PET/CT Imaging Characteristics of Gastric-Type Endocervical Adenocarcinoma: Findings from a Small Exploratory Series
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
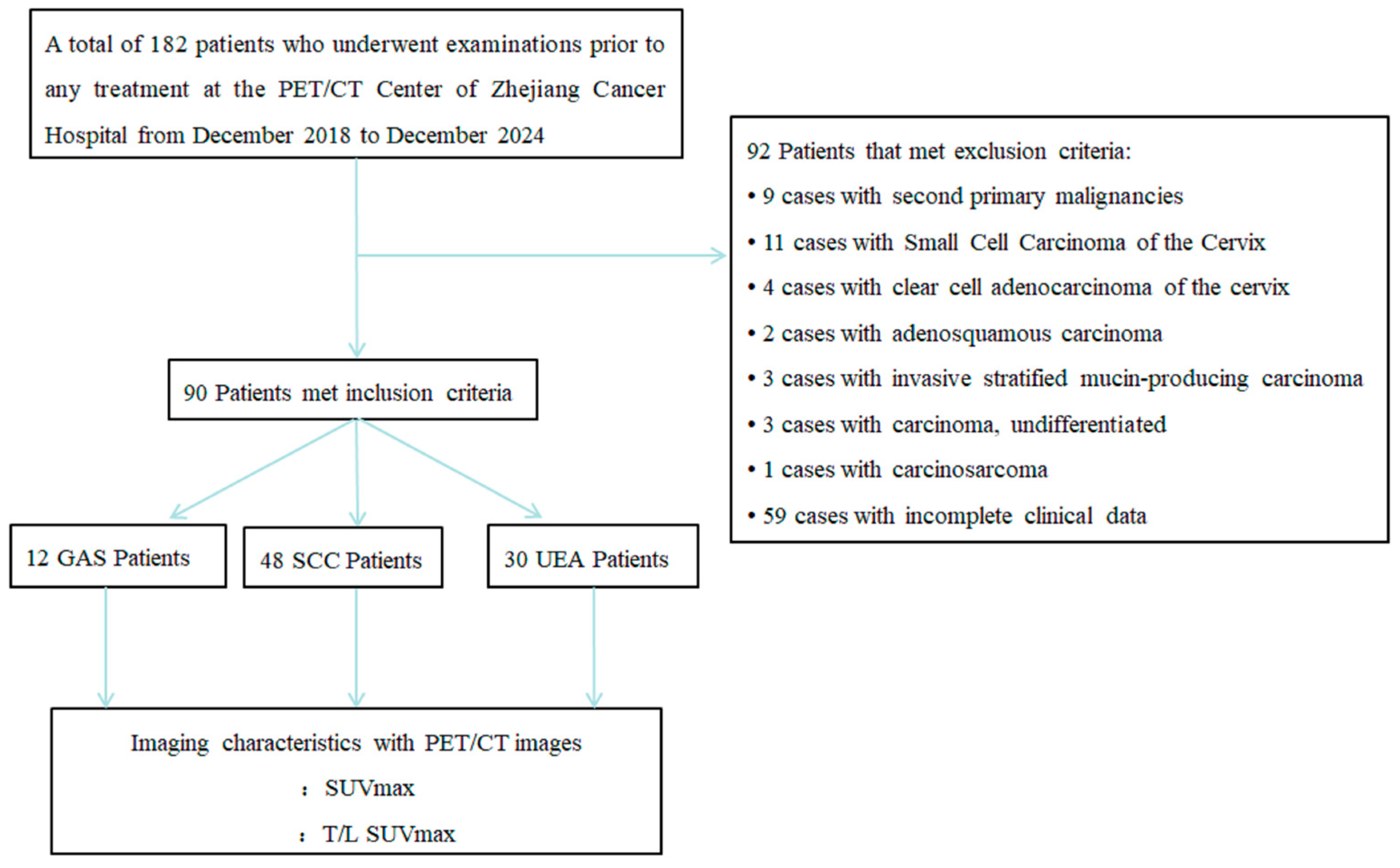
2.1. Patient Selection
2.2. 18F-FDG PET/CT Image Acquisition
2.3. Data Collection and Image Analysis
2.4. Statistical Analysis
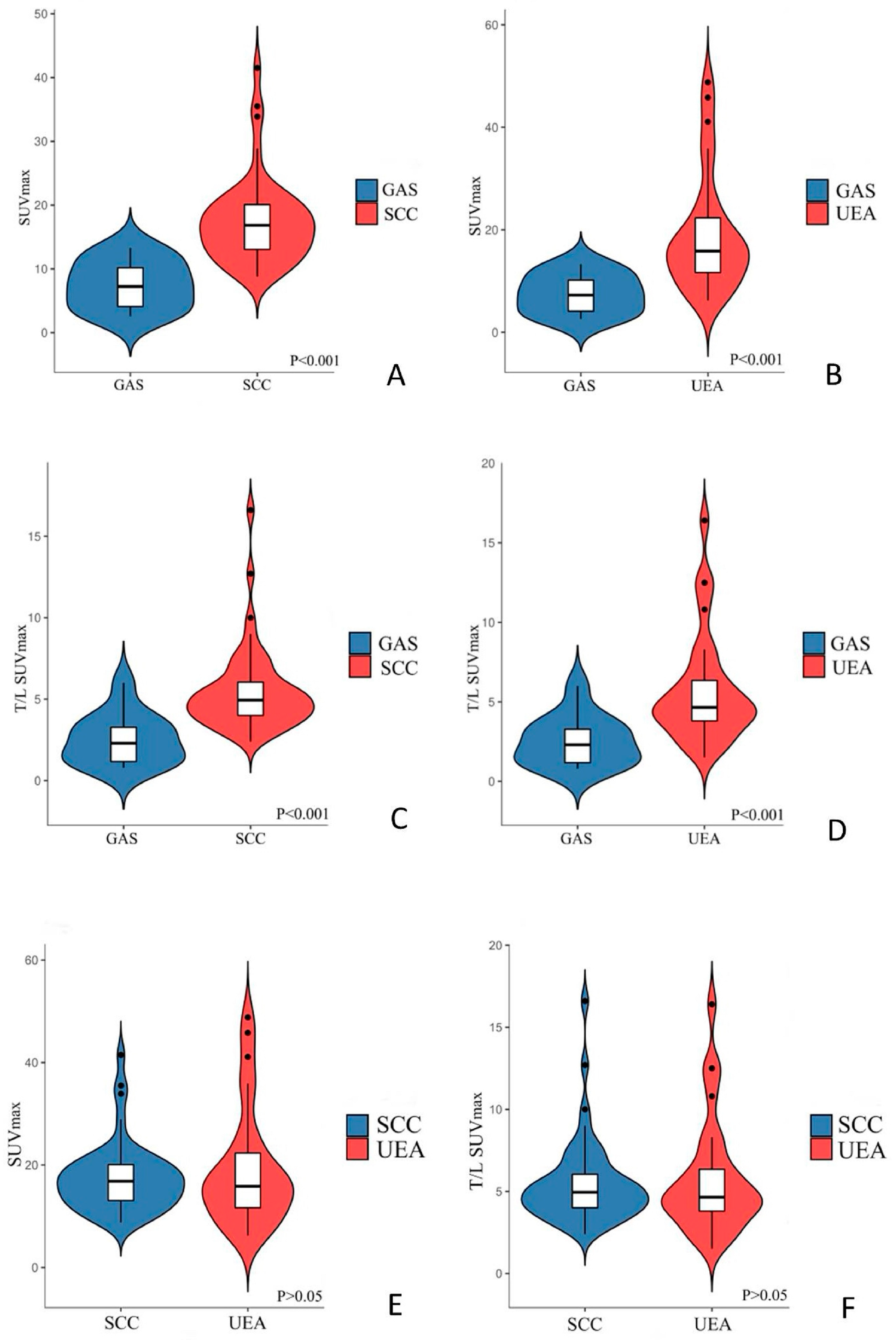
3. Results
3.1. Patient Characteristics
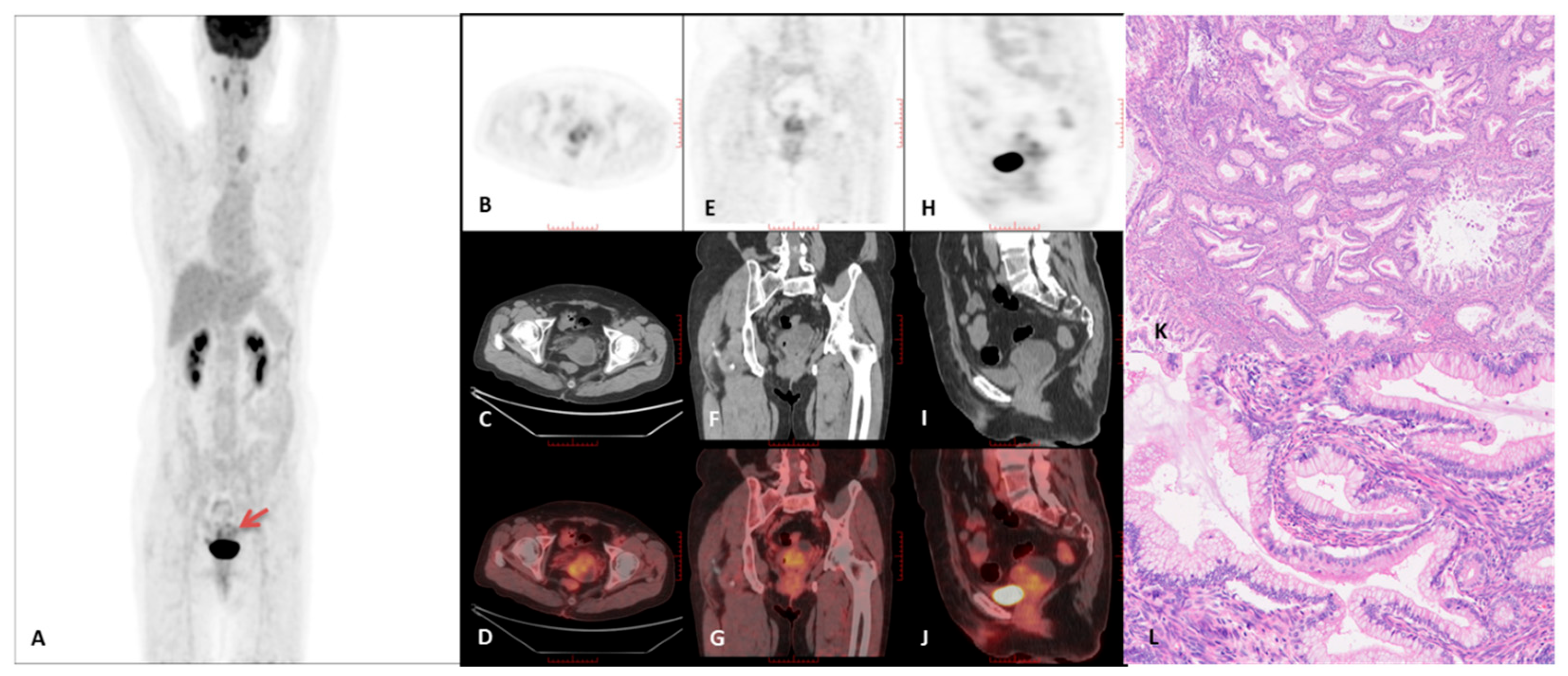
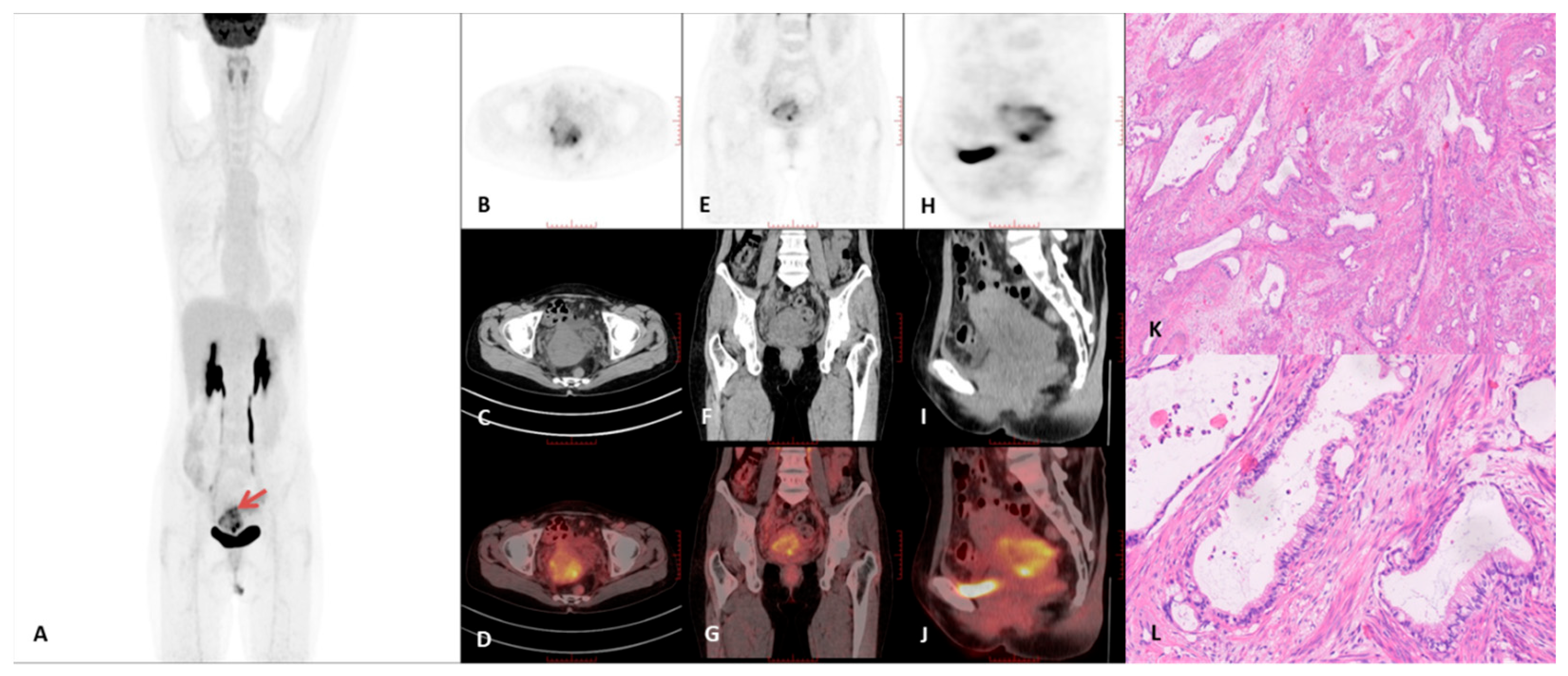
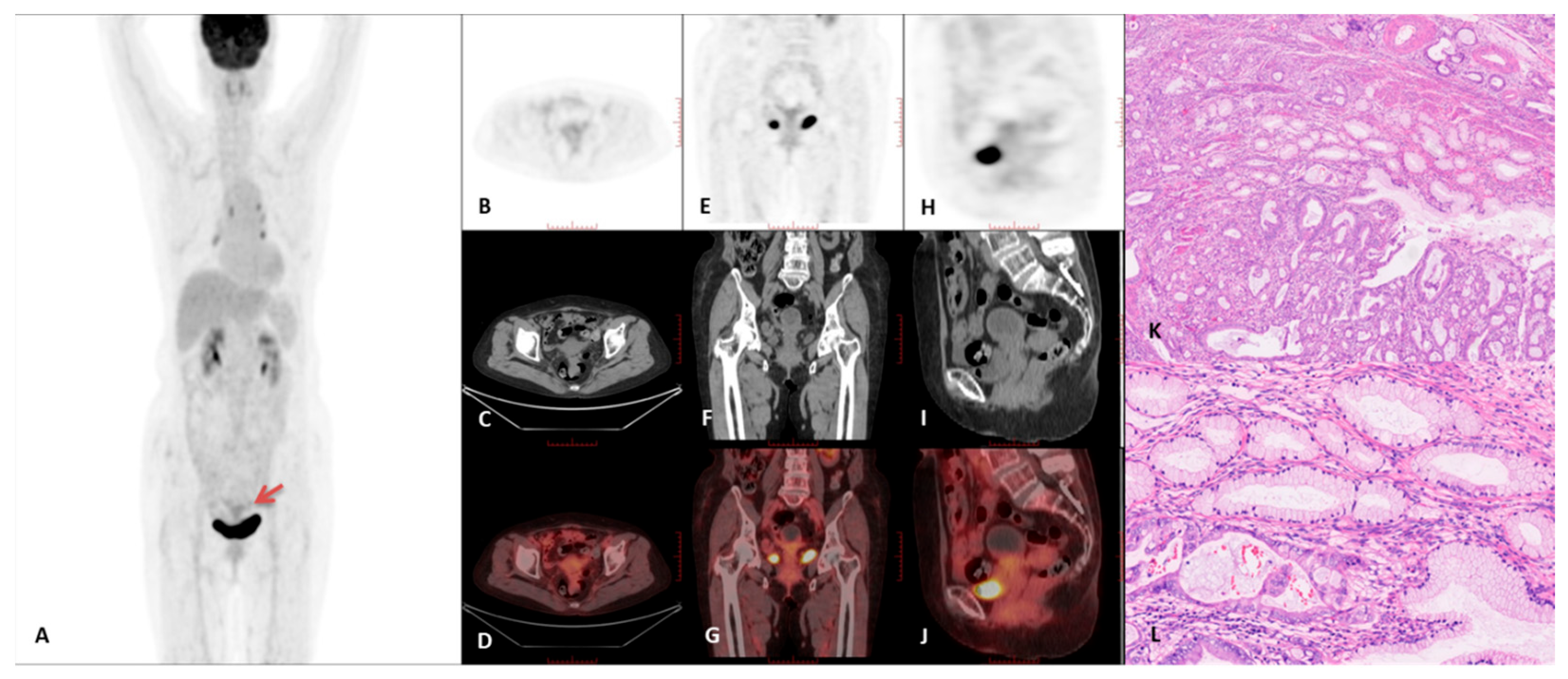
3.2. PET/CT Imaging Characteristics
3.3. Immunohistochemical Characteristics of the GAS Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GAS | gastric-type endocervical adenocarcinoma |
| SCC | squamous cell carcinoma |
| UEA | usual-type endocervical adenocarcinoma |
| FIGO | Federation of Gynecology and Obstetrics |
| AJCC | American Joint Committee on Cancer |
| PET/CT | positron emission tomography/computed tomography |
| SUVmax | the max standardized uptake value |
| T/L SUVmax | the ratio of cervical SUVmax to liver SUVmax. |
References
- Yoshino, A.; Kobayashi, E.; Tsuboyama, T.; Fukui, H.; Tomiyama, N.; Sato, K.; Morii, E.; Nakatani, E.; Komura, N.; Sawada, I.; et al. Novel Strategy for the Management of Cervical Multicystic Diseases. Ann. Surg. Oncol. 2023, 30, 2964–2973. [Google Scholar] [CrossRef]
