The Quality of Life after Endometrial Cancer Study: Baseline Characteristics and Patient-Reported Outcomes
Abstract
1. Introduction
2. Methods
2.1. Study Design Overview
2.2. Baseline Measures
2.3. Statistical Analysis
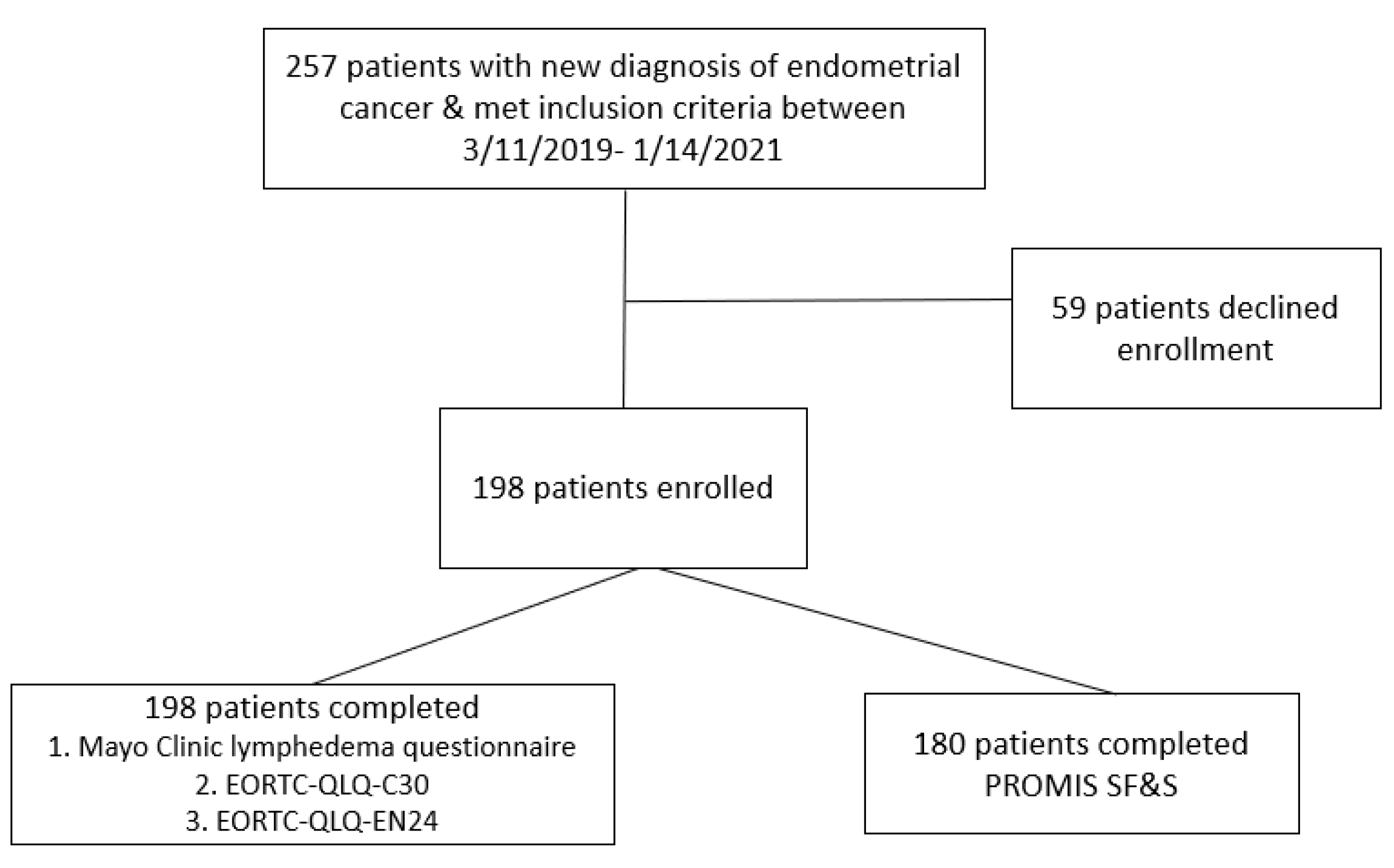
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.A.; Althouse, A.D.; Freese, K.E.; Soisson, S.; Edwards, R.P.; Welburn, S.; Sukumvanich, P.; Comerci, J.; Kelley, J.; LaPorte, R.E.; et al. USA endometrial cancer projections to 2030: Should we be concerned? Future Oncol. 2014, 10, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Creasman, W.T.; Odicino, F.; Maisonneuve, P.; Quinn, M.A.; Beller, U.; Benedet, J.L.; Heintz, A.P.M.; Ngan, H.Y.S.; Pecorelli, S. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006, 95 (Suppl. S1), S105–S143. [Google Scholar] [PubMed]
- Omichi, C.; Nakamura, K.; Haraga, J.; Ida, N.; Saijo, M.; Nishida, T.; Kusumoto, T.; Masuyama, H. The Influence of Adverse Effects on Quality of Life of Survivors of Gynecologic Cancer. Int. J. Gynecol. Cancer 2017, 27, 2014–2019. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Horak, F.; Jacobs, P.G.; Trubowitz, P.; Dieckmann, N.F.; Stoyles, S.; Faithfull, S. Falls, Functioning, and Disability among Women with Persistent Symptoms of Chemotherapy-Induced Peripheral Neuropathy. J. Clin. Oncol. 2017, 35, 2604–2612. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xiao, M.; Bai, H.; Zhang, Z. Sexual Function and Quality of Life Among Patients with Endometrial Cancer after Surgery. Int. J. Gynecol. Cancer. 2017, 27, 608–612. [Google Scholar] [CrossRef] [PubMed]
- De Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Yost, K.J.; Cheville, A.L.; Al-Hilli, M.M.; Mariani, A.; Barrette, B.A.; Mc Gree, M.E.; Weaver, A.L.; Dowdy, S.C. Lymphedema after surgery for endometrial cancer: Prevalence, risk factors, and quality of life. Obstet. Gynecol. 2014, 124, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Kolotkin, R.L.; Meter, K.; Williams, G.R. Quality of life and obesity. Obes. Rev. 2001, 2, 219–229. [Google Scholar] [CrossRef]
- Kolotkin, R.L.; Andersen, J.R. A systematic review of reviews: Exploring the relationship between obesity, weight loss and health-related quality of life. Clin. Obes. 2017, 7, 273–289. [Google Scholar] [CrossRef]
- Cella, D.; Yount, S.; Rothrock, N.; Gershon, R.; Cook, K.; Reeve, B.; Ader, D.; Fries, J.F.; Bruce, B.; Rose, M.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Med. Care 2007, 45, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.; Yashar, C.