
- Vural, M.; Acikalin, M.F.; Adapinar, B.; Atasoy, M.A. Congenital cavernous hemangioma of the calvaria: Case report. J. Neurosurg. Pediatr. 2009, 3, 41–45. [Google Scholar] [PubMed]
- Costanzo, R.; Rosetti, V.; Tomassini, A.; Fuschillo, D.; Lofrese, G.; Iacopino, D.G.; Tosatto, L.; D’Andrea, M. Hypothalamic hemangioma-like pilocytic astrocytoma in an adult patient: A systematic review with a focus on differential diagnosis and neurological presentation. J. Clin. Med. 2024, 13, 3536. [Google Scholar] [CrossRef]
- Kotowski, M. The differential diagnosis of congenital developmental midline nasal masses: Histopathological, clinical, and radiological aspects. Diagnostics 2023, 13, 2796. [Google Scholar] [CrossRef]
- EScheerlinck, L.M.; MMuradin, M.S.; van der Bilt, A.; Meijer, G.J.; Koole, R.; Van Cann, E.M. Donor site complications in bone grafting: Comparison of iliac crest, calvarial, and mandibular ramus bone. Int. J. Oral Maxillofac. Implant. 2013, 28, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, D.E.; Klein-Nulend, J.; van Ruijven, L.J.; Schortinghuis, J.; Vissink, A.; Raghoebar, G.M. Incorporation of anterior iliac crest or calvarial bone grafts in reconstructed atrophied maxillae: A randomized clinical trial with histomorphometric and micro-CT analyses. Clin. Implant. Dent. Relat. Res. 2021, 23, 492–502. [Google Scholar] [PubMed]
- Araújo, C.R.; Astarita, C.; D’Aquino, R.; Pelegrine, A.A. Evaluation of bone regeneration in rat calvaria using bone autologous micrografts and xenografts: Histological and histomorphometric analysis. Materials 2020, 13, 4284. [Google Scholar]
- Alexiou, G.A.; Lampros, M.; Gavra, M.M.; Vlachos, N.; Ydreos, J.; Boviatsis, E.J. Primary intraosseous cavernous hemangioma of the cranium: A systematic review of the literature. World Neurosurg. 2022, 164, 323–329. [Google Scholar] [CrossRef]
- Bagherzadegan, N.; Hohlweg-Majert, B.; Mücke, T.; Haerle, S.; Deppe, H.; Wolff, K.D.; Hölzle, F. Microvascular bone grafting: A new long-term solution for intraosseous arteriovenous malformations of the mandible in children. J. Cranio-Maxillofac. Surg. 2011, 39, 431–434. [Google Scholar]
- Zhang, J.; Li, S.; He, H.; Han, L.; Zhang, S.; Yang, L.; Han, W.; Wang, X.; Gao, J.; Zhao, J.; et al. Clinical guidelines for indications, techniques, and complications of autogenous bone grafting. Chin. Med. J. 2024, 137, 5–7. [Google Scholar]
- Crespi, R.; Vinci, R.; Cappare, P.; Gherlone, E.; Romanos, G.E. Calvarial versus iliac crest for autologous bone graft material for a sinus lift procedure: A histomorphometric study. Int. J. Oral Maxillofac. Implant. 2007, 22, 527–532. [Google Scholar]
- Carinci, F.; Farina, A.; Zanetti, U.; Vinci, R.; Negrini, S.; Calura, G.; Laino, G.; Piattelli, A. Alveolar ridge augmentation: A comparative longitudinal study between calvaria and iliac crest bone grafts. J. Oral Implantol. 2005, 31, 39–45. [Google Scholar] [CrossRef]
- Neovius, E.; Engstrand, T. Craniofacial reconstruction with bone and biomaterials: Review over the last 11 years. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 1615–1623. [Google Scholar] [CrossRef]
- Zins, J.E.; Türegün, M.C.; Hosn, W.; Bauer, T.W. Reconstruction of intraosseous hemangiomas of the midface using split calvarial bone grafts. Plast. Reconstr. Surg. 2006, 117, 948–953. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.G.; Zide, B.M. The spectrum of calvarial bone grafting: Introduction of the vascularized calvarial bone flap. Plast. Reconstr. Surg. 1984, 74, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Simsek, E.; Bingöl, F.; Kilic, K.; Çalik, I. Mixed hemangioma of the nasal cavity destructing the nasal bone. J. Craniofacial Surg. 2017, 28, 846. [Google Scholar] [CrossRef] [PubMed]
- Liberale, C.; Rozell-Shannon, L.; Moneghini, L.; Nocini, R.; Tombris, S.; Colletti, G. Stop calling me cavernous hemangioma! A literature review on misdiagnosed bony vascular anomalies. J. Investig. Surg. 2022, 35, 141–150. [Google Scholar]
- Baudoin, M.E.; Palines, P.A.; Stalder, M.W. Frontal cranioplasty with vascularized split-iliac crest bone flap. Plast. Reconstr. Surg.-Glob. Open 2021, 9, e3934. [Google Scholar]
- Prasad, G.L.; Pai, K. Pediatric cranial intraosseous hemangiomas: A review. Neurosurg. Rev. 2018, 41, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Salazar Davern, M.; Lovell, M.A.; Wilkinson, C.C. Cavernous hemangiomas of the pediatric calvaria. Pediatr. Neurosurg. 2015, 50, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Badhey, A.; Kadakia, S.; Mourad, M.; Inman, J.; Ducic, Y. Calvarial reconstruction. Semin. Plast. Surg. 2017, 31, 222–226. [Google Scholar] [CrossRef]
- Chattopadhyay, C. Reconstruction of acquired frontal bone defects using titanium mesh implants: A retrospective study. J. Maxillofac. Oral Surg. 2019, 18, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Antúnez-Conde, R.; Navarro Cuéllar, C.; Salmerón Escobar, J.I.; Díez-Montiel, A.; Navarro Cuéllar, I.; Dell’Aversana Orabona, G.; de Vera, J.L.D.C.P.; Vila, C.N.; Cebrián Carretero, J.L. Intraosseous venous malformation of the zygomatic bone: Comparison between virtual surgical planning and standard surgery with review of the literature. J. Clin. Med. 2021, 10, 4565. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gigov, K.; Ginev, I.; Shopova, D. Removal of a Recurrent Calvarial Hemangioma Followed by Autologous Iliac Crest Bone Reconstruction: A Case-Based Experience. Curr. Oncol. 2025, 32, 551. https://doi.org/10.3390/curroncol32100551
Gigov K, Ginev I, Shopova D. Removal of a Recurrent Calvarial Hemangioma Followed by Autologous Iliac Crest Bone Reconstruction: A Case-Based Experience. Current Oncology. 2025; 32(10):551. https://doi.org/10.3390/curroncol32100551
Chicago/Turabian StyleGigov, Kostadin, Ivan Ginev, and Dobromira Shopova. 2025. "Removal of a Recurrent Calvarial Hemangioma Followed by Autologous Iliac Crest Bone Reconstruction: A Case-Based Experience" Current Oncology 32, no. 10: 551. https://doi.org/10.3390/curroncol32100551
APA StyleGigov, K., Ginev, I., & Shopova, D. (2025). Removal of a Recurrent Calvarial Hemangioma Followed by Autologous Iliac Crest Bone Reconstruction: A Case-Based Experience. Current Oncology, 32(10), 551. https://doi.org/10.3390/curroncol32100551