- Stolnicu, S.; Barsan, I.; Hoang, L.; Patel, P.; Terinte, C.; Pesci, A.; Aviel-Ronen, S.; Kiyokawa, T.; Alvarado-Cabrero, I.; Pike, M.C.; et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): A New Pathogenetic Classification for Invasive Adenocarcinomas of the Endocervix. Am. J. Surg. Pathol. 2018, 42, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, C.M.; Markese, M.; Moroney, M.R.; Smith, L.P.; Patel, N.U. Adenocarcinoma of the uterine cervix, gastric-type (GAS): A review of the literature focused on pathology and multimodality imaging. Abdom. Radiol. 2023, 48, 713–723. [Google Scholar] [CrossRef]
- Park, E.; Kim, S.W.; Kim, S.; Kim, H.S.; Lee, J.Y.; Kim, Y.T.; Cho, N.H. Genetic characteristics of gastric-type mucinous carcinoma of the uterine cervix. Mod. Pathol. 2021, 34, 637–646. [Google Scholar] [CrossRef]
- Gordhandas, S.B.; Kahn, R.; Sassine, D.; Aviki, E.M.; Baltich Nelson, B.; Catchings, A.; Liu, Y.L.; Lakhman, Y.; Abu-Rustum, N.R.; Park, K.J.; et al. Gastric-type adenocarcinoma of the cervix in patients with Peutz-Jeghers syndrome: A systematic review of the literature with proposed screening guidelines. Int. J. Gynecol. Cancer 2022, 32, 79–88. [Google Scholar] [CrossRef]
- Ohya, A.; Kobara, H.; Miyamoto, T.; Komatsu, M.; Shiozawa, T.; Fujinaga, Y. Usefulness of the ‘cosmos pattern’ for differentiating between cervical gastric-type mucin-positive lesions and other benign cervical cystic lesions in magnetic resonance images. J. Obstet. Gynaecol. Res. 2021, 47, 745–756. [Google Scholar] [CrossRef]