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; et al. Uterine Neoplasms, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Nolte, S.; Liegl, G.; Petersen, M.; Aaronson, N.; Costantini, A.; Fayers, P.; Groenvold, M.; Holzner, B.; Johnson, C.; Kemmler, G.; et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur. J. Cancer 2019, 107, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Greimel, E.; Nordin, A.; Lanceley, A.; Creutzberg, C.L.; van de Poll-Franse, L.V.; Radisic, V.B.; Galalae, R.; Schmalz, C.; Barlow, E.; Jensen, P.T.; et al. Psychometric Validation of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Endometrial Cancer Module (EORTC QLQ-EN24). Eur. J. Cancer 2011, 47, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Weinfurt, K.P.; Lin, L.; Bruner, D.W.; Cyranowski, J.M.; Dombeck, C.B.; Hahn, E.A.; Jeffery, D.D.; Luecht, R.M.; Magasi, S.; Porter, L.S.; et al. Development and Initial Validation of the PROMIS(®) Sexual Function and Satisfaction Measures Version 2.0. J. Sex. Med. 2015, 12, 1961–1974. [Google Scholar] [CrossRef]
- Yost, K.J.; Cheville, A.L.; Weaver, A.L.; Al Hilli, M.; Dowdy, S.C. Development and validation of a self-report lower-extremity lymphedema screening questionnaire in women. Phys. Ther. 2013, 93, 694–703. [Google Scholar] [CrossRef]
- Armbrust, R.; Auletta, V.; Cichon, G.; Vercellino, G.; Yost, K.; Sehouli, J. Lymphedema after pelvic and para-aortic lymphadenectomy-results of a systematic evaluation in patients with cervical and endometrial carcinoma. Arch. Gynecol. Obstet. 2023, 307, 1557–1565. [Google Scholar] [CrossRef]
- Lokich, E. Gynecologic Cancer Survivorship. Obstet. Gynecol. Clin. North Am. 2019, 46, 165–178. [Google Scholar] [CrossRef]
- Denlinger, C.S.; Sanft, T.; Baker, K.S.; Broderick, G.; Demark-Wahnefried, W.; Friedman, D.L.; Goldman, M.; Hudson, M.; Khakpour, N.; King, A.; et al. Survivorship, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 1216–1247. [Google Scholar] [CrossRef]
- Bødtcher, H.; Bidstrup, P.E.; Andersen, I.; Christensen, J.; Mertz, B.G.; Johansen, C.; Dalton, S.O. Fatigue trajectories during the first 8 months after breast cancer diagnosis. Qual. Life Res. 2015, 24, 2671–2679. [Google Scholar] [CrossRef]
- Juraskova, I.; Butow, P.; Robertson, R.; Sharpe, L.; McLeod, C.; Hacker, N. Post-treatment sexual adjustment following cervical and endometrial cancer: A qualitative insight. Psychooncology 2003, 12, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, A.; Krychman, M.L. Sexual dysfunction in patients with gynecologic neoplasms: A retrospective pilot study. J. Sex. Med. 2006, 3, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Ratner, E.S.; Foran, K.A.; Schwartz, P.E.; Minkin, M.J. Sexuality and intimacy after gynecological cancer. Maturitas 2010, 66, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, T.L.; Heckman, S.R.; Qualls, C.; Muller, C.Y.; Rogers, R.G. Pelvic floor disorders and sexual function in gynecologic cancer survivors: A cohort study. Am. J. Obstet. Gynecol. 2010, 203, 514.e1–514.e7. [Google Scholar] [CrossRef]
- Abbott-Anderson, K.; Kwekkeboom, K.L. A systematic review of sexual concerns reported by gynecological cancer survivors. Gynecol. Oncol. 2012, 124, 477–489. [Google Scholar] [CrossRef]
- Kulkarni, A.; Sun, G.; Manuppelli, S.; Mendez, H.; Rezendes, J.; Marin, C.; Raker, C.A.; Robison, K. Sexual health and function among patients receiving systemic therapy for primary gynecologic cancers. Gynecol. Oncol. 2022, 165, 323–329. [Google Scholar] [CrossRef]
