1. Introduction
As the prevalence of advanced prostate cancer increases (CCS), its management continues to rapidly evolve. One of the most significant changes in management over the last decade is the use of systemic therapies that were initially approved for metastatic castration-resistant prostate cancer (mCRPC) in earlier disease states, such as metastatic castration-sensitive prostate cancer (mCSPC). Since the landmark TAX-327 and SWOG 99-16 phase III trials, docetaxel has been used in mCRPC [
1,
2]. The benefits of the early use of docetaxel in addition to androgen deprivation therapy (ADT) in newly diagnosed patients with mCSPC were demonstrated approximately ten years later with the publication of the CHAARTED and STAMPEDE trials [
3,
4]. Similarly, while androgen receptor pathway inhibitors (ARPIs) such as abiraterone and enzalutamide were first approved in mCRPC, they have demonstrated significant benefits in mCSPC [
3,
4,
5,
6,
7,
8]. Recent evidence suggests that there may also be further benefit of combination strategies with both abiraterone and darolutamide demonstrating improved survival when added to ADT and docetaxel [
6,
7,
9,
10]. Additionally, the number of systemic therapy options for patients is significantly expanding, with several novel agents, such as PARP inhibitors and radioligand therapies, receiving regulatory approval in mCRPC and being actively investigated in mCSPC [
11]. Such addition of further therapies along with ADT in mCSPC, such as ARPIs and/or chemotherapy, is referred to as treatment intensification. This approach has shown gradual uptake and is now considered the standard of care for most patients with mCSPC due to the demonstrated improvements in overall survival and time to progression [
12].
Despite these advances, cytotoxic chemotherapy remains a relevant option for patients. Though approved before many of the currently available therapies, both docetaxel and cabazitaxel have demonstrated improved OS in modern clinical trials [
3,
5,
13]. It has been demonstrated that cabazitaxel does not improve OS when used in place of docetaxel but does when used sequentially after progression on docetaxel when both are used in mCRPC [
13,
14]. However, the optimal sequence has not been established for when docetaxel is used initially in the mCSPC setting [
15], where we are likely to see increased use given recent data. In a post-treatment analysis of patients participating in GETUG-AFU 15, only 14% of patients who received docetaxel intensification (DI) in mCSPC had a ≥50% PSA response to the subsequent use of docetaxel in mCRPC [
16]. The efficacy of docetaxel relative to cabazitaxel after DI has not been demonstrated. It is important to demonstrate the outcomes of docetaxel and cabazitaxel in mCRPC after DI in mCSPC to ensure efficacy and tolerance are demonstrated when re-challenging with a taxane in this setting, as well as to guide treatment sequencing efforts for mCRPC therapies to apply to individual patients.
In this study, we aim to characterize the activity of docetaxel and cabazitaxel in mCRPC patients who have previously received docetaxel in mCSPC. By comparing the outcomes of patients who receive these agents as their first subsequent taxane (FST) after DI in mCSPC, we hope to add to the existing literature and inform treatment selection for patients and clinicians.
2. Methods
2.1. Patient Population
This study was approved by the Health Research Ethics Board of Alberta. Following approval, all new patient consults seen by the genitourinary medical oncology group at the Cross Cancer Institute, Edmonton, Canada, from 1 July 2014 to 31 December 2020 were reviewed to identify eligible patients. Patients were considered eligible for this study if they (1) received DI for mCSPC and (2) later received either docetaxel or cabazitaxel for mCRPC, irrespective of the line of therapy.
2.2. Data Collection Outcome Measures
Clinical data were retrieved from the regional electronic medical record (EMR). Variables of interest, including patient characteristics, treatment regimens, laboratory and imaging results, and survival outcomes, were collected from the EMR. Data were aggregated and anonymized for statistical analysis. The primary endpoint was the proportion of patients achieving ≥50% PSA response at 12 weeks relative to the baseline for FST. Secondary endpoints included OS from mCSPC diagnosis, as well as PFS and OS from the FST start date. PFS was defined as either PSA progression using Prostate Cancer Working Group criteria 3 (Scher), radiographic progression using RECIST v1.1 for soft tissue disease (Eisenhauer) and PCWG3 criteria for bone or death from any cause.