- Board WC of TE. WHO classification of tumours. In Female Genital Tumours, 5th ed.; World Health Organization: Geneva, Switzerland, 2020; Volume 4. [Google Scholar]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Kikkawa, N.; Sugawara, H.; Yoshida, H.; Kobayashi-Kato, M.; Tanase, Y.; Uno, M.; Ishikawa, M.; Kato, T.; Kusumoto, M. Characteristics of the magnetic resonance imaging findings of cervical gastric-type adenocarcinoma. Clin. Radiol. 2024, 79, e1189–e1195. [Google Scholar] [CrossRef] [PubMed]
- Kido, A.; Mikami, Y.; Koyama, T.; Kataoka, M.; Shitano, F.; Konishi, I.; Togashi, K. Magnetic resonance appearance of gastric-type adenocarcinoma of the uterine cervix in comparison with that of usual-type endocervical adenocarcinoma: A pitfall of newly described unusual subtype of endocervical adenocarcinoma. Int. J. Gynecol. Cancer 2014, 24, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Kim, M.H.; Kim, J.K.; Cho, K.S. Gastric-Type Adenocarcinoma of the Uterine Cervix: Magnetic Resonance Imaging Features, Clinical Outcomes, and Prognostic Factors. Int. J. Gynecol. Cancer 2018, 28, 1203–1210. [Google Scholar] [CrossRef]
- Ryu, A.; Nagata, S.; Kubo, C.; Ueda, Y.; Tanada, S.; Idota, A.; Kamiura, S.; Honma, K.; Yamasaki, T. Conventional Direct Smear Yields Diagnostic Indicators of Gastric-Type Mucinous Carcinoma Compared with Cytomorphological Features Identified by Liquid-Based Cervical Cytology. Acta Cytol. 2021, 65, 150–157. [Google Scholar] [CrossRef]
