A Phase Ib Study of Indirect Immunization with Oregovomab and Toll-like-Receptor-3 Stimulation with Hiltonol® in Patients with Recurrent Platinum-Resistant Ovarian Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Study Variables and Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, A.N.; Schultes, B.C.; Gallion, H.; Edwards, R.; Whiteside, T.L.; Cermak, J.M.; Nicodemus, C.F. CA125- and tumor-specific T-cell responses correlate with prolonged survival in oregovomab-treated recurrent ovarian cancer patients. Gynecol. Oncol. 2004, 94, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.; Taylor, P.; McGuire, W.; Smith, L.M.; Schultes, B.; Nicodemus, C.F. Oregovomab Maintenance Monoimmunotherapy Does Not Improve Outcomes in Advanced Ovarian Cancer. J. Clin. Oncol. 2009, 27, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, A.; Buzzonetti, A.; Fossati, M.; Scambia, G.; Fattorossi, A.; Madiyalakan, M.R.; Mahnke, Y.D.; Nicodemus, C. Translational immune correlates of indirect antibody immunization in a randomized phase II study using scheduled combination therapy with carboplatin/paclitaxel plus oregovomab in ovarian cancer patients. Cancer Immunol. Immunother. 2020, 69, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.; Angioli, R.; Scambia, G.; Lorusso, D.; Terranova, C.; Panici, P.B.; Raspagliesi, F.; Scollo, P.; Plotti, F.; Ferrandina, G.; et al. Front-line chemo-immunotherapy with carboplatin-paclitaxel using oregovomab indirect immunization in advanced ovarian cancer: A randomized phase II study. Gynecol. Oncol. 