- Bretschneider, C.E.; Bensen, J.T.; Geller, E.J.; Gehrig, P.A.; Wu, J.M.; Doll, K.M. Perioperative sexual interest in women with suspected gynecologic malignancies. Gynecol. Oncol. 2017, 146, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, C.E.; Doll, K.M.; Bensen, J.T.; Gehrig, P.A.; Wu, J.M.; Geller, E.J. Sexual Health Before Treatment in Women with Suspected Gynecologic Malignancy. J. Womens Health 2017, 26, 1326–1332. [Google Scholar] [CrossRef]
- Cianci, S.; Rosati, A.; Capozzi, V.A.; Tarascio, M.; Uccella, S.; Palumbo, M.; Caruso, S. Quality of life and sexual functioning of patient affected by endometrial cancer. Minerva Med. 2021, 112, 81–95. [Google Scholar] [CrossRef]
- Guedes, T.S.R.; Guedes, M.B.O.G.; Santana, R.d.C.; da Silva, J.F.C.; Dantas, A.A.G.; Ochandorena-Acha, M.; Terradas-Monllor, M.; Jerez-Roig, J.; de Souza, D.L.B. Sexual Dysfunction in Women with Cancer: A Systematic Review of Longitudinal Studies. Int. J. Environ. Res. Public Health 2022, 19, 11921. [Google Scholar] [CrossRef]
- Faubion, S.S.; Fairbanks, F.; Kuhle, C.L.; Sood, R.; Kling, J.M.; Vencill, J.A.; Mara, K.C.; Kapoor, E. Association between Body Mass Index and Female Sexual Dysfunction: A Cross-sectional Study from the Data Registry on Experiences of Aging, Menopause, and Sexuality. J. Sex. Med. 2020, 17, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Mehrara, B.J.; Greene, A.K. Lymphedema and obesity: Is there a link? Plast. Reconstr. Surg. 2014, 134, 154e–160e. [Google Scholar] [CrossRef] [PubMed]
- Balasubbramanian, D.; Mitchell, B.M. Lymphatics in Cardiovascular Physiology. Cold Spring Harb. Perspect. Med. 2022, 12, a041173. [Google Scholar] [CrossRef] [PubMed]
- von Gruenigen, V.E.; Gil, K.M.; Frasure, H.E.; Jenison, E.L.; Hopkins, M.P. The impact of obesity and age on quality of life in gynecologic surgery. Am. J. Obstet. Gynecol. 2005, 193, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Fader, A.N.; Frasure, H.E.; Gil, K.M.; Berger, N.A.; von Gruenigen, V.E. Quality of life in endometrial cancer survivors: What does obesity have to do with it? Obstet. Gynecol. Int. 2011, 2011, 308609. [Google Scholar] [CrossRef]
- Oldenburg, C.S.; Boll, D.; Nicolaije, K.A.; Vos, M.C.; Pijnenborg, J.M.; Coebergh, J.-W.; Beijer, S.; van de Poll-Franse, L.V.; Ezendam, N.P. The relationship of body mass index with quality of life among endometrial cancer survivors: A study from the population-based PROFILES registry. Gynecol. Oncol. 2013, 129, 216–221. [Google Scholar] [CrossRef]
- Smits, A.; Lopes, A.; Das, N.; Bekkers, R.; Galaal, K. The impact of BMI on quality of life in obese endometrial cancer survivors: Does size matter? Gynecol. Oncol. 2014, 132, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Karvinen, K.H.; Campbell, K.L.; Pearcey, R.G.; Dundas, G.; Capstick, V.; Tonkin, K.S. Associations among exercise, body weight, and quality of life in a population-based sample of endometrial cancer survivors. Gynecol. Oncol. 2005, 97, 422–430. [Google Scholar] [CrossRef]
