High-Grade Serous Ovarian Cancer during Pregnancy: From Diagnosis to Treatment
Abstract
1. Introduction
2. Materials and Methods
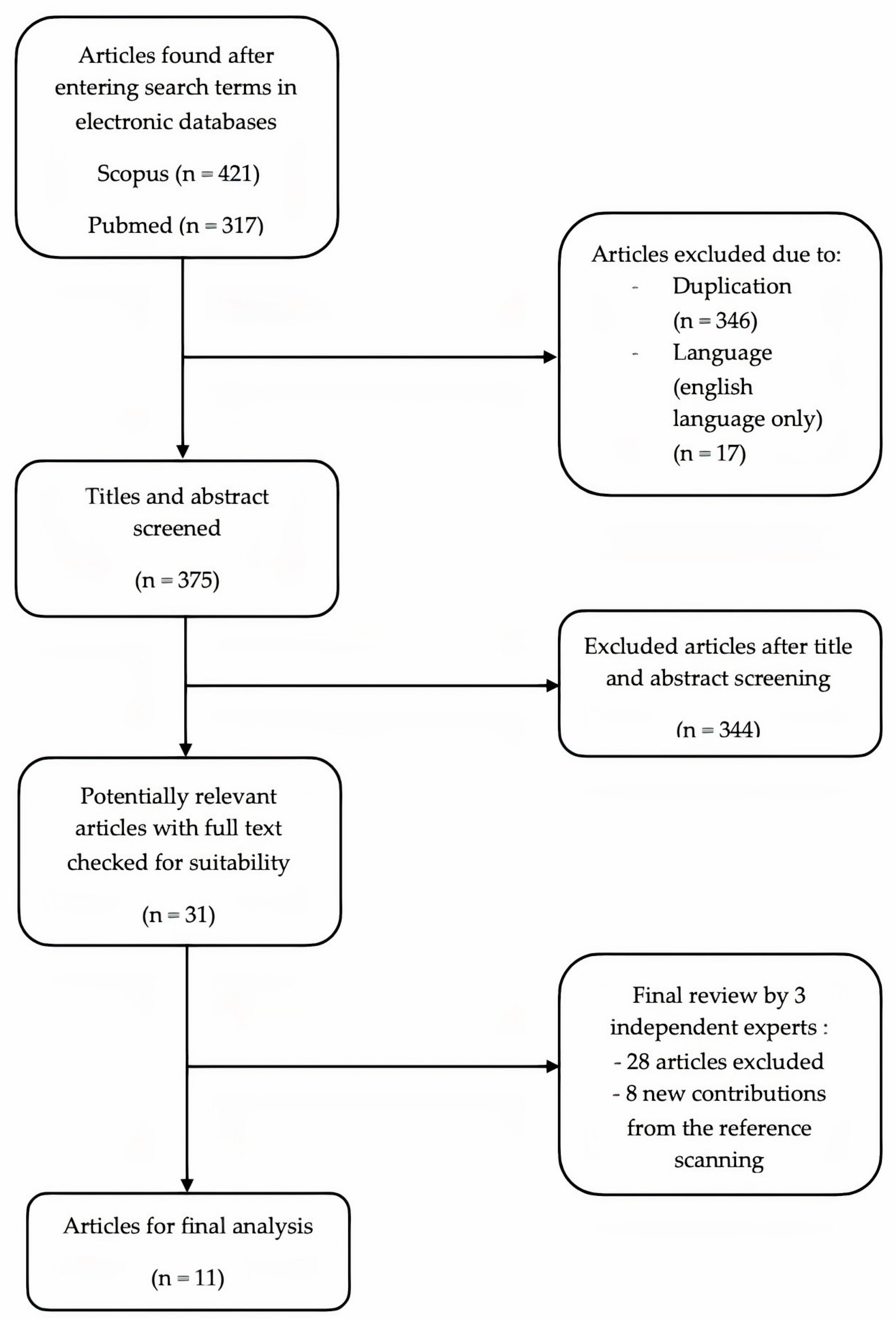
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction
3. Results
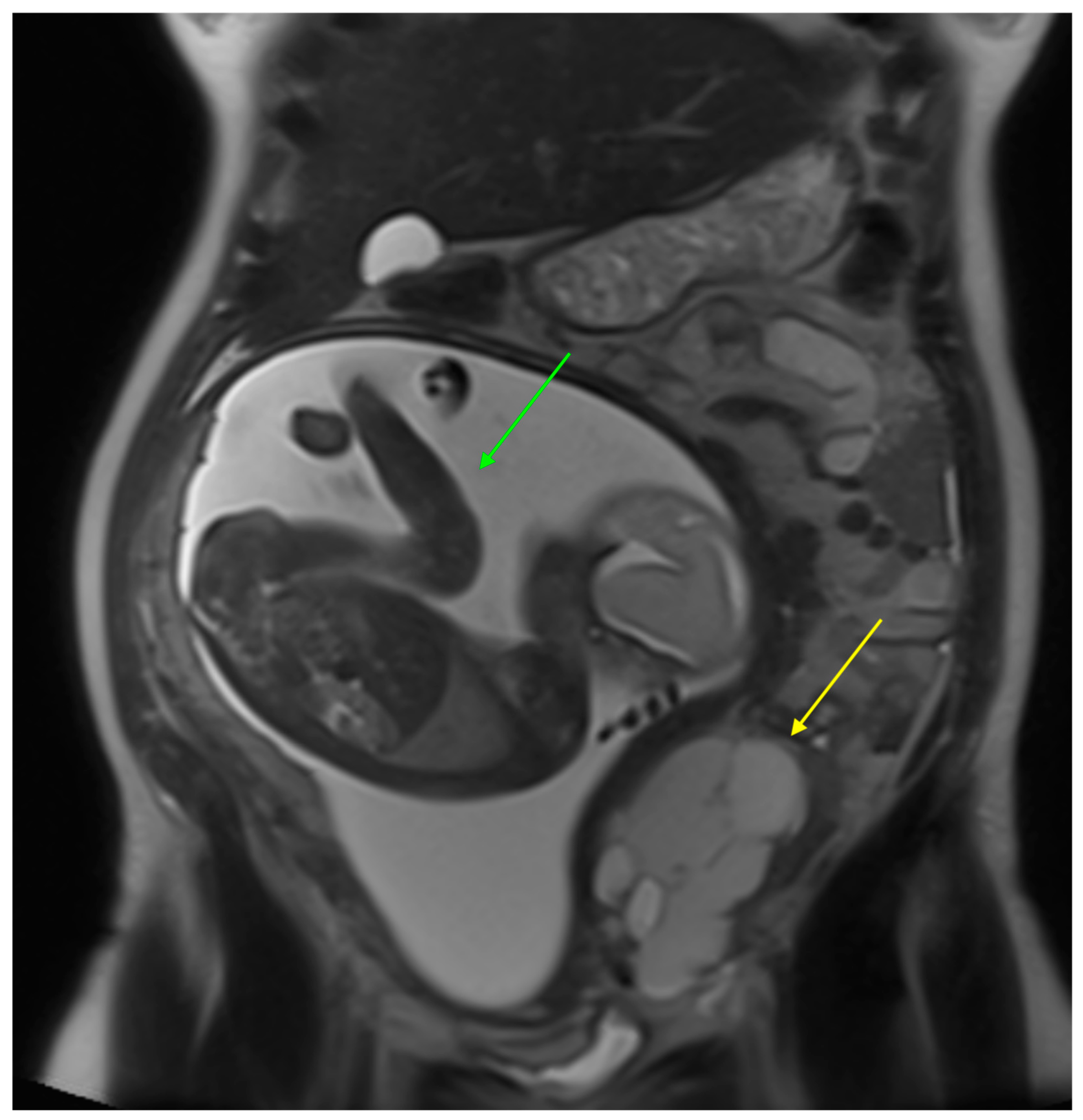
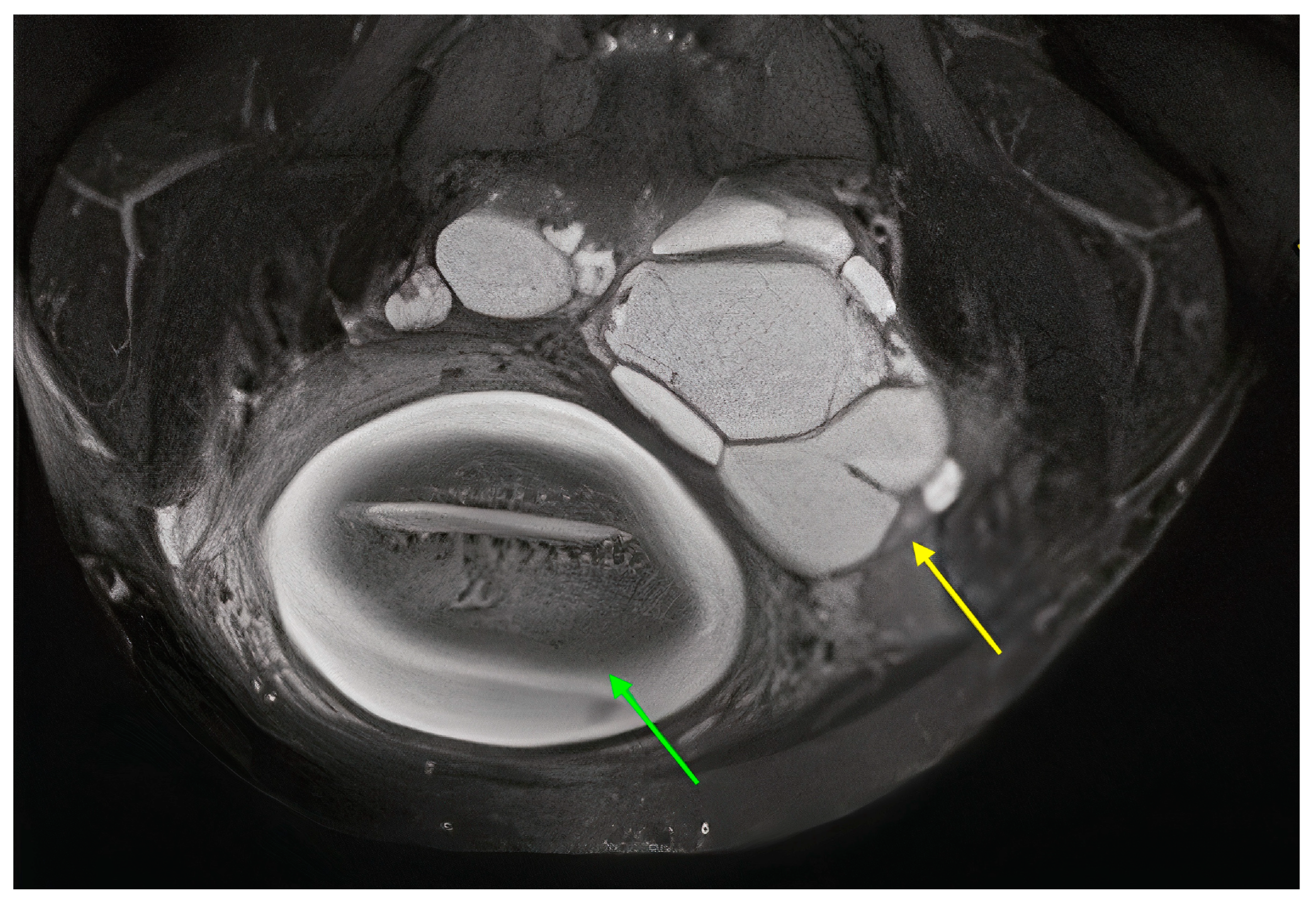
3.1. Our Case
3.2. Review of the Literature
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Calsteren, K.; Heyns, L.; De Smet, F.; Van Eycken, L.; Gziri, M.M.; Van Gemert, W.; Halaska, M.; Vergote, I.; Ottevanger, N.; Amant, F. Cancer during Pregnancy: An Analysis of 215 Patients Emphasizing the Obstetrical and the Neonatal Outcomes. J. Clin. Oncol. 2010, 28, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Verheecke, M.; Wlodarska, I.; Dehaspe, L.; Brady, P.; Brison, N.; Van Den Bogaert, K.; Dierickx, D.; Vandecaveye, V.; Tousseyn, T.; et al. Presymptomatic Identification of Cancers in Pregnant Women During Noninvasive Prenatal Testing. JAMA Oncol. 2015, 1, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, A.M.; Nezhat, F.R.; Emerson, J.; Pejovic, T.; Nezhat, C.H.; Nezhat, C.R. Adnexal Masses during Pregnancy: Diagnosis, Treatment, and Prognosis. Am. J. Obstet. Gynecol. 