2020, 156, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Pietragalla, A.; Duranti, S.; Daniele, G.; Nero, C.; Ciccarone, F.; Lorusso, D.; Fagotti, A.; Scambia, G. Oregovomab: An investigational agent for the treatment of advanced ovarian cancer. Expert Opin. Investig. Drugs 2021, 30, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.N.; Sarvestani, A.L.; Ryan, C.E.; Teke, M.E.; Akmal, S.R.; Hernandez, J.M.; Gupta, S. Oregovomab Plus Chemo in Newly Diagnosed Patients with Advanced Epithelial Ovarian Cancer Following Optimal Debulking Surgery (FLORA-5/GOG-3035). Ann. Surg. Oncol. 2022, 30, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Noujaim, A.A.; Schultes, B.C.; Baum, R.P.; Madiyalakan, R. Induction of CA125-Specific B and T Cell Responses in Patients Injected with MAb-B43.13—Evidence for Antibody-Mediated Antigen-Processing and Presentation of CA125 in Vivo. Cancer Biother. Radiopharm. 2001, 16, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Braly, P.; Nicodemus, C.F.; Chu, C.; Collins, Y.; Edwards, R.; Gordon, A.; McGuire, W.; Schoonmaker, C.; Whiteside, T.; Smith, L.M.; et al. The Immune Adjuvant Properties of Front-line Carboplatin-Paclitaxel: A Randomized Phase 2 Study of Alternative Schedules of Intravenous Oregovomab Chemoimmunotherapy in Advanced Ovarian Cancer. J. Immunother. 