- McBride, A.A. Human papillomaviruses: Diversity, infection and host interactions. Nat. Rev. Microbiol. 2022, 20, 95–108. [Google Scholar] [CrossRef]
- Li, X.; Qi, Y.; Zhang, W.; Rao, Y.; Zhang, N.; Qu, P. Peutz-Jeghers syndrome with gastric-type mucinous endocervical adenocarcinoma and sex-cord tumor with annular tubules: A case report. Front. Med. 2023, 10, 1094839. [Google Scholar] [CrossRef]
- Yang, L.; Duan, D.; Xiong, Y.; Liu, T.; Zhao, L.; Lai, F.; Gu, D.; Zhou, L. Preoperative multimodal ultrasonic imaging in a case of Peutz-Jeghers syndrome complicated by atypical lobular endocervical glandular hyperplasia: A case report and literature review. Hered. Cancer Clin. Pract. 2024, 22, 3. [Google Scholar] [CrossRef]
- Mikami, Y. Gastric-type mucinous carcinoma of the cervix and its precursors—Historical overview. Histopathology 2020, 76, 102–111. [Google Scholar] [CrossRef]
- Kojima, A.; Mikami, Y.; Sudo, T.; Yamaguchi, S.; Kusanagi, Y.; Ito, M.; Nishimura, R. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am. J. Surg. Pathol. 2007, 31, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.L.; Fu, L.; Ma, F.H.; Li, Y.A.; Zhang, G.F.; Qiang, J.W. Comparison of MRI features among squamous cell carcinoma, adenocarcinoma and adenosquamous carcinoma, usual-type endocervical adenocarcinoma and gastric adenocarcinoma of cervix. Magn. Reson. Imaging 2024, 112, 10–17. [Google Scholar] [CrossRef]
