Bridging Knowledge Gaps in Small Cell Lung Cancer: Data, Challenges and Priorities
Simple Summary
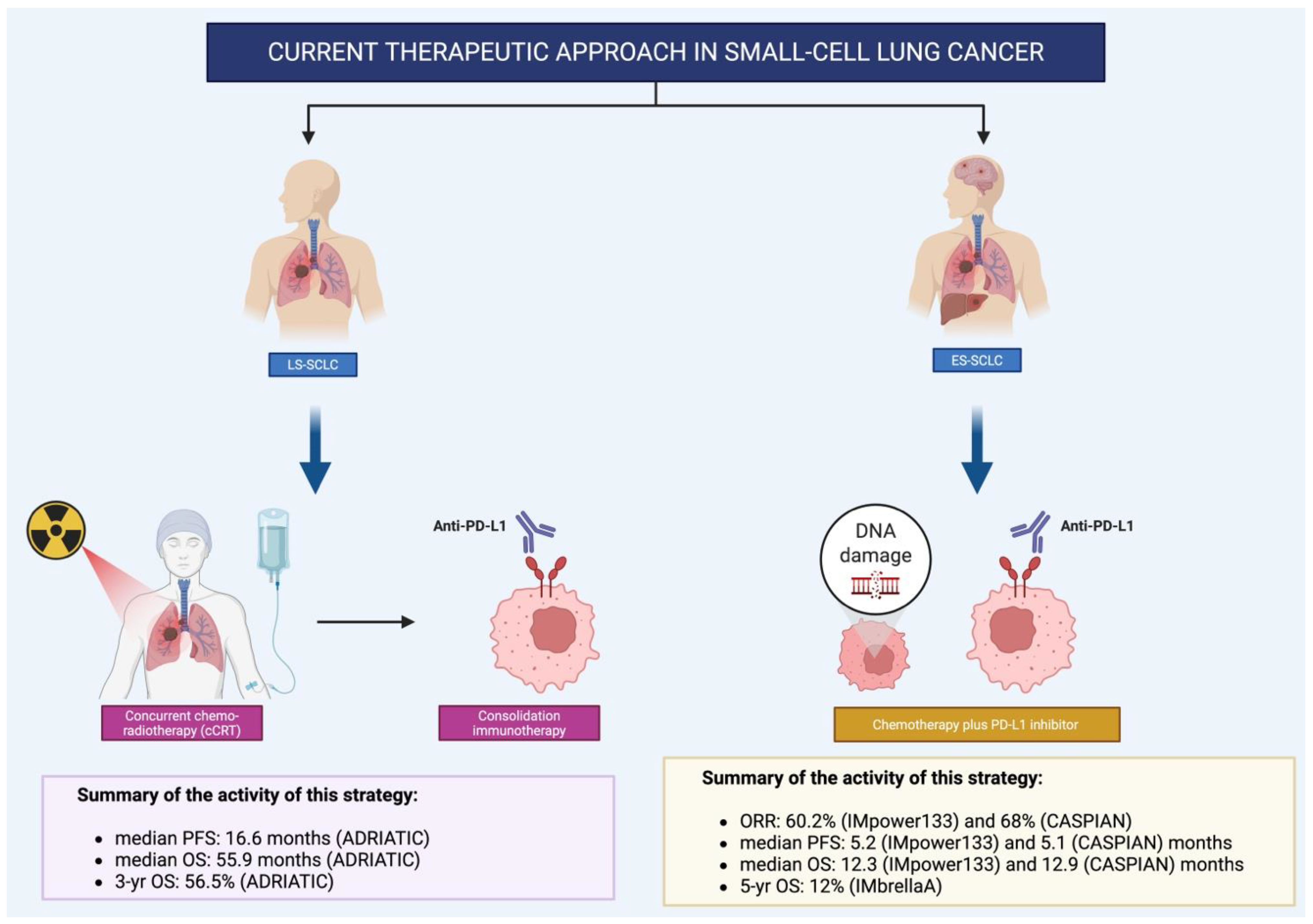
Abstract
1. Introduction
2. Classification of Pulmonary Neuroendocrine Tumors According to Grading (G)
2.1. Rationale for the Addition of Immunotherapy to Chemotherapy
- Increasing T-cell infiltration into the tumor microenvironment, thereby improving immune surveillance and the potential for tumor cell eradication.