- Lin, L.L.; Brown, J.C.; Segal, S.; Schmitz, K.H. Quality of life, body mass index, and physical activity among uterine cancer patients. Int. J. Gynecol. Cancer 2014, 24, 1027–1032. [Google Scholar] [CrossRef]
- Zhang, X.; Brown, J.C.; Schmitz, K.H. Association between Body Mass Index and Physical Function among Endometrial Cancer Survivors. PLoS ONE 2016, 11, e0160954. [Google Scholar] [CrossRef]
- Arem, H.; Irwin, M.L. Obesity and endometrial cancer survival: A systematic review. Int. J. Obes. 2013, 37, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, C.R.; Noll, M.; de Carvalho Santos, A.; Rodrigues, A.; Silveira, E.A. High prevalence of musculoskeletal pain in individuals with severe obesity: Sites, intensity, and associated factors. Korean J. Pain 2020, 33, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Onyemaechi, N.O.; Anyanwu, G.E.; Obikili, E.N.; Onwuasoigwe, O.; Nwankwo, O.E. Impact of overweight and obesity on the musculoskeletal system using lumbosacral angles. Patient Prefer. Adherence 2016, 10, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.C.; Xia, R.; Reynolds, E.; Banerjee, M.; Rothberg, A.E.; Burant, C.F.; Villegas-Umana, E.; Pop-Busui, R.; Feldman, E.L. Association Between Metabolic Syndrome Components and Polyneuropathy in an Obese Population. JAMA Neurol. 2016, 73, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.C.; Reynolds, E.; Banerjee, M.; Chant, E.; Villegas-Umana, E.; Feldman, E.L. Central Obesity Is Associated with Neuropathy in the Severely Obese. Mayo Clin. Proc. 2020, 95, 1342–1353. [Google Scholar] [CrossRef]
- Callaghan, B.; Kerber, K.; Langa, K.M.; Banerjee, M.; Rodgers, A.; McCammon, R.; Burke, J.; Feldman, E. Longitudinal patient-oriented outcomes in neuropathy: Importance of early detection and falls. Neurology 2015, 85, 71–79. [Google Scholar] [CrossRef]
- Census Bureau Releases New Educational Attainment Data. 2023. Available online: https://www.census.gov/newsroom/press-releases/2023/educational-attainment-data.html (accessed on 16 September 2024).
- Williams, C.P.; Senft Everson, N.; Shelburne, N.; Norton, W.E. Demographic and Health Behavior Factors Associated with Clinical Trial Invitation and Participation in the United States. JAMA Netw. Open 2021, 4, e2127792. [Google Scholar] [CrossRef]
- Thomas, C.; Mulnick, S.; Krucien, N.; Marsh, K. How do study design features and participant characteristics influence willingness to participate in clinical trials? Results from a choice experiment. BMC Med. Res. Methodol. 2022, 22, 323. [Google Scholar] [CrossRef]
- Agoritsas, T.; Deom, M.; Perneger, T.V. Study design attributes influenced patients’ willingness to participate in clinical research: A randomized vignette-based study. J. Clin. Epidemiol. 2011, 64, 107–115. [Google Scholar] [CrossRef]
| Demographic Characteristics | n = 198 |