- Saida, T.; Sakata, A.; Tanaka, Y.O.; Ochi, H.; Ishiguro, T.; Sakai, M.; Takahashi, H.; Satoh, T.; Minami, M. Clinical and MRI Characteristics of Uterine Cervical Adenocarcinoma: Its Variants and Mimics. Korean J. Radiol. 2019, 20, 364–377. [Google Scholar] [CrossRef]
- Yang, J.; Peng, Y.; Ding, Y.; Liu, Y.; Wang, Y.; Liu, Y.; Liu, C. The Clinicopathological and Molecular Characteristics of Endocervical Gastric-Type Adenocarcinoma and the Use of Claudin18.2 as a Potential Therapeutic Target. Mod. Pathol. 2024, 37, 100569. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Yamaguchi, K.; Minamiguchi, S.; Murakami, R.; Abiko, K.; Hamanishi, J.; Kondoh, E.; Baba, T.; Mandai, M.; Matsumura, N. Mucinous adenocarcinoma, gastric type of the uterine cervix: Clinical features and HER2 amplification. Med. Mol. Morphol. 2019, 52, 52–59. [Google Scholar] [CrossRef]
- Cho, H.; Park, S.; Kim, H.S. Gastric-type Endocervical Adenocarcinoma: Comprehensive Cytopathological Analysis and Comparison With Usual-type Endocervical Adenocarcinoma. In Vivo 2023, 37, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Kato, H.; Kawaguchi, M.; Kanayama, T.; Furui, T.; Noda, Y.; Hyodo, F.; Matsuo, M. MRI characteristics for predicting histological subtypes in patients with uterine cervical adenocarcinoma. Eur. J. Radiol. 2023, 158, 110612. [Google Scholar] [CrossRef] [PubMed]