2009, 32, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Butowski, N.; Chang, S.M.; Junck, L.; DeAngelis, L.M.; Abrey, L.; Fink, K.; Cloughesy, T.; Lamborn, K.R.; Salazar, A.M.; Prados, M.D. A phase II clinical trial of poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: A North American Brain Tumor Consortium (NABTC01-05). J. Neuro-Oncol. 2008, 91, 175–182. [Google Scholar] [CrossRef]
- Butowski, N.; Lamborn, K.R.; Lee, B.L.; Prados, M.D.; Cloughesy, T.; DeAngelis, L.M.; Abrey, L.; Fink, K.; Lieberman, F.; Mehta, M.; et al. A North American brain tumor consortium phase II study of poly-ICLC for adult patients with recurrent anaplastic gliomas. J. Neuro-Oncol. 2008, 91, 183–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caskey, M.; Lefebvre, F.; Filali-Mouhim, A.; Cameron, M.J.; Goulet, J.-P.; Haddad, E.K.; Breton, G.; Trumpfheller, C.; Pollak, S.; Shimeliovich, I.; et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J. Exp. Med. 2011, 208, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, C.F.; Berek, J.S. TLR3 Agonists as Immunotherapeutic Agents. Immunotherapy 2010, 2, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Sistigu, A.; Yamazaki, T.; Vacchelli, E.; Chaba, K.; Enot, D.P.; Adam, J.; Vitale, I.; Goubar, A.; Baracco, E.E.; Remédios, C.; et al. Cancer