|---|---|
| Age (years), mean (SD) | 63.6 (9.8) |
| BMI (kg/m2), median (IQR) | 36.0 (29.3, 41.5) |
| Race | |
| White | 197 (99.5) |
| Other | 1 (0.5) |
| Education | |
| High school graduate/GED or less | 44 (22.2) |
| Some college or 2-year degree | 71 (35.9) |
| 4-year degree | 54 (27.3) |
| Master’s or Ph.D. | 27 (13.6) |
| Unknown | 2 (1.0) |
| ASA score | |
| ≤2 | 101 (51.0) |
| >2 | 97 (49.0) |
| Treatment characteristics | |
| Procedure type | |
| Laparotomy | 14 (7.1) |
| Vaginal | 6 (3.0) |
| Robot-assisted | 162 (81.8) |
| Laparoscopic | 16 (8.1) |
| Procedures | |
| Oophorectomy | 191 (96.5) |
| Pelvic LND | 21 (10.6) |
| Paraaortic LND | 4 (2.0) |
| SLN biopsy ± LND | 186 (93.9) |
| Number of pelvic nodes removed (n = 21), mean (SD) | 11.5 (7.6) |
| Number of paraaortic nodes removed (n = 4), mean (SD) | 8.3 (5.6) |
| SLN nodes removed (n = 186), mean (SD) | 3.3 (2.2) |
| Disease characteristics | |
| Histology | |
| Endometrioid | 179 (90.4) |
| Serous | 7 (3.5) |
| Clear cell | 2 (1.0) |
| No residual cancer identified | 4 (2.0) |
| Other * | 6 (3.0) |
| Stage (FIGO 2009) | |
| IA | 154 (77.8) |
| IB | 20 (10.1) |
| II | 6 (3.0) |
| IIIA | 6 (3.0) |
| IIIC | 2 (1.0) |
| IVA | 1 (0.5) |
| No residual cancer identified | 9 (4.5) |
| Grade | |
| Grade 1 | 120 (60.6) |
| Grade 2 | 53 (26.8) |
| Grade 3 | 8 (4.0) |
| Not applicable | 17 (8.6) |
| Postoperative outcomes | |
| Readmission within 30 days of surgery | 2 (1.0) |
| Reoperation within 30 days of surgery | 0 (0.0) |
| Adjuvant therapy | |
| None | 149 (75.3) |
| VB only | 27 (13.6) |
| EBRT ± VB | 6 (3.0) |
| Chemotherapy ± VB | 5 (2.5) |
| Chemotherapy and EBRT ± VB | 11 (5.6) |
| Characteristic | Total (N = 198) | Underweight/ Normal/Overweight (BMI < 30.0 kg/m2) (N = 55) | Obesity Class I/II (BMI 30.0–39.9 kg/m2) (N = 84) | Obesity Class III (BMI 40.0+ kg/m2) (N = 59) | p * |
|---|---|---|---|---|---|
| Total # participants completed survey | 198 | 55 | 84 | 59 | |
| LEL screen-positive (prorated score > 4), N (%) | 63 (31.8) | 8 (14.5) | 28 (33.3) | 27 (45.8) | <0.01 |
| LEL screen-positive (prorated score > 8), N (%) | 26 (13.1) | 1 (1.8) | 13 (15.5) | 12 (20.3) | <0.01 |
| Domain * | Total | T-Score, Mean (SE) †(1) | Underweight/ Normal/Overweight (BMI < 30.0 kg/m2) (N = 51) | Obesity Class I/II (BMI 30.0–39.9 kg/m2) (N = 77) | Obesity Class III (BMI 40.0+ kg/m2) (N = 52) | p ‡ | |
|---|---|---|---|---|---|---|---|
| N | T-Score, Mean (SD) | T-Score, Median (IQR) | T-Score, Median (IQR) | T-Score, Median (IQR) | |||
| Interest in sexual activity | 180 | 31.9 (9.8) | 47.5 (0.70) | 32.9 (21.9, 43.9) | 27.4 (21.9, 38.4) | 32.9 (21.9, 38.4) | 0.26 |
| Orgasm ability | 42 | 43.6 (11.2) | 47.4 (0.74) | 39.6 (30.2, 58.4) | 39.6 (39.6, 49.0) | 49.0 (39.6, 53.7) | 0.48 |
| Orgasm pleasure | 39 | 44.6 (7.1) | 48.8 (0.84) | 47.5 (40.2, 58.6) | 40.2 (40.2, 47.5) | 43.9 (40.2, 47.5) | 0.61 |
| Satisfaction with sex life | 46 | 46.8 (6.8) | 47.5 (0.86) | 45.3 (39.8, 51.9) | 45.3 (39.8, 51.9) | 45.3 (42.5, 51.9) | 0.93 |
