From Chronic Lymphocytic Leukemia to Plasmablastic Myeloma: Beyond the Usual Richter Transformation
Simple Summary
Abstract
1. Introduction
2. Detailed Case Presentation
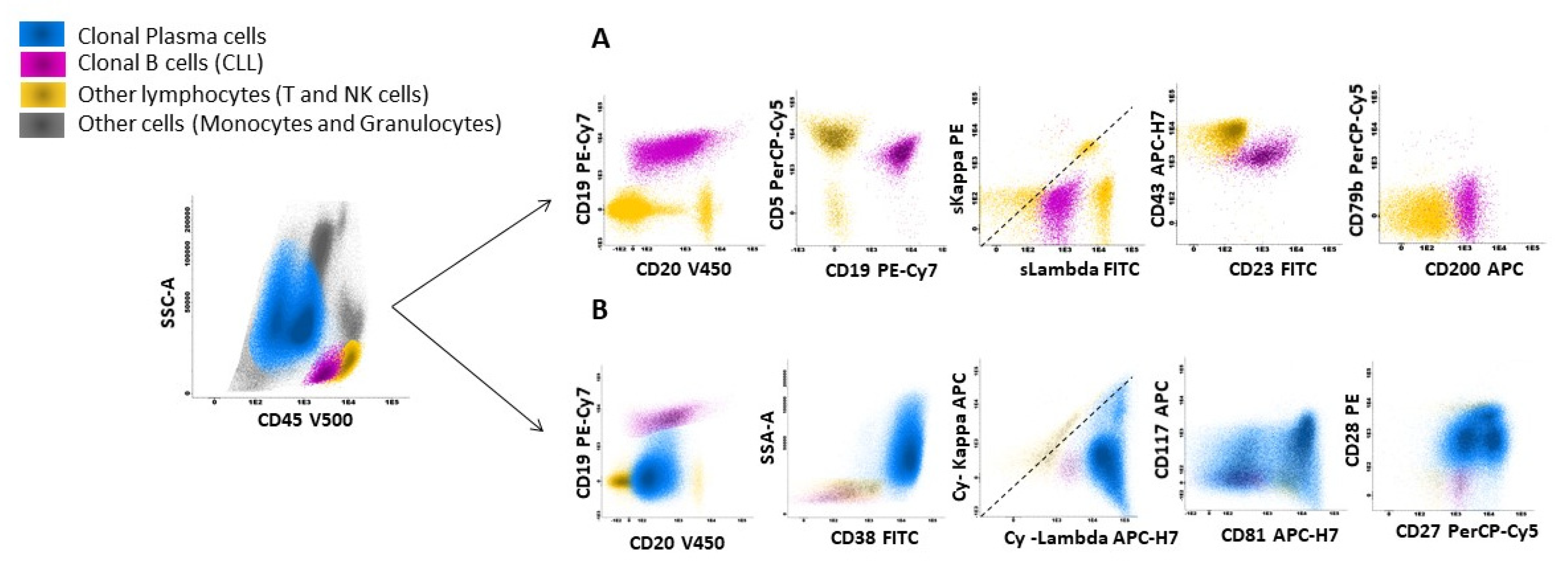
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CLL | Chronic Lymphocytic Leukemia |
| DLBCL | Diffuse Large B-Cell Lymphoma |
| MM | Multiple Myeloma |
| PBL | Plasmablastic Lymphoma |
| PBM | Plasmablastic Myeloma |
| RT | Richter Transformation |
| SLL | Small Lymphocytic Lymphoma |
| WHO | World Health Organization |
References
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.; De, O.; Berti, E.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Lenartova, A.; Randen, U.; Johannesen, T.B.; Tjønnfjord, G.E. Richter syndrome epidemiology in a large population based chronic lymphocytic leukemia cohort from Norway. Cancer Epidemiol. 2019, 60, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Spina, V.; Gaidano, G. Biology and treatment of Richter syndrome. Blood 2018, 131, 2761–2772. [Google Scholar] [CrossRef]
- Zhou, J.; Nassiri, M. Lymphoproliferative Neoplasms with Plasmablastic Morphology. Arch. Pathol. Lab. Med. 2021, 146, 407–414. [Google Scholar] [CrossRef]
- Li, J.-W.; Peng, H.-L.; Zhou, X.-Y.; Wang, J.-J. Plasmablastic lymphoma: Current knowledge and future directions. Front. Immunol. 2024, 15, 1354604. [Google Scholar] [CrossRef] [PubMed]
- Greipp, P.R.; Raymond, N.M.; Kyle, R.A.; O’Fallon, W.M. Multiple myeloma: Significance of plasmablastic subtype in morphological classification. Blood 1985, 65, 305–310. [Google Scholar] [CrossRef]
- Ahn, J.S.; Okal, R.; Vos, J.A.; Smolkin, M.; Kanate, A.S.; Rosado, F.G. Plasmablastic lymphoma versus plasmablastic myeloma: An ongoing diagnostic dilemma. J. Clin. Pathol. 2017, 70, 775–780. [Google Scholar] [CrossRef]
- Chen, B.-J.; Yuan, C.-T.; Yang, C.-F.; Ho, C.-H.; Lin, Y.-K.; Su, Y.-Z.; Chou, H.-C.; Chuang, S.-S. Plasmablastic myeloma in Taiwan frequently presents with extramedullary and extranodal mass mimicking plasmablastic lymphoma. Virchows Arch. 2022, 481, 283–293. [Google Scholar] [CrossRef]
- Møller, H.E.H.; Preiss, B.S.; Pedersen, P.; Kristensen, I.B.; Hansen, C.T.; Frederiksen, M.; Abildgaard, N.; Møller, M.B. Clinicopathological features of plasmablastic multiple myeloma: A population-based cohort. APMIS 2015, 123, 652–658. [Google Scholar] [CrossRef]