cell–autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 2014, 20, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.M.; Levy, H.B.; Ondra, S.; Kende, M.; Scherokman, B.; Brown, D.; Mena, H.; Martin, N.; Schwab, K.; Donovan, D.; et al. Long-term Treatment of Malignant Gliomas with Intramuscularly Administered Polyinosinic-Polycytidylic Acid Stabilized with Polylysine and Carboxymethylcellulose: An Open Pilot Study. Neurosurgery 1996, 38, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Giantonio, B.J.; Hochster, H.; Blum, R.; Wiernik, P.H.; Hudes, G.R.; Kirkwood, J.; Trump, D.; Oken, M.M. Toxicity and Response Evaluation of the Interferon Inducer Poly ICLC Administered at Low Dose in Advanced Renal Carcinoma and Relapsed or Refractory Lymphoma: A Report of Two Clinical Trials of the Eastern Cooperative Oncology Group. Investig. New Drugs 2001, 19, 89–92. [Google Scholar] [CrossRef] [PubMed]
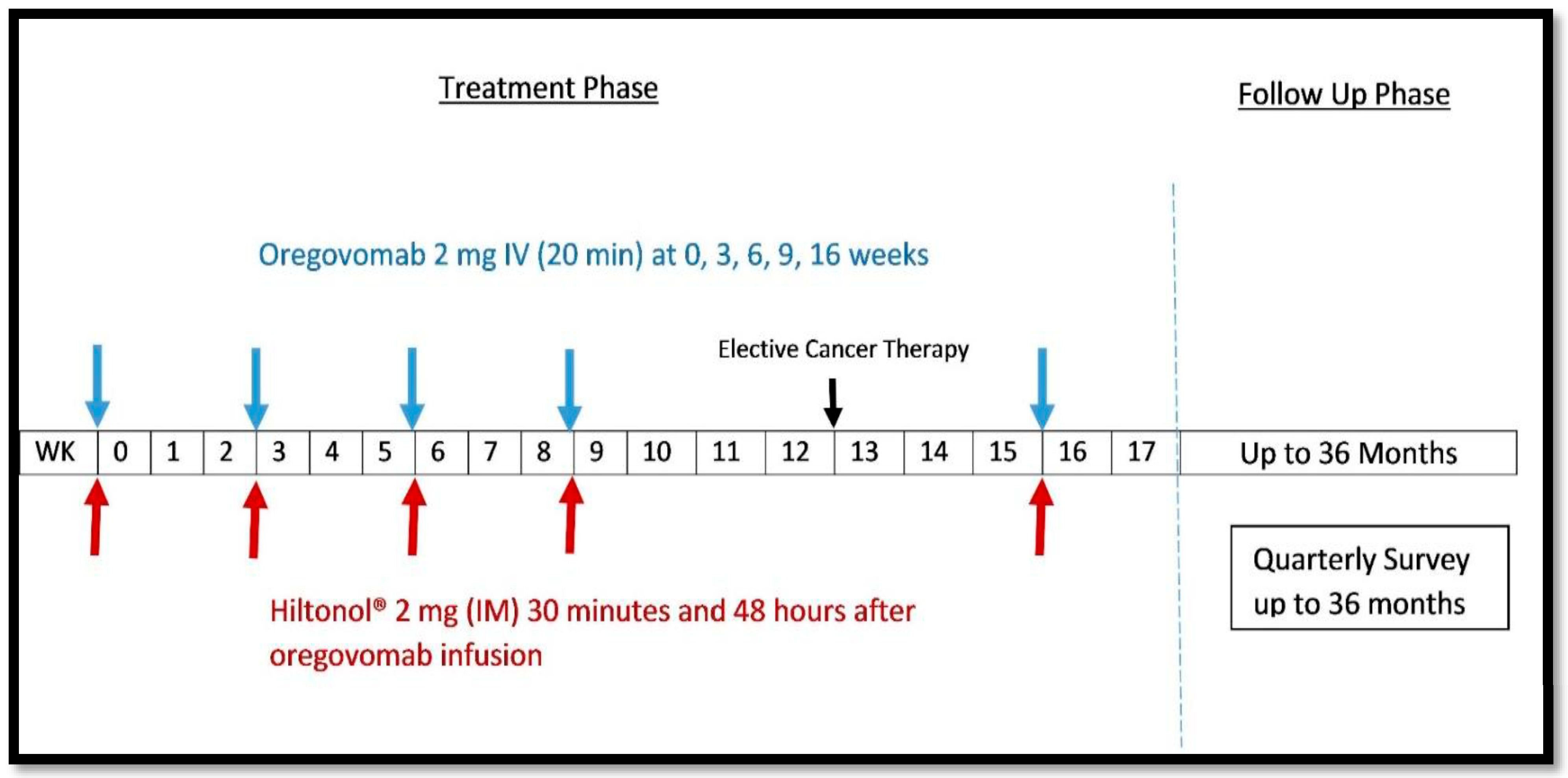
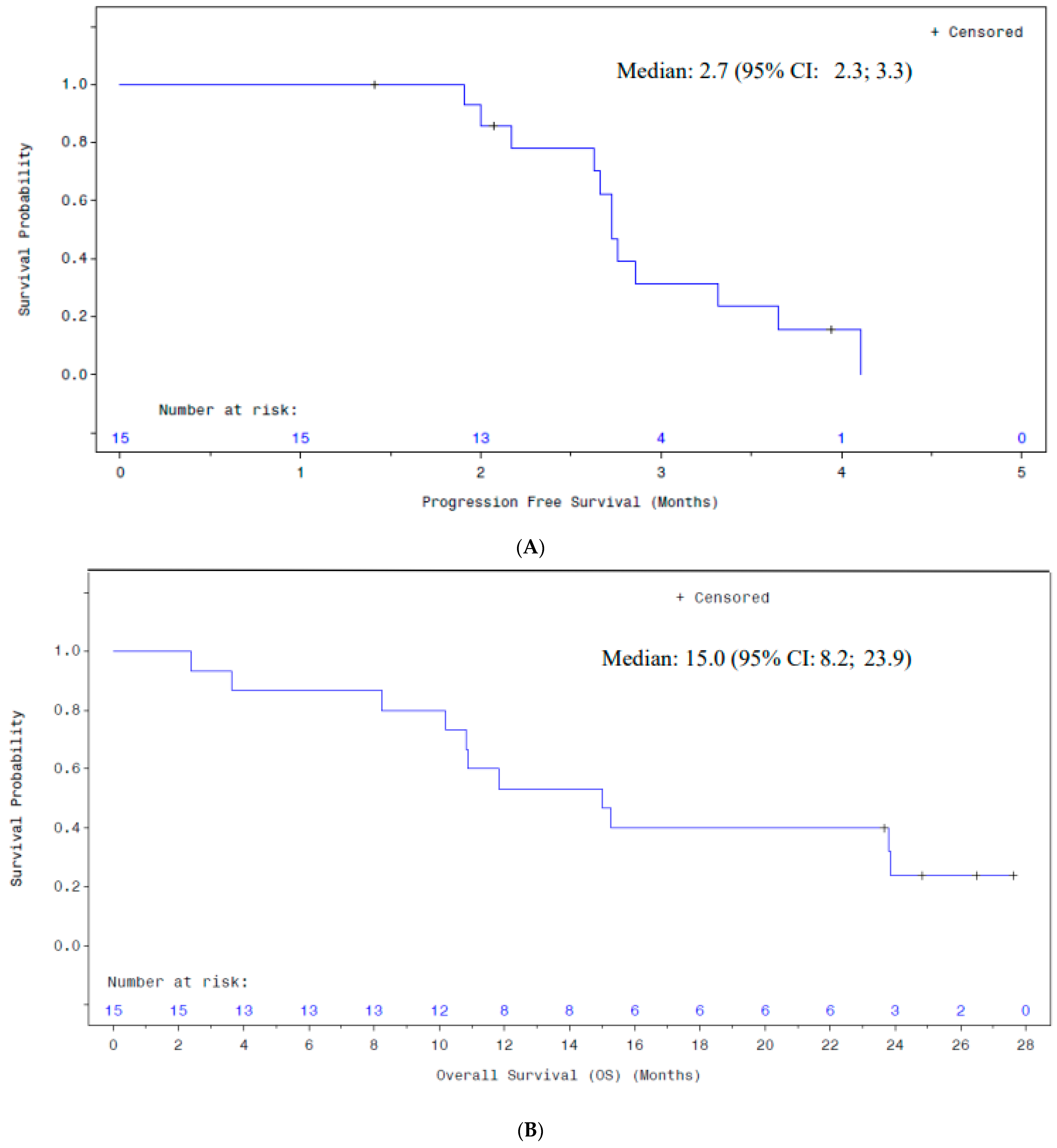
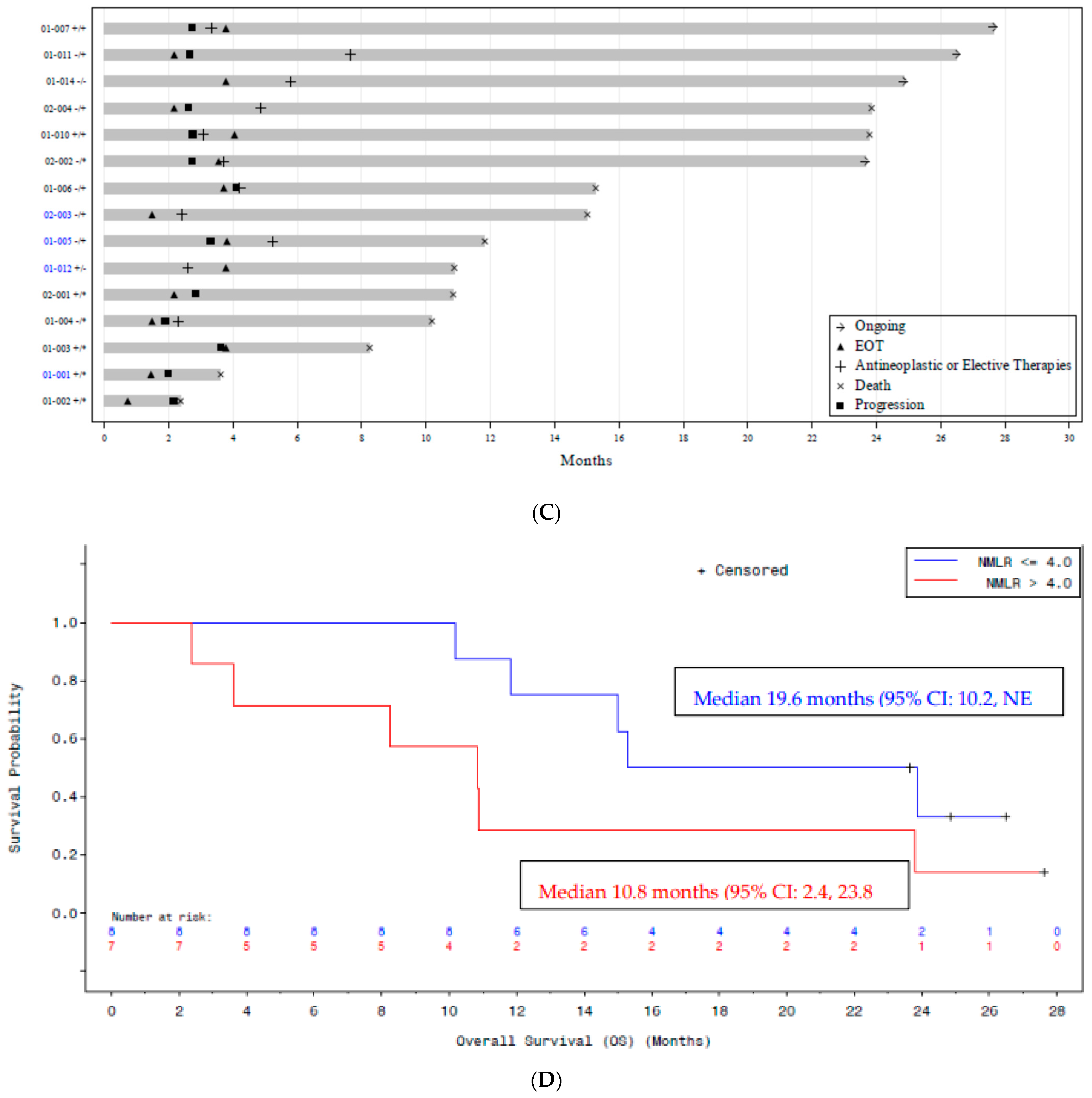
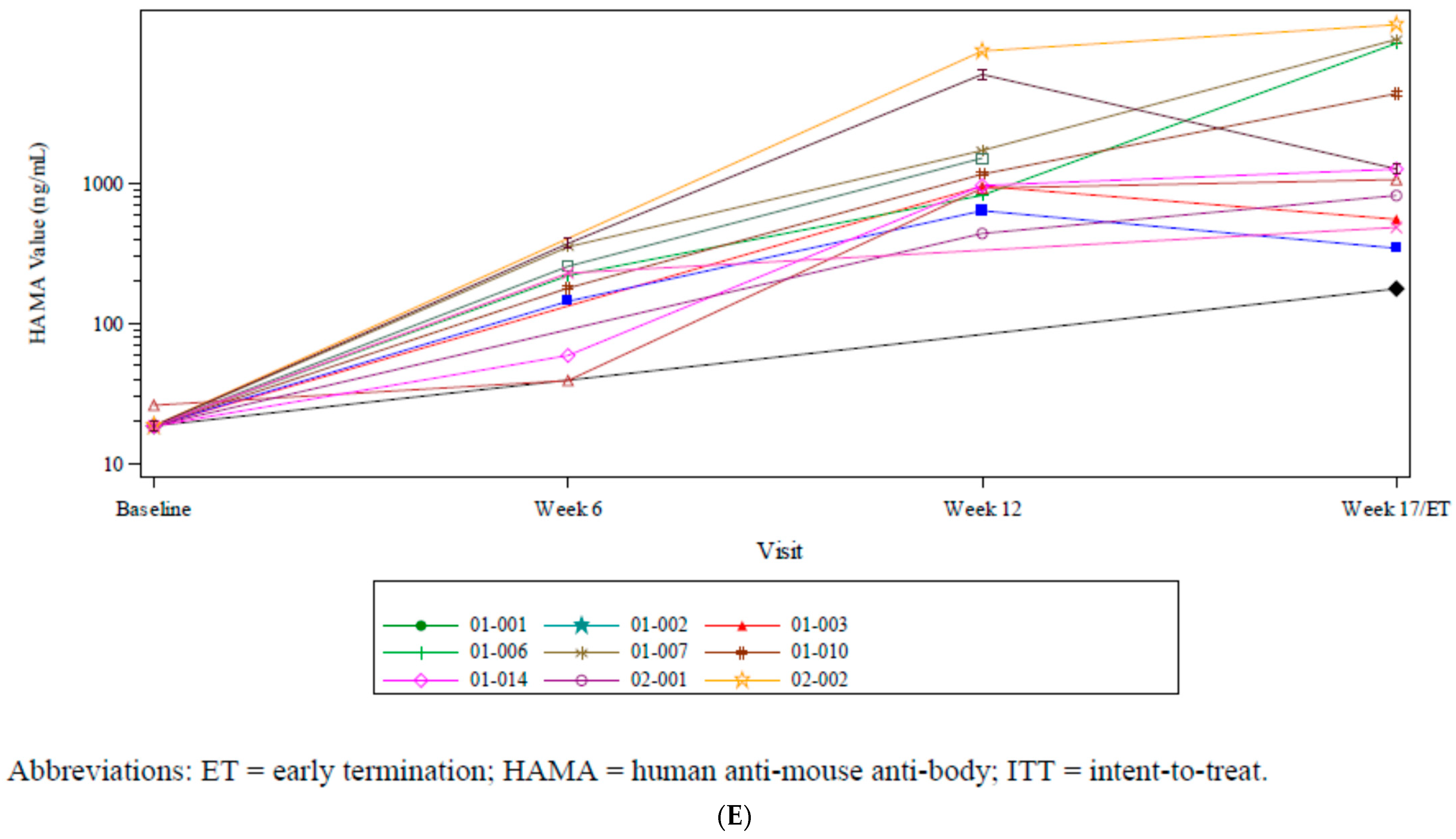
| Characteristics | Subjects (N = 15) |
|---|---|
| Disposition (of 18 screened) | |
| Entered the study and dosed | 15 |
| Received 3 doses of immunotherapy | 13 |
| Age at study entry (year) | |
| Median | 68 |
| Range | 42–83 |
| ECOG performance status, n (%) | |
| 0 | 10 (66.7) |
| 1 | 5 (33.3) |
| Race, n (%) | |
| Black | 1 (6.7) |