| Vaginal lubrication for sexual activity | 43 | 49.7 (8.7) | 46.2 (0.83) | 51.2 (40.3, 51.2) | 50.2 (41.8, 58.4) | 58.4 (51.9, 58.4) | 0.11 |
| Vaginal discomfort with sexual activity | 42 | 50.5 (8.6) | 51.9 (0.80) | 53.3 (43.3, 57.1) | 48.1 (43.3, 56.9) | 43.3 (43.3, 48.3) | 0.27 |
| Labial discomfort with sexual activity | 44 | 50.4 (6.5) | 50.9 (0.92) | 47.4 (47.4, 47.4) | 47.4 (47.4, 47.4) | 47.4 (47.4, 47.4) | 0.81 |
| Clitoral discomfort with sexual activity | 44 | 50.2 (5.8) | 50.4 (0.81) | 48.2 (48.2, 48.2) | 48.2 (48.2, 48.2) | 48.2 (48.2, 48.2) | 0.99 |
| Total (N = 198) | Reference Mean (SD) (2) | Underweight/Normal/ Overweight (BMI < 30.0 kg/m2) (N = 55) | Obesity Class I/II (BMI 30.0–39.9 kg/m2) (N = 84) | Obesity Class III (BMI 40.0+ kg/m2) (N = 59) | p * | |
|---|---|---|---|---|---|---|
| Functional scales † | ||||||
| Global health status | <0.01 | |||||
| Mean (SD) | 72.6 (17.6) | 64.3 (21.8) | 79.5 (15.9) | 74.6 (16.6) | 63.1 (16.8) | |
| Median (IQR) | 75.0 (66.7, 83.3) | 83.3 (66.7, 91.7) | 75.0 (66.7, 83.3) | 66.7 (58.3, 75.0) | ||
| No. score of 100, N (%) | 13 (6.6) | 7 (12.7) | 6 (7.1) | 3 (5.1) | ||
| Physical functioning | <0.01 | |||||
| Mean (SD) | 87.5 (16.5) | 84.3 (18.5) | 94.7 (8.5) | 87.8 (15.5) | 80.3 (20.2) | |
| Median (IQR) | 93.3 (86.7, 100.0) | 100.0 (93.3, 100.0) | 93.3 (86.7, 100.0) | 86.7 (73.3, 93.3) | ||
| No. score of 100, N (%) | 84 (42.4) | 34 (61.8) | 36 (42.9) | 14 (23.7) | ||
| Role functioning | 0.08 | |||||
| Mean (SD) | 91.9 (17.6) | 84.1 (24.6) | 95.5 (12.2) | 92.3 (18.0) | 87.9 (20.7) | |
| Median (IQR) | 100.0 (100.0, 100.0) | 100.0 (100.0, 100.0) | 100.0 (100.0, 100.0) | 100.0 (83.3, 100.0) | ||
| No. score of 100, N (%) | 152/197 (77.2) | 47 (85.5) | 66 (78.6) | 39/58 (67.2) | ||
| Emotional functioning | 0.46 | |||||
| Mean (SD) | 76.3 (19.8) | 71.9 (25.3) | 75.3 (18.5) | 77.6 (20.5) | 75.3 (20.3) | |
| Median (IQR) | 83.3 (66.7, 91.7) | 83.3 (66.7, 91.7) | 83.3 (66.7, 91.7) | 83.3 (66.7, 91.7) | ||
| No. score of 100, N (%) | 27/196 (13.8) | 6/54 (11.1) | 16/83 (19.3) | 5 (8.5) | ||
| Cognitive functioning | 0.42 | |||||
| Mean (SD) | 86.5 (18.7) | 84.3 (20.9) | 89.2 (14.5) | 86.7 (17.8) | 83.6 (22.8) | |
| Median (IQR) | 100.0 (83.3, 100.0) | 100.0 (83.3, 100.0) | 100.0 (83.3, 100.0) | 83.3 (83.3, 100.0) | ||
| No. score of 100, N (%) | 101/196 (51.5) | 29/54 (53.7) | 44/83 (53.0) | 28 (47.5) | ||
| Social functioning | 0.57 | |||||
| Mean (SD) | 87.2 (21.8) | 85.7 (24.6) | 89.8 (16.3) | 89.6 (19.1) | 81.4 (28.2) | |
| Median (IQR) | 100.0 (83.3, 100.0) | 100.0 (83.3, 100.0) | 100.0 (83.3, 100.0) | 100.0 (66.7, 100.0) | ||
| No. score of 100, N (%) | 126/196 (64.3) | 36/54 (66.7) | 58/83 (69.9) | 32 (54.2) | ||
| Symptom scales and items ‡ | ||||||
| Fatigue | 0.02 | |||||
| Mean (SD) | 21.3 (19.8) | 31.7 (25.9) | 15.2 (14.2) | 22.4 (21.1) | 25.4 (21.3) | |
| Median (IQR) | 22.2 (11.1, 33.3) | 11.1 (0.0, 22.2) | 22.2 (11.1, 33.3) | 22.2 (11.1, 33.3) | ||