- Liu, Y.; Jelloul, F.; Zhang, Y.; Bhavsar, T.; Ho, C.; Rao, M.B.; Lewis, N.E.; Cimera, R.B.; Baik, J.B.; Sigler, A.B.; et al. Genetic Basis of Extramedullary Plasmablastic Transformation of Multiple Myeloma. Am. J. Surg. Pathol. 2020, 44, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-J.; Chuang, S.-S. Lymphoid Neoplasms With Plasmablastic Differentiation: A Comprehensive Review and Diagnostic Approaches. Adv. Anat. Pathol. 2019, 27, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- van Dongen, J.J.M.; Langerak, A.W.; Brüggemann, M.; Evans, P.A.S.; Hummel, M.; Lavender, F.L.; Delabesse, E.; Davi, F.; Schuuring, E.; García-Sanz, R.; et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003, 17, 2257–2317. [Google Scholar] [CrossRef]
- Ghia, P.; Stamatopoulos, K.; Belessi, C.; Moreno, C.; Stilgenbauer, S.; Stevenson, F.; Davi, F.; Rosenquist, R.; on behalf of the European Research Initiative on CLL (ERIC). ERIC recommendations on IGHV gene mutational status analysis in chronic lymphocytic leukemia. Leukemia 2006, 21, 1–3. [Google Scholar] [CrossRef]
- Ailawadhi, S.; Dholaria, B.R.; Khurana, S.; Sher, T.; Alegria, V.; Paulus, A.; Ailawadhi, M.; Mehta, A.; Chanan-Khan, A.; Roy, V. Outcomes of patients with simultaneous diagnosis of chronic lymphocytic leukaemia/small lymphocytic lymphoma and multiple myeloma. Br. J. Haematol. 2019, 185, 347–350. [Google Scholar] [CrossRef] [PubMed]
- van der Straten, L.; Levin, M.-D.; Dinnessen, M.A.W.; Visser, O.; Posthuma, E.F.M.; Doorduijn, J.K.; Langerak, A.W.; Kater, A.P.; Dinmohamed, A.G. Risk of second primary malignancies in patients with chronic lymphocytic leukemia: A population-based study in the Netherlands, 1989–2019. Blood Cancer J. 2023, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Chattopadhyay, S.; Sud, A.; Sundquist, K.; Sundquist, J.; Försti, A.; Houlston, R.; Hemminki, A.; Hemminki, K. Second primary cancers in patients with acute lymphoblastic, chronic lymphocytic and hairy cell leukaemia. Br. J. Haematol. 2019, 185, 232–239. [Google Scholar] [CrossRef]
- Bond, D.A.; Huang, Y.; Fisher, J.L.; Ruppert, A.S.; Owen, D.H.; Bertino, E.M.; Rogers, K.A.; Bhat, S.A.; Grever, M.R.; Jaglowski, S.M.; et al. Second cancer incidence in CLL patients receiving BTK inhibitors. Leukemia 2020, 34, 3197–3205. [Google Scholar] [CrossRef]