- Enhancing the functional capacity of effector T cells, which are critical for mediating cytotoxic responses against tumor cells.
- Improving the recognition of tumor-associated antigens by T cells, facilitating a more targeted and effective immune response.
- Eliminating immunosuppressive cell populations, such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and M2-polarized macrophages, which collectively contribute to immune evasion by the tumor.
- Inducing immunogenic cell death, a process by which dying tumor cells release danger signals and antigens that stimulate a robust immune response.
- Promoting the maturation and activation of dendritic cells, thereby enhancing antigen presentation and the priming of naïve T cells.
- Targeting and depleting regulatory T cells, which play a central role in maintaining an immunosuppressive tumor microenvironment [8].
2.2. IMpower133: First-Line Atezolizumab Plus Chemotherapy in Extensive-Stage Small Cell Lung Cancer (ES-SCLC)
2.3. Durvalumab in Extensive-Stage Small-Cell Lung Cancer: Long-Term Outcomes from the CASPIAN Trial
2.4. ADRIATIC Trial: Redefining the Standard of Care in Limited-Stage SCLC with Durvalumab Consolidation
2.5. Key Efficacy Outcomes
2.6. Triple Modality in SCLC: Is There a Rationale for Combining Chemotherapy, Radiotherapy, and Immunotherapy?
2.6.1. Biological and Clinical Rationale Behind KEYLYNK LD-SCLC
2.6.2. KEYLYNK LD-SCLC: Is Triple Modality Therapy the Key?
2.7. Early Maintenance Therapy in ES-SCLC: Insights from the IMforte Trial
2.8. Shorter or Longer? Defining the Optimal Chemotherapy Course with Immunotherapy in SCLC
2.9. Challenges, Unmet Needs, and the Path Toward Precision
2.10. Transformed Small-Cell Lung Cancer (t-SCLC)
2.11. Future Perspectives in Biomarker Discovery for SCLC
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sadeghi, M.S.; Lotfi, M.; Soltani, N.; Farmani, E.; Fernandez, J.H.O.; Akhlaghitehrani, S.; Mohammed, S.H.; Yasamineh, S.; Kalajahi, H.G.; Gholizadeh, O. Recent advances on high-efficiency of microRNAs in different types of lung cancer: A comprehensive review. Cancer Cell Int. 2023, 23, 284. [Google Scholar] [CrossRef]
- Huang, D.; Wang, J.; Chen, L.; Jiang, W.; Inuzuka, H.; Simon, D.K.; Wei, W. Molecular subtypes and targeted therapeutic strategies in small cell lung cancer: Advances, challenges, and future perspectives. Molecules 2025, 30, 1731. [Google Scholar] [CrossRef]
- Reck, M.; Dziadziuszko, R.; Sugawara, S.; Kao, S.; Hochmair, M.; Huemer, F.; de Castro, G.; Havel, L.; Caro, R.B.; Losonczy, G.; et al. Five-year survival in patients with extensive-stage small cell lung cancer treated with atezolizumab in the Phase III IMpower133 study and the Phase III IMbrella A extension study. Lung Cancer 2024, 196, 107924. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. (Eds.) WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th ed.; World Health Organization Classification of Tumours; IARC Press: Lyon, France, 2015; Volume 7. [Google Scholar]
- WHO Classification of Tumours Editorial Board. Thoracic Tumours, 5th ed.; WHO Classification of Tumours series; International Agency for Research on Cancer: Lyon, France, 2021; Volume 5. [Google Scholar]
- Qiang, M.; Liu, H.; Yang, L.; Wang, H.; Guo, R. Immunotherapy for small cell lung cancer: The current state and future trajectories. Discov. Oncol. 2024, 15, 355. [Google Scholar] [CrossRef]