2.3. Statistical Analysis
Descriptive statistics were reported for the study variables. Mean and SD or median (range) were reported for continuous variables; frequencies and proportions were reported for categorical variables. PSA responses between the two groups were compared using chi-squared tests. Mann–Whitney U tests were used for median comparison between the two groups. Time-based endpoints were compared using the Kaplan–Meier method, and the survival curves were compared using log-rank tests. Univariate analysis was used to determine the factors associated with PSA response to FST. For the statistical analysis, a p-value < 0.05 was used for statistical significance. All statistical analyses were conducted using SPSS software version 29 (IBM Corp. Released 2022. IBM SPSS Statistics for Windows. Version 29.0. Armonk, NY, USA: IBM Corp.).
3. Results
Thirty-four patients (cabazitaxel = twelve; docetaxel = twenty-two) were identified, with their baseline characteristics shown in
Table 1. Almost all patients (97%) received ADT and docetaxel as initial systemic therapy in mCSPC, while one patient who later received cabazitaxel as FST received an ARPI in addition to ADT and docetaxel. Nearly all patients (91%: cabazitaxel = 92%; docetaxel = 91%) received six total cycles of docetaxel in mCSPC. The median age at mCSPC diagnosis for patients was 67.1 years for cabazitaxel and 63.1 years for docetaxel (
p = 0.236). Most patients (cabazitaxel = 100%; docetaxel = 81.8%) had bone metastases at diagnosis, with a minority having lymph node only (cabazitaxel = 0%; docetaxel = 4.5%) or visceral metastases (cabazitaxel = 0%; docetaxel =13.6%) at diagnosis with no significant difference between groups (
p = 0.215). Baseline PSA, LDH, ALP, and neutrophil-to-lymphocyte ratio before FST were not significantly different between groups (
Table 1).
At the time of the last follow-up, almost all (total 97.1%: cabazitaxel = 100%; docetaxel = 95.5%; p = 0.453) had disease progression on FST therapy. One patient who had received docetaxel as FST continued to be progression-free and alive at the last follow-up. Further, most patients had died at the time of the last follow-up (88.2%: cabazitaxel = 100%; docetaxel = 81.8%, p = 0.116).
The median time to CRPC was 17.6 months (cabazitaxel = 14.2 mos; docetaxel = 18.6 mos, p = 0.079). Most patients (91.2%, cabazitaxel = 83.3%; docetaxel = 95.5%) received their FST as second-line treatment for mCRPC, with few patients receiving their FST as first (5.9%: cabazitaxel = 8.3%; docetaxel = 4.6%) or third (2.9%: cabazitaxel = 8.3%; docetaxel = 0%) treatment lines in mCRPC. There was no significant difference in the line of therapy in which the FST was received between groups (p = 0.680).
As shown in
Table 2, the median time to FST from the date of mCSPC diagnosis was significantly shorter for patients receiving cabazitaxel as FST (cabazitaxel = 24.1 mos; docetaxel = 34.6 mos;
p = 0.036). Similarly, the time from the last docetaxel in mCSPC to FST was significantly shorter for patients receiving cabazitaxel between groups (cabazitaxel = 18.9 mos; docetaxel = 29.8 mos;
p = 0.041). Of those who received the FST as second- or third-line therapies in mCRPC, all patients received an androgen receptor pathway inhibitor (ARPI) (28% abiraterone; 72% enzalutamide) as first-line therapy.
PSA responses, identified as ≥50% PSA reductions, at 12 weeks after the baseline from FST occurred in 35.3%, with no significant difference between groups (cabazitaxel = 25.0%; docetaxel = 40.9%; p = 0.465). The median PSA change at 12 weeks relative to the FST increased by 19.8% (cabazitaxel = 3.0%; docetaxel = 19.8%; p = 0.490) due to several patients having drastic biochemical progression since the initiation of FST. There was no significant difference between groups for PSA response as a continuous variable to FST (p = 0.645).