- Pepe, S.; Vitale, C.; Giannarelli, D.; Visentin, A.; Sanna, A.; Frustaci, A.M.; Olivieri, J.; Quaglia, F.M.; Gozzetti, A.; Sportoletti, P.; et al. Richter transformation in diffuse large B-cell lymphoma in patients with chronic lymphocytic leukemia receiving ibrutinib: Risk factors and outcomes. Leukemia 2025, 39, 1883–1891. [Google Scholar] [CrossRef]
- Kittai, A.S.; Huang, Y.; Beckwith, K.A.; Bhat, S.A.; Bond, D.A.; Byrd, J.C.; Goldstein, D.; Grever, M.R.; Miller, C.; Rogers, K.A.; et al. Patient characteristics that predict Richter’s transformation in patients with chronic lymphocytic leukemia treated with ibrutinib. Am. J. Hematol. 2022, 98, 56–65. [Google Scholar] [CrossRef]
- Ramsey, M.C.; Sabatini, P.J.B.; Smith, A.C.; Sakhdari, A. Molecular characterization and clonal evolution in Richter transformation: Insights from a case of plasmablastic lymphoma (RT-PBL) arising from chronic lymphocytic leukaemia (CLL) and review of the literature. eJHaem 2023, 4, 1203–1207. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castonguay, M.; Gagnon, M.-F.; Le Nguyen, A.; Terra, R.; Pilon, S.-J.; Lépine, G.; LeBlanc, R.; Roy, J.; Cohen, S.; Fleury, I.; et al. From Chronic Lymphocytic Leukemia to Plasmablastic Myeloma: Beyond the Usual Richter Transformation. Curr. Oncol. 2025, 32, 550. https://doi.org/10.3390/curroncol32100550
Castonguay M, Gagnon M-F, Le Nguyen A, Terra R, Pilon S-J, Lépine G, LeBlanc R, Roy J, Cohen S, Fleury I, et al. From Chronic Lymphocytic Leukemia to Plasmablastic Myeloma: Beyond the Usual Richter Transformation. Current Oncology. 2025; 32(10):550. https://doi.org/10.3390/curroncol32100550
Chicago/Turabian StyleCastonguay, Mathias, Marie-France Gagnon, Alexandre Le Nguyen, Rafik Terra, Sarah-Jeanne Pilon, Guylaine Lépine, Richard LeBlanc, Jean Roy, Sandra Cohen, Isabelle Fleury, and et al. 2025. "From Chronic Lymphocytic Leukemia to Plasmablastic Myeloma: Beyond the Usual Richter Transformation" Current Oncology 32, no. 10: 550. https://doi.org/10.3390/curroncol32100550
APA StyleCastonguay, M., Gagnon, M.-F., Le Nguyen, A., Terra, R., Pilon, S.-J., Lépine, G., LeBlanc, R., Roy, J., Cohen, S., Fleury, I., Mollica, L., Veilleux, O., & Claveau, J.-S. (2025). From Chronic Lymphocytic Leukemia to Plasmablastic Myeloma: Beyond the Usual Richter Transformation. Current Oncology, 32(10), 550. https://doi.org/10.3390/curroncol32100550