- Nabet, B.Y.; Hamidi, H.; Lee, M.C.; Banchereau, R.; Morris, S.; Adler, L.; Gayevskiy, V.; Elhossiny, A.M.; Srivastava, M.K.; Patil, N.S.; et al. Transcriptomic profiling defines immune-inflamed subsets of small cell lung cancer with therapeutic relevance. Cancer Cell 2024, 42, 429–443. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum–etoposide vs platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomized, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. Durvalumab plus platinum–etoposide vs platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): Updated analysis. Lancet Oncol. 2021, 22, 486–498. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.; Özgüroğlu, M.; Ji, J.; Garassino, M.; et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year OS update from CASPIAN. ESMO Open 2022, 7, 100408. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Spigel, D.R.; Cho, B.C.; Laktionov, K.K.; Fang, J.; Chen, Y.; Zenke, Y.; Lee, K.H.; Wang, Q.; Navarro, A.; et al. Durvalumab after chemoradiotherapy in limited-stage small-cell lung cancer. N. Engl. J. Med. 2024, 391, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Higgins, K.; Hu, C.; Ross, H.J.; Jabbour, S.K.; Kozono, D.E.; Owonikoko, T.K.; Dib, E.G.; Brownstein, J.M.; Kuzma, C.; Kotecha, R.; et al. Concurrent Chemoradiation § Atezolizumab (atezo) in Limited-Stage Small Cell Lung Cancer (LS-SCLC): Results of NRG Oncology/Alliance LU005. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, S2. [Google Scholar] [CrossRef]
- Bradley, J.; Sugawara, S.; Lee, K.; Ostoros, G.; Demirkazik, A.; Zemanova, M.; Sriuranpong, V.; Gelatti, A.; Menezes, J.; Zurawski, B.; et al. Durvalumab in Combination with Chemoradiotherapy for Patients with Unresectable, Stage III NSCLC: Final Results from PACIFIC-2. ESMO Open 2024, 9, 102986. [Google Scholar] [CrossRef]
- Rimner, A.; Lai, W.V.V.; Califano, R.; Jabbour, S.K.; Faivre-Finn, C.; Cho, B.C.; Kato, T.; Yu, J.M.; Yu, L.; Zhao, B.; et al. KEYLYNK-013: A phase 3 study of pembrolizumab in combination with concurrent chemoradiation therapy followed by pembrolizumab with or without olaparib versus concurrent chemoradiation therapy in patients with newly diagnosed limited-stage SCLC. J. Clin. Oncol. 2021, 39 (Suppl. S15), TPS8587. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Borghaei, H.; Liu, S.V.; Peters, S.; Herbst, R.S.; Stencel, K.; Majem, M.; Şendur, M.A.N.; Czyżewicz, G.; Caro, R.B.; et al. IMforte investigators. Efficacy and safety of first-line maintenance therapy with lurbinectedin plus atezolizumab in extensive-stage small-cell lung cancer (IMforte): A randomised, multicentre, open-label, phase 3 trial. Lancet 2025, 405, 2129–2143. [Google Scholar] [CrossRef]
- Bria, E.; Morgillo, F.; Garassino, M.C.; Ciardiello, F.; Ardizzoni, A.; Stefani, A.; Verderame, F.; Morabito, A.; Chella, A.; Tonini, G.; et al. Atezolizumab plus carboplatin and etoposide in untreated extensive-stage SCLC: Interim MAURIS results. Oncologist 2024, 29, e690–e698. [Google Scholar] [CrossRef] [PubMed]
- Masago, K.; Kuroda, H.; Seto, K.; Sasaki, E.; Fujita, Y.; Horio, Y.; Fujita, S.; Matsushita, H. Durvalumab plus platinum–etoposide in extensive-stage SCLC: Final LUMINANCE results. Clin. Lung Cancer 2025, 26, e321–e330. [Google Scholar] [CrossRef]
- Bolte, F.J.; Dougherty, S.C.; Danos, A.O.; Lynch, A.C.; Shvorak, Y.; Statler, S.; Gentzler, R.D.; Hall, R.D. Real-world outcomes of tarlatamab in SCLC, including patients with untreated brain metastases. Clin. Lung Cancer 2025, 26, e421–e430. [Google Scholar] [CrossRef]
- Feldman, J. Ifinatamab Deruxtecan Shows Favorable Efficacy and Tolerability in SCLC. Targeted Oncol. 2023. Available online: https://www.targetedonc.com/view/ifinatamab-deruxtecan-shows-favorable-efficacy-and-tolerability-in-sclc (accessed on 10 September 2023).