| No. score of 0, N (%) | 47 (23.7) | 21 (38.2) | 20 (23.8) | 6 (10.2) | ||
| Pain | 0.06 | |||||
| Mean (SD) | 17.9 (23.7) | 25.3 (27.9) | 13.6 (22.2) | 18.7 (24.0) | 20.9 (24.5) | |
| Median (IQR) | 16.7 (0.0, 33.3) | 0.0 (0.0, 16.7) | 16.7 (0.0, 33.3) | 16.7 (0.0, 33.3) | ||
| No. score of 0, N (%) | 97 (49.0) | 32 (58.2) | 39 (46.4) | 26 (44.1) | ||
| Dyspnea, N (%) | <0.01 | |||||
| 0 | 149 (75.3) | 50 (90.9) | 66 (78.6) | 33 (55.9) | ||
| 33.3 | 45 (22.7) | 5 (9.1) | 17 (20.2) | 23 (39.0) | ||
| 66.7 | 3 (1.5) | - | 1 (1.2) | 2 (3.4) | ||
| 100 | 1 (0.5) | - | - | 1 (1.7) |
| Scale | Total (N = 198) | Underweight/ Normal/ Overweight (BMI < 30.0 kg/m2) (N = 55) | Obesity Class I/II (BMI 30.0–39.9 kg/m2) (N = 84) | Obesity Class III (BMI 40.0+ kg/m2) (N = 59) | p * |
|---|---|---|---|---|---|
| Functional scales † | |||||
| Sexual interest functioning | 0.02 | ||||
| Mean (SD) | 18.5 (21.5) | 25.6 (23.4) | 15.4 (21.7) | 16.4 (18.0) | |
| Median (IQR) | 0.0 (0.0, 33.3) | 33.3 (0.0, 33.3) | 0.0 (0.0, 33.3) | 0.0 (0.0, 33.3) | |
| No. score of 100, N (%) | 1/191 (0.5) | 1/52 (1.9) | 0/82 (0.0) | 0/57 (0.0) | |
| Sexual activity functioning | 0.19 | ||||
| Mean (SD) | 10.8 (19.0) | 15.0 (23.4) | 10.6 (18.1) | 7.5 (15.3) | |
| Median (IQR) | 0.0 (0.0, 33.3) | 0.0 (0.0, 33.3) | 0.0 (0.0, 33.3) | 0.0 (0.0, 0.0) | |
| No. score of 100, N (%) | 1/191 (0.5) | 1/51 (2.0) | 0/82 (0.0) | 0/58 (0.0) | |
| Sexual enjoyment functioning ¥ | 0.40 | ||||
| Mean (SD) | 57.6 (26.4) | 56.9 (32.8) | 54.0 (24.7) | 66.7 (15.7) | |
| Median (IQR) | 66.7 (33.3, 66.7) | 66.7 (33.3, 66.7) | 66.7 (33.3, 66.7) | 66.7 (66.7, 66.7) | |
| No. score of 100, N (%) | 7/48 (14.6) | 4/17 (23.5) | 2/21 (9.5) | 1/10 (10.0) | |
| Symptom scales ‡ | |||||
| Lymphedema | <0.01 | ||||
| Mean (SD) | 9.1 (15.0) | 2.1 (7.2) | 10.3 (15.5) | 13.8 (17.3) | |
| Median (IQR) | 0.0 (0.0, 16.7) | 0.0 (0.0, 0.0) | 0.0 (0.0, 16.7) | 0.0 (0.0, 33.3) | |
| No. score of 0, N (%) | 133 (67.2) | 50 (90.9) | 53 (63.1) | 30 (50.8) | |
| Urological | <0.01 | ||||
| Mean (SD) | 19.3 (19.6) | 13.8 (15.7) | 18.4 (19.4) | 25.8 (21.6) | |
| Median (IQR) | 16.7 (0.0, 25.0) | 8.3 (0.0, 25.0) | 16.7 (0.0, 25.0) | 25.0 (8.3, 41.7) | |
| No. score of 0, N (%) | 52 (26.3) | 18 (32.7) | 24 (28.6) | 10 (16.9) | |
| Gastrointestinal | 0.17 | ||||
| Mean (SD) | 13.8 (14.0) | 11.3 (13.1) | 15.4 (15.0) | 13.9 (13.2) | |
| Median (IQR) | 13.3 (0.0, 20.0) | 6.7 (0.0, 13.3) | 13.3 (6.7, 20.0) | 13.3 (6.7, 20.0) | |
| No. score of 0, N (%) | 51 (25.8) | 18 (32.7) | 19 (22.6) | 14 (23.7) | |
| Poor body image | 0.92 | ||||
| Mean (SD) | 8.6 (16.6) | 8.3 (17.1) | 8.1 (14.3) | 9.6 (19.4) | |
| Median (IQR) | 0.0 (0.0, 16.7) | 0.0 (0.0, 0.0) | 0.0 (0.0, 16.7) | 0.0 (0.0, 16.7) | |
| No. score of 0, N (%) | 145/197 (73.6) | 41/54 (75.9) | 60 (71.4) | 44 (74.6) | |
| Sexual/vaginal problems ¥ | 0.25 | ||||
| Mean (SD) | 17.1 (23.1) | 26.1 (30.4) | 13.8 (17.5) | 8.9 (14.6) | |