| White | 12 (80) |
| Spanish | 1 (6.7) |
| Indian | 1 (6.7) |
| Ethnicity, n (%) | |
| Non-Hispanic | 13 (86.7) |
| Hispanic | 2 (13.3) |
| Origin of epithelial cancer, n (%) | |
| Ovarian | 12 (80) |
| Peritoneal | 2 (13.3) |
| Tubal | 1 (6.7) |
| Time since the initial diagnosis (months) | |
| Median | 53.6 |
| Min–Max | 25–110 |
| FIGO stage n (%) | |
| IIIC | 11 (73.3) |
| IV | 4 (26.7) |
| Pre-immunization CA-125, U/mL | |
| Median | 357 |
| Min–Max | 63–2140 |
| Lines of prior therapy | |
| Median | 5 |
| Min–Max | 3–8 |
| Time Since Most Recent Relapse (months) | |
| Median | 0.5 |
| Min–Max | 0–11 |
| Time from last platinum therapy | |
| <1 month | 9 (60%) |
| 1–6 months | 6 (40%) |
| Parameter | N (%) |
|---|---|
| Indirect immunizations | 15 (100) |
| Oregovomab | |
| 2 | 2 (13.3) |
| 3 | 2 (13.3) |
| 4 | 3 (20) |
| 5 | 8 (53.3) |
| Hiltonol® injections | |
| 4 | 2 (13.3) |
| 6 | 2 (13.3) |
| 8 | 3 (20) |
| 10 | 8 (53.3) |
| Continuing antineoplastic therapy | |
| Started chemotherapy and stopped immunotherapy | 3 (20) |
| Started chemotherapy during immunotherapy | 4 (26.6) |
| Started chemotherapy post-immunotherapy | 6 (40) |
| Went to hospice prior to week 12 | 2 (13.3) |
| Went to hospice post week 17 | 1 (6.7) |
| Events | n (%) |
|---|---|
| At Least 1 TEAE | 15 (100) |
| Relationship a | |
| Not Related | 14 (93.3) |
| Unlikely Related | 8 (53.3) |