- Shao, N. Research progress on human papillomavirus-negative cervical cancer: A review. Medicine 2024, 103, e39957. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | GAS Patients | SCC Patients | UEA Patients | |
|---|---|---|---|---|
| Number | 12 | 48 | 30 | |
| Age (years), median (range) | 63 (29–77) | 56 (25–74) | 55 (28–75) | |
| FIGO (2018), n | IB | 1 | 13 | 0 |
| IIA | 1 | 7 | 7 | |
| IIIB | 0 | 0 | 3 | |
| IIIC | 1 | 7 | 12 | |
| IVA | 1 | 0 | 0 | |
| IVB | 8 | 21 | 8 | |
| GAS (N = 12) | SCC (N = 48) | UEA (N = 30) | p-Value | ||
|---|---|---|---|---|---|
| GAS vs. SCC | GAS vs. UEA | ||||
| Growth pattern | <0.001 | <0.001 | |||
| Mass forming | 1 | 37 | 24 | ||
| Diffuse infiltration | 11 | 11 | 6 | ||
| Intrauterine fluid | <0.001 | 0.0024 | |||
| Present | 11 | 11 | 12 | ||
| Absent | 1 | 37 | 18 | ||
| Cyst | <0.001 | <0.001 | |||
| Microcyst | 1 | 0 | 0 | ||
| Macrocyst | 11 | 9 | 9 | ||
| None | 0 | 39 | 21 | ||
| N-stage | 0.7334 | 0.5041 | |||
| N0 | 5 | 20 | 12 | ||
| N1 | 1 | 9 | 10 | ||
| N2 | 6 | 19 | 8 | ||
| M-stage | 0.0528 | 0.0061 | |||
| M0 | 3 | 27 | 22 | ||
| M1 | 9 | 21 | 8 | ||
| Peritoneal metastasis | 0.0001 | 0.0009 | |||
| Present | 5 | 0 | 0 | ||
| Absent | 7 | 48 | 30 | ||
| tumor diameter mean value (cm) | 4.4 ± 1.5 | 4.8 ± 1.8 | 3.9 ± 1.5 | 0.4678 | 0.3643 |
| SUVmax | 7.5 ± 3.8 | 17.4 ± 6.7 | 19.1 ± 11.4 | <0.001 | <0.001 |