| Median (IQR) | 11.1 (0.0, 27.8) | 22.2 (0.0, 33.3) | 11.1 (0.0, 22.2) | 0.0 (0.0, 22.2) | |
| No. score of 0, N (%) | 23/48 (47.9) | 7/17 (41.2) | 9/21 (42.9) | 7/10 (70.0) | |
| Pain in back and pelvis | 0.02 | ||||
| Mean (SD) | 29.5 (28.7) | 20.0 (23.7) | 34.9 (31.4) | 30.5 (27.2) | |
| Median (IQR) | 33.3 (0.0, 33.3) | 0.0 (0.0, 33.3) | 33.3 (0.0, 50.0) | 33.3 (0.0, 66.7) | |
| No. score of 0, N (%) | 76 (38.4) | 29 (52.7) | 26 (31.0) | 21 (35.6) | |
| Tingling/numbness | <0.01 | ||||
| Mean (SD) | 14.5 (22.9) | 9.1 (19.7) | 13.1 (21.3) | 21.5 (26.1) | |
| Median (IQR) | 0.0 (0.0, 33.3) | 0.0 (0.0, 0.0) | 0.0 (0.0, 33.3) | 0.0 (0.0, 33.3) | |
| No. score of 0, N (%) | 131 (66.2) | 43 (78.2) | 57 (67.9) | 31 (52.5) | |
| Muscular pain | 0.01 | ||||
| Mean (SD) | 25.5 (26.1) | 17.6 (23.0) | 26.3 (25.7) | 31.6 (28.0) | |
| Median (IQR) | 33.3 (0.0, 33.3) | 0.0 (0.0, 33.3) | 33.3 (0.0, 33.3) | 33.3 (0.0, 66.7) | |
| No. score of 0, N (%) | 82/195 (42.1) | 31 (56.4) | 31/81 (38.3) | 20 (33.9) | |
| Hair loss | 0.10 | ||||
| Mean (SD) | 9.6 (18.5) | 5.5 (12.4) | 12.3 (20.6) | 9.8 (19.8) | |
| Median (IQR) | 0.0 (0.0, 33.3) | 0.0 (0.0, 0.0) | 0.0 (0.0, 33.3) | 0.0 (0.0, 0.0) | |
| No. score of 0, N (%) | 147/197 (74.6) | 46 (83.6) | 57 (67.9) | 44/58 (75.9) | |
| Taste change | 0.40 | ||||
| Mean (SD) | 4.0 (13.7) | 1.8 (7.6) | 5.6 (17.8) | 4.0 (10.9) | |
| Median (IQR) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | |
| No. score of 0, N (%) | 178 (89.9) | 52 (94.5) | 74 (88.1) | 52 (88.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warring, S.; Yost, K.J.; Cheville, A.L.; Dowdy, S.C.; Faubion, S.S.; Kumar, A.; Lemens, M.A.; Van Oort, C.C.; Fought, A.J.; Mc Gree, M.E.; et al. The Quality of Life after Endometrial Cancer Study: Baseline Characteristics and Patient-Reported Outcomes. Curr. Oncol. 2024, 31, 5557-5572. https://doi.org/10.3390/curroncol31090412
Warring S, Yost KJ, Cheville AL, Dowdy SC, Faubion SS, Kumar A, Lemens MA, Van Oort CC, Fought AJ, Mc Gree ME, et al. The Quality of Life after Endometrial Cancer Study: Baseline Characteristics and Patient-Reported Outcomes. Current Oncology. 2024; 31(9):5557-5572. https://doi.org/10.3390/curroncol31090412
Chicago/Turabian StyleWarring, Simrit, Kathleen J. Yost, Andrea L. Cheville, Sean C. Dowdy, Stephanie S. Faubion, Amanika Kumar, Maureen A. Lemens, Chelsie C. Van Oort, Angela J. Fought, Michaela E. Mc Gree, and et al. 2024. "The Quality of Life after Endometrial Cancer Study: Baseline Characteristics and Patient-Reported Outcomes" Current Oncology 31, no. 9: 5557-5572. https://doi.org/10.3390/curroncol31090412
APA StyleWarring, S., Yost, K. J., Cheville, A. L., Dowdy, S. C., Faubion, S. S., Kumar, A., Lemens, M. A., Van Oort, C. C., Fought, A. J., Mc Gree, M. E., Mariani, A., & Glaser, G. (2024). The Quality of Life after Endometrial Cancer Study: Baseline Characteristics and Patient-Reported Outcomes. Current Oncology, 31(9), 5557-5572. https://doi.org/10.3390/curroncol31090412