| Possibly Related | 11 (73.3) |
| Probably Related | 6 (40) |
| Definitely Related | 2 (13.3) |
| Missing | 0 |
| CTCAE Grade | |
| 1 | 15 (100) |
| 2 | 13 (86.7) |
| 3 | 7 (46.7) |
| 4 | 0 |
| 5 | 0 |
| At Least 1 TEAE | 14 (93.3) |
| At Least 1 Grade ≥3 TEAE | 7 (46.7) |
| At Least 1 Serious TEAE | 5 (33.3) |
| At Least 1 TEAE Leading to Study Withdrawal | 0 |
| MedDRA System Organ Class Preferred Term | All Subjects (n = 15) n (%) |
|---|---|
| At least 1 TEAE with CTCAE Grade ≥3 | 7 (46.7) |
| Gastrointestinal disorders | 3 (20) |
| Large intestinal obstruction | 1 (6.7) |
| Nausea | 1 (6.7) |
| Small intestinal obstruction | 1 (6.7) |
| Vomiting | 1 (6.7) |
| General disorders and administration site conditions | 2 (13.3) |
| Asthenia | 1 (6.7) |
| Fatigue | 1 (6.7) |
| Hepatobiliary disorders | 1 (6.7) |
| Bile duct obstruction | 1 (6.7) |
| Investigations | 2 (13.3) |
| Alanine aminotransferase increased * | 1 (6.7) |
| Aspartate aminotransferase increased * | 1 (6.7) |
| Blood alkaline phosphatase increased * | 1 (6.7) |
| Blood bilirubin increased * | 1 (6.7) |
| Platelet count increased * | 1 (6.7) |
| Metabolic and nutritional disorders | 2 (13.3) |
| Dehydration | 1 (6.7) |
| Failure to thrive | 1 (6.7) |
| Respiratory, thoracic, and mediastinal disorders | 1 (6.7) |