Univariate analysis was conducted to determine if any factors were associated with PSA response to FST. There was no association between time to FST (p = 0.294) and time from the last docetaxel in mCSPC to FST (p = 0.220) and response. Baseline ALP before FST, though nearing significance, was not associated with response (p = 0.057). Baseline PSA (p = 0.164), LDH (p = 0.606), and baseline neutrophil-to-lymphocyte ratio (p = 0.229) before FST were not significantly associated with response. As no factors were significant in the univariate analysis, a multivariate analysis was not conducted.
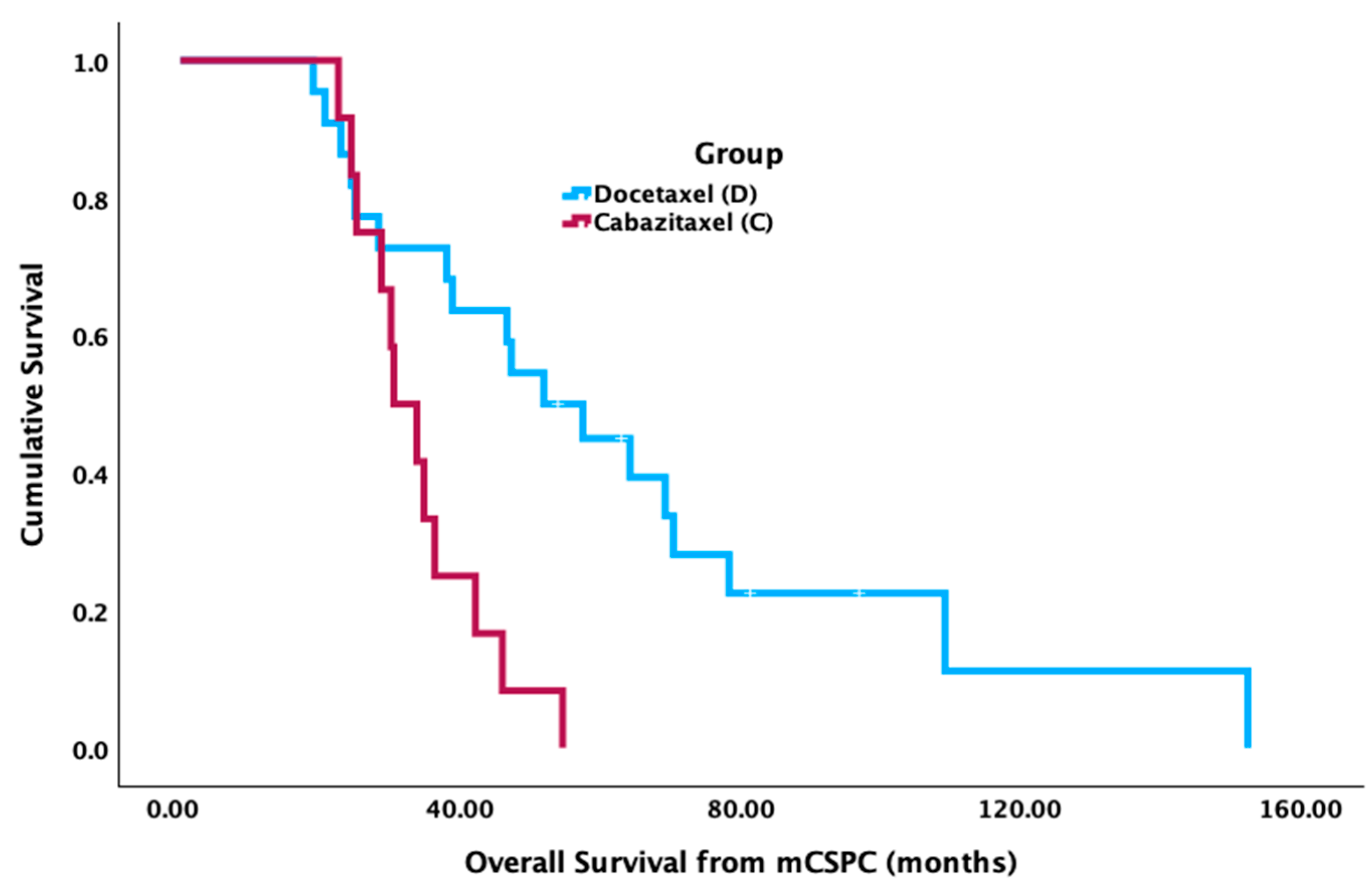
Patients who received docetaxel in FST had significantly longer median OS from mCSPC diagnosis (52.7 mos [95% CI 36.4–69.0 mos]) compared to cabazitaxel (31.0 mos [95% CI 24.6–37.3 mos] (
p = 0.002) (
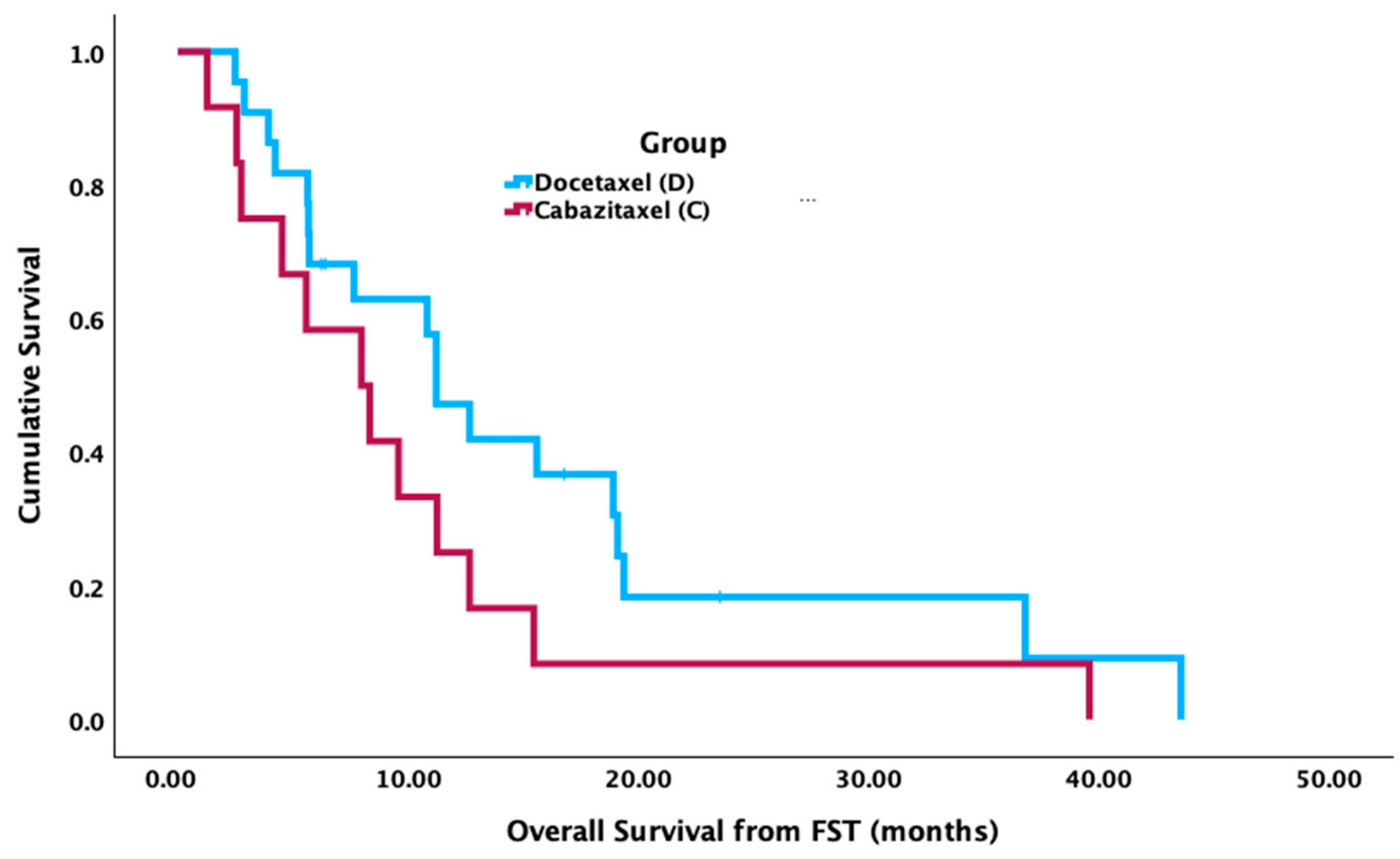