| T/L SUVmax | 2.5 ± 1.6 | 5.5 ± 2.6 | 5.7 ± 3.4 | <0.001 | <0.001 |
| Ca199 (U/mL) | <0.001 | 0.7186 | |||
| ≤37 | 3 | 41 | 11 | ||
| >37 | 9 | 7 | 19 | ||
| Ca125 (U/mL) | 0.5081 | 0.292 | |||
| ≤35 | 6 | 31 | 9 | ||
| >35 | 6 | 17 | 21 | ||
| SCC (U/mL) | <0.001 | 0.09 | |||
| ≤1.5 | 10 | 6 | 16 | ||
| >1.5 | 2 | 42 | 14 | ||
| Case # | FIGO 2018 | Tumor Diameter Mean Value (cm) | Macrocysts | Tumor Growth Pattern | Intrauterine Fluid | SUVmax | T/L SUVmax |
|---|---|---|---|---|---|---|---|
| 1 | IVB | 2.9 | Present | Diffuse | Present | 3.0 | 1.0 |
| 2 | IVA | 2.4 | Present | Diffuse | Present | 2.8 | 1.1 |
| 3 | IB | 3.7 | Present | Diffuse | Present | 2.6 | 0.8 |
| 4 | IVB | 6.5 | Present | Diffuse | Present | 9.3 | 3.9 |
| 5 | IVB | 3.3 | Present | Mass | Present | 9.7 | 2.7 |
| 6 | IIIC | 4.8 | Present | Diffuse | Present | 11.6 | 3.1 |
| 7 | IIA | 4.6 | Present | Diffuse | Present | 6.8 | 1.7 |
| 8 | IVB | 2.9 | Present | Diffuse | Present | 12.6 | 3.8 |
| 9 | IVB | 5.2 | Microcysts | Diffuse | Present | 6.2 | 1.9 |
| 10 | IVB | 5.9 | Present | Diffuse | Absent | 13.3 | 6.0 |
| 11 | IVB | 6.8 | Present | Diffuse | Present | 7.7 | 3.1 |
| 12 | IVB | 3.7 | Present | Diffuse | Present | 4.5 | 1.2 |
| Case # | p16 | MUC6 | Claudin18.2 | PAX8 | p53 | ER | PR |
|---|---|---|---|---|---|---|---|
| 1 | - | + | - | - | + | - | - |
| 2 | - | + | ++~+++ | - | + | - | - |
| 3 | + | + | +++ | - | - | - | - |