- Mountzios, G.; Sun, L.; Cho, B.C.; Demirci, U.; Baka, S.; Gümüş, M.; Lugini, A.; Zhu, B.; Yu, Y.; Korantzis, I.; et al. DeLLphi-304 Investigators. Tarlatamab in SCLC after platinum-based chemotherapy. N. Engl. J. Med. 2025, 392, 1123–1135. [Google Scholar] [CrossRef]
- Ceresoli, G.L.; Rossi, G.; Agustoni, F.; Bonomi, L.; Borghetti, P.; Bulotta, A.; Casartelli, C.; Cerea, G.; Colonese, F.; del Signore, E.; et al. Management of extensive-stage SCLC in the immunotherapy era: Italian Delphi consensus. Crit. Rev. Oncol. Hematol. 2024, 199, 104247. [Google Scholar] [CrossRef] [PubMed]
- Saalfeld, F.C.; Möller, J.; Christopoulos, P.; Wenzel, C.; Rasokat, A.; Wang, X.A.; Vathiotis, I.; König, D.; Illini, O.; Grohé, C.; et al. Correlation between treatments and outcomes of patients with EGFR-mutated non-small-cell lung cancer that transitioned into small-cell lung cancer: An international retrospective study. ESMO Open 2025, 10, 105326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saalfeld, F.C.; Möller, J.; Christopoulos, P.; Wenzel, C.; Rasokat, A.; Wang, X.A.; Vathiotis, I.; König, D.; Illini, O.; Grohé, C.; et al. Small cell transformation in EGFR-mutated non-small cell lung cancer: DLL3 expression and efficacy of immune checkpoint inhibitors or tyrosine kinase inhibitors combined with chemotherapy. Eur. J. Cancer 2024, 213, 115065. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, Z.; Xu, X.; Tian, Y.; Feng, L.; Huang, X.; Du, Y.; Li, Z. Single-cell and spatial transcriptomics integration: New frontiers in tumor microenvironmen and cellular communication. Front. Immunol. 2025, 16, 1649468. [Google Scholar] [CrossRef]
- Yin, H.; Wang, X.; Zhang, X.; Zeng, Y.; Xu, Q.; Wang, W.; Zhou, F.; Zhou, Y. Proteomics in lung cancer: Current status and future directions. Cancer Lett. 2022, 543, 215792. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-Y.; You, L.; Hardillo, J.A.U.; Chien, M.-P. Spatial Transcriptomic Technologies. Cells 2023, 12, 2042. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catania, C.; Cascetta, P.; Russo, A.; Governini, E.; Bendoni, M.; Laffi, A.; Piloni, I.; Conforti, F.; Pala, L.; Cocorocchio, E.; et al. Bridging Knowledge Gaps in Small Cell Lung Cancer: Data, Challenges and Priorities. Curr. Oncol. 2025, 32, 536. https://doi.org/10.3390/curroncol32100536
Catania C, Cascetta P, Russo A, Governini E, Bendoni M, Laffi A, Piloni I, Conforti F, Pala L, Cocorocchio E, et al. Bridging Knowledge Gaps in Small Cell Lung Cancer: Data, Challenges and Priorities. Current Oncology. 2025; 32(10):536. https://doi.org/10.3390/curroncol32100536
Chicago/Turabian StyleCatania, Chiara, Priscilla Cascetta, Alessandro Russo, Emily Governini, Marzia Bendoni, Alice Laffi, Ilaria Piloni, Fabio Conforti, Laura Pala, Emilia Cocorocchio, and et al. 2025. "Bridging Knowledge Gaps in Small Cell Lung Cancer: Data, Challenges and Priorities" Current Oncology 32, no. 10: 536. https://doi.org/10.3390/curroncol32100536
APA StyleCatania, C., Cascetta, P., Russo, A., Governini, E., Bendoni, M., Laffi, A., Piloni, I., Conforti, F., Pala, L., Cocorocchio, E., Ceresoli, G., Locatelli, M., Laszlo, D., Facella, F., & De Pas, T. (2025). Bridging Knowledge Gaps in Small Cell Lung Cancer: Data, Challenges and Priorities. Current Oncology, 32(10), 536. https://doi.org/10.3390/curroncol32100536