Figure 1). There was no significant difference in median OS from the FST start date between groups (cabazitaxel = 8.1 mos [95% CI 3.3–12.9 mos]; docetaxel = 11.4 mos [95% CI 8.8–14.0 mos],
p = 0.132) (
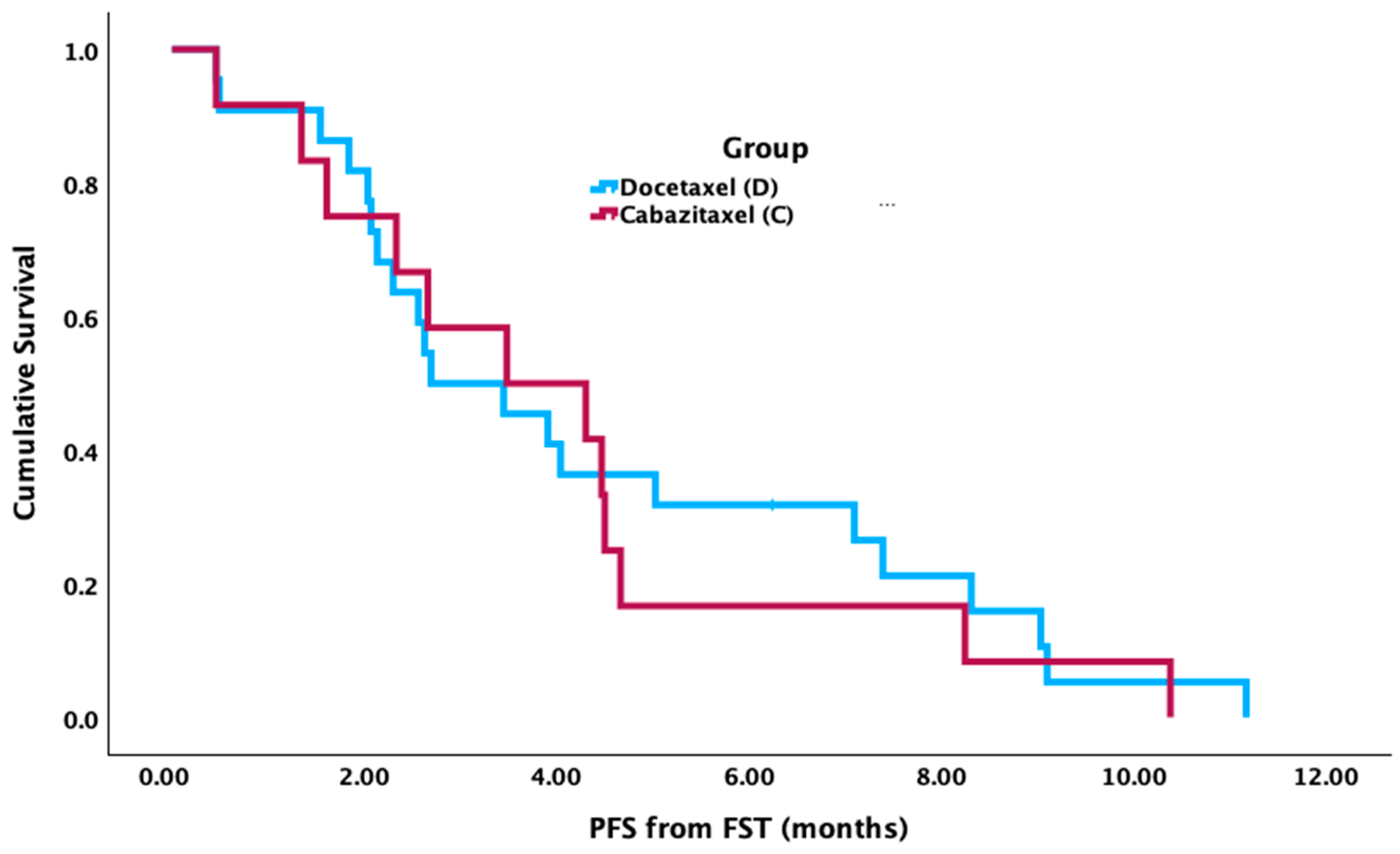
Figure 2). There was no significant difference between groups for median PFS from the FST start date (cabazitaxel = 3.5 mos [95% CI 0.7–6.4 mos]; docetaxel = 2.7 mos [95% CI 1.2–4.3 mos],
p = 0.727) (
Figure 3).