| 4 | + | + | + | - | + | - | - |
| 5 | - | + | +++ | - | - | - | - |
| 6 | + | + | +++ | - | - | - | - |
| 7 | + | + | + | - | - | - | - |
| 8 | + | + | ++~+++ | + | + | - | - |
| 9 | - | + | - | - | + | - | - |
| 10 | + | + | + | + | + | +~++ | - |
| 11 | - | + | ++ | + | + | - | - |
| 12 | - | + | - | - | + | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhou, X.; Xi, Y.; Lou, H.; Li, L.; Yi, H.; Zhu, T. PET/CT Imaging Characteristics of Gastric-Type Endocervical Adenocarcinoma: Findings from a Small Exploratory Series. Curr. Oncol. 2025, 32, 530. https://doi.org/10.3390/curroncol32100530
Wang Y, Zhou X, Xi Y, Lou H, Li L, Yi H, Zhu T. PET/CT Imaging Characteristics of Gastric-Type Endocervical Adenocarcinoma: Findings from a Small Exploratory Series. Current Oncology. 2025; 32(10):530. https://doi.org/10.3390/curroncol32100530
Chicago/Turabian StyleWang, Yun, Xuan Zhou, Yun Xi, Hanmei Lou, Linfa Li, Heqing Yi, and Tao Zhu. 2025. "PET/CT Imaging Characteristics of Gastric-Type Endocervical Adenocarcinoma: Findings from a Small Exploratory Series" Current Oncology 32, no. 10: 530. https://doi.org/10.3390/curroncol32100530
APA StyleWang, Y., Zhou, X., Xi, Y., Lou, H., Li, L., Yi, H., & Zhu, T. (2025). PET/CT Imaging Characteristics of Gastric-Type Endocervical Adenocarcinoma: Findings from a Small Exploratory Series. Current Oncology, 32(10), 530. https://doi.org/10.3390/curroncol32100530