| Pneumonia | 1 (6.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holloway, R.W.; Temkin, S.M.; Gordon, S.W.; Gupta, S.; Jada, S.R.; Ahmad, S.; McGuire, W.P. A Phase Ib Study of Indirect Immunization with Oregovomab and Toll-like-Receptor-3 Stimulation with Hiltonol® in Patients with Recurrent Platinum-Resistant Ovarian Cancer. Curr. Oncol. 2025, 32, 532. https://doi.org/10.3390/curroncol32100532
Holloway RW, Temkin SM, Gordon SW, Gupta S, Jada SR, Ahmad S, McGuire WP. A Phase Ib Study of Indirect Immunization with Oregovomab and Toll-like-Receptor-3 Stimulation with Hiltonol® in Patients with Recurrent Platinum-Resistant Ovarian Cancer. Current Oncology. 2025; 32(10):532. https://doi.org/10.3390/curroncol32100532
Chicago/Turabian StyleHolloway, Robert W., Sarah M. Temkin, Sarah W. Gordon, Sunil Gupta, Srinivasa R. Jada, Sarfraz Ahmad, and William P. McGuire. 2025. "A Phase Ib Study of Indirect Immunization with Oregovomab and Toll-like-Receptor-3 Stimulation with Hiltonol® in Patients with Recurrent Platinum-Resistant Ovarian Cancer" Current Oncology 32, no. 10: 532. https://doi.org/10.3390/curroncol32100532
APA StyleHolloway, R. W., Temkin, S. M., Gordon, S. W., Gupta, S., Jada, S. R., Ahmad, S., & McGuire, W. P. (2025). A Phase Ib Study of Indirect Immunization with Oregovomab and Toll-like-Receptor-3 Stimulation with Hiltonol® in Patients with Recurrent Platinum-Resistant Ovarian Cancer. Current Oncology, 32(10), 532. https://doi.org/10.3390/curroncol32100532