4. Discussion
This study found that both docetaxel and cabazitaxel were active in mCRPC after DI in mCSPC, with PSA response rates comparable to the registration trials for these agents that were conducted in mCRPC patients who did not receive DI [
1,
13]. No significant difference in response rates was seen between docetaxel and cabazitaxel, nor were differences in PFS or OS from the start of FST. Specifically, in this study, docetaxel showed a PSA ≥50% response rate of 40.9%, which is notably higher than what was reported by Lavaud et al. [
16], where a PSA ≥ 50% response was seen in only four of twenty-nine patients (14%). There did not appear to be a clear advantage of using cabazitaxel as FST after DI, which showed a numerically lower response rate compared to docetaxel. Baseline characteristics were similar between patients who received docetaxel or cabazitaxel, including known prognostic factors. Baseline ALP, LDH, neutrophil/lymphocyte ratio (NLR), PSA, total PSA response, and the presence of visceral metastases have previously been identified as important prognostic factors in mCRPC patients [
17,
18,
19,
20,
21,
22,
23]. In this study, baseline ALP (
p = 0.057), baseline PSA (
p = 0.164), LDH (
p = 0.606), and NLR (
p = 0.229) before FST were not predictive of biochemical treatment response but were not analyzed prognostically.
Notably, the time to FST and time from the last docetaxel was significantly shorter for patients receiving cabazitaxel compared to docetaxel. This may reflect clinicians’ perception that cabazitaxel is more active after docetaxel and a preference to select this in patients they believe to have more aggressive disease due to the relatively rapid development of mCRPC. The patients in the cabazitaxel group had a worse prognosis, as reflected in a worse OS from mCSPC diagnosis. This, however, did appear to not impact outcomes with FST after DI with no baseline factor, including time to mCRPC or time from the last docetaxel, associated with probability of response on univariate analysis. Similarly, there was no significant difference in PFS or OS from FST, which would suggest the differences in OS from mCSPC were related to prognostic factors rather than their outcome with FST therapy. The p-value, however, was low (0.132), which may indicate a trend toward differences in overall survival from FST between docetaxel and cabazitaxel, which would be better assessed through a larger sample size.
Upon a review of the literature, FIRSTANA is the only study to directly compare the efficacy of cabazitaxel and docetaxel in mCRPC patients; however, this study used a chemotherapy-naïve population. FIRSTANA found no significant difference in OS between groups [
14]. The findings of this study are consistent with this research, suggesting that the prior use of DI in mCSPC does not influence the outcome of taxane chemotherapy in mCRPC. These findings could be used to guide patient-specific taxane selection, acknowledging a patient preference for cabazitaxel with enhanced quality of life and reduced fatigue, pain, nausea/vomiting, and hair/nail changes when compared to docetaxel with reduced diarrhea [
14,
24].
This study is limited by its retrospective nature and small sample size, as patients had to receive taxane chemotherapy in mCSPC and mCRPC to meet eligibility. Another possible limitation is its lack of standardization of drug sequencing, where patients received cabazitaxel and docetaxel in various treatment lines after different systemic therapies. Further studies are needed to determine the best sequencing of and comparisons between survival-prolonging systemic therapies in mCRPC. Additionally, further studies are needed to determine outcomes following the use of FST in mCRPC after DI in mCSPC. There is evidence for the re-challenge of taxanes, specifically docetaxel, in mCRPC, but little is known regarding the use of taxanes in mCRPC after DI in mCSPC due to the timing of initial studies.
5. Conclusions
This study compared the use of taxane chemotherapies, specifically docetaxel and cabazitaxel, in mCRPC following DI in mCSPC. Its findings contribute to patient-specific treatment selection and sequencing efforts in mCRPC. Both docetaxel and cabazitaxel demonstrated activity as FST after DI in mCSPC. Patients who received cabazitaxel had a significantly shorter time to FST and a shorter median OS from mCSPC. No difference was found between docetaxel and cabazitaxel for PSA response, PFS, or OS from the FST. While limited by its retrospective nature and small sample size, this study suggests that docetaxel and cabazitaxel are active as FST after treatment with DI in mCSPC. Further research, such as a multicentre analysis, is needed to compare docetaxel and cabazitaxel as FST in mCRPC after DI in mCSPC.
Author Contributions
Conceptualization, M.K.; Methodology, M.K.; Validation, M.K.; Formal Analysis, G.R. and S.G.; Investigation, G.R.; Data Curation, G.R.; Writing—original draft, G.R.; Writing—review and editing, N.S.B., S.N., S.G. and M.K.; Visualization, G.R.; Supervision, M.K.; Project Administration, G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethics approval was waived in accordance with the Health Research Ethics Board of Alberta as it was deemed a quality assurance study.
Informed Consent Statement
Patient consent was waived as it was impractical to seek consent from these patients with many being deceased, minimal risk was posed to patients through this retrospective data review, and collected data were anonymized and protected and collected by trained investigators.
Data Availability Statement
The data presented in this study are not available due to privacy or ethical restrictions.
Acknowledgments
This project was presented at the 2023 ASCO Genitourinary Cancers Symposium. Abstract, figures, and findings were originally published by the American Society of Clinical Oncology. Gabrielle Robin, Naveen S. Basappa, Scott A. North, Sunita Ghosh, Michael Paul Kolinsky: 41, 2023: 72-72. ©ASCO. All Rights Reserved.
Conflicts of Interest
Gabrielle Robin: No disclosures. Naveen S. Basappa: Astellas, AstraZeneca, Bayer, BMS, Eisai, EMD Serono, Ipsen, Janssen, Merck, Pfizer, Seagen, Takeda. Scott North: No disclosures. Sunita Ghosh: No disclosures. Michael Kolinsky: AstraZeneca, Astellas, Bayer, BMS, Eisai, EMD Serono, Ipsen, Janssen, Merck.
References
- Tannock, I.F.; De Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; Lara, P.N.J.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, C.E.; Chen, Y.-H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022, 399, 1695–1707. [Google Scholar] [CrossRef]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N. Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef]
- de Wit, R.; de Bono, J.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Wülfing, C.; Kramer, G.; Eymard, J.-C.; Bamias, A.; Carles, J.; et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef]
- Assi, T.; Rassy, E.; Farhat, F.; Kattan, C.; Kattan, J. Docetaxel rechallenge in patients with metastatic prostate cancer: A comprehensive review. Oncol. Res. Treat. 2020, 43, 299–306. [Google Scholar] [CrossRef] [PubMed]
- McManus, H.D.; Armstrong, A.J. The past, present, and future of treatment intensification for metastatic hormone–sensitive prostate cancer. J. Clin. Oncol. 2023, 41, 3576–3579. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.-P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Oudard, S.; Fizazi, K.; Sengeløv, L.; Daugaard, G.; Saad, F.; Hansen, S.; Hjälm-Eriksson, M.; Jassem, J.; Thiery-Vuillemin, A.; Caffo, O.; et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: A randomized phase III trial—FIRSTANA. J. Clin. Oncol. 2017, 35, 3189–3197. [Google Scholar] [CrossRef] [PubMed]
- Caffo, O.; Maines, F.; Kinspergher, S.; Veccia, A.; Messina, C. Sequencing strategies in the new treatment landscape of prostate cancer. Futur. Oncol. 2019, 15, 2967–2982. [Google Scholar] [CrossRef]
- Lavaud, P.; Gravis, G.; Foulon, S.; Joly, F.; Oudard, S.; Priou, F.; Latorzeff, I.; Mourey, L.; Soulié, M.; Delva, R.; et al. Anticancer activity and tolerance of treatments received beyond progression in men treated upfront with androgen deprivation therapy with or without docetaxel for metastatic castration-naïve prostate cancer in the GETUG-AFU 15 phase 3 trial. Eur. Urol. 2018, 73, 696–703. [Google Scholar] [CrossRef]
- Halabi, S.; Lin, C.-Y.; Kelly, W.K.; Fizazi, K.S.; Moul, J.W.; Kaplan, E.B.; Morris, M.J.; Small, E.J. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2014, 32, 671–677. [Google Scholar] [CrossRef]
- Berry, W.R.; Laszlo, J.; Cox, E.; Walker, A.; Paulson, D. Prognostic factors in metastatic and hormonally unresponsive carcinoma of the prostate. Cancer 1979, 44, 763–775. [Google Scholar] [CrossRef]
- Pei, X.-Q.; He, D.-L.; Tian, G.; Lv, W.; Jiang, Y.-M.; Wu, D.-P.; Fan, J.-H.; Wu, K.-J. Prognostic factors of first-line docetaxel treatment in castration-resistant prostate cancer: Roles of neutrophil-to-lymphocyte ratio in patients from Northwestern China. Int. Urol. Nephrol. 2017, 49, 629–635. [Google Scholar] [CrossRef]
- Hiew, K.; Hart, C.A.; Ali, A.; Elliott, T.; Ramani, V.; Sangar, V.; Lau, M.; Maddineni, S.; Brown, M.; Clarke, N. Primary mutational landscape linked with pre-docetaxel lactate dehydrogenase levels predicts docetaxel response in metastatic castrate-resistant prostate cancer. Eur. Urol. Focus 2019, 5, 831–841. [Google Scholar] [CrossRef]
- Qu, Y.-Y.; Dai, B.; Kong, Y.-Y.; Ye, D.-W.; Yao, X.-D.; Zhang, S.-L.; Zhang, H.-L.; Ma, C.-G.; Yang, W.-Y. Prognostic factors in Chinese patients with metastatic castration-resistant prostate cancer treated with docetaxel-based chemotherapy. Asian J. Androl. 2013, 15, 110–115. [Google Scholar] [CrossRef]
- Kelly, W.K.; Scher, H.I.; Mazumdar, M.; Vlamis, V.; Schwartz, M.; Fossa, S.D. Prostate-specific antigen as a measure of disease outcome in metastatic hormone-refractory prostate cancer. J. Clin. Oncol. 1993, 11, 607–615. [Google Scholar] [CrossRef]
- Smaletz, O.; Scher, H.I.; Small, E.J.; Verbel, D.A.; McMillan, A.; Regan, K.; Kelly, W.K.; Kattan, M.W. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J. Clin. Oncol. 2002, 20, 3972–3982. [Google Scholar] [CrossRef]
- Baciarello, G.; Delva, R.; Gravis, G.; Tazi, Y.; Beuzeboc, P.; Gross-Goupil, M.; Bompas, E.; Joly, F.; Greilsamer, C.; Hon, T.N.T.; et al. Patient preference between cabazitaxel and docetaxel for first-line chemotherapy in metastatic castration-resistant prostate cancer: The CABADOC trial. Eur. Urol. 2022, 81, 234–240. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).