2023, 228, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, K.; Cymbaluk-Płoska, A. Approaches to the Diagnosis and Management of Ovarian Cancer in Pregnancy. Cancer Manag. Res. 2021, 13, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Korenaga, T.-R.K.; Tewari, K.S. Gynecologic Cancer in Pregnancy. Gynecol. Oncol. 2020, 157, 799–809. [Google Scholar] [CrossRef]
- Leiserowitz, G.S.; Xing, G.; Cress, R.; Brahmbhatt, B.; Dalrymple, J.L.; Smith, L.H. Adnexal Masses in Pregnancy: How Often Are They Malignant? Gynecol. Oncol. 2006, 101, 315–321. [Google Scholar] [CrossRef]
- Fruscio, R.; de Haan, J.; Van Calsteren, K.; Verheecke, M.; Mhallem, M.; Amant, F. Ovarian Cancer in Pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Vermeulen, M.J.; Bharatha, A.; Montanera, W.J.; Park, A.L. Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. JAMA 2016, 316, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Committee Opinion No. 723: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet. Gynecol. 2017, 130, e210–e216. [CrossRef]
- Parpinel, G.; Laudani, M.E.; Giunta, F.P.; Germano, C.; Zola, P.; Masturzo, B. Use of Positron Emission Tomography for Pregnancy-Associated Cancer Assessment: A Review. J. Clin. Med. 2022, 11, 3820. [Google Scholar] [CrossRef]
- Despierres, M.; Boudy, A.-S.; Selleret, L.; Gligorov, J.; Richard, S.; Thomassin, I.; Dabi, Y.; Zilberman, S.; Touboul, C.; Montravers, F.; et al. Feasibility, Safety and Impact of (18F)-FDG PET/CT in Patients with Pregnancy-Associated Cancer: Experience of the French CALG (Cancer Associé à La Grossesse) Network. Acta Oncol. 2022, 61, 302–308. [Google Scholar] [CrossRef]
- Han, S.N.; Lotgerink, A.; Gziri, M.M.; Van Calsteren, K.; Hanssens, M.; Amant, F. Physiologic Variations of Serum Tumor Markers in Gynecological Malignancies during Pregnancy: A Systematic Review. BMC Med. 2012, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Sarandakou, A.; Protonotariou, E.; Rizos, D. Tumor Markers in Biological Fluids Associated with Pregnancy. Crit. Rev. Clin. Lab. Sci. 2007, 44, 151–178. [Google Scholar] [CrossRef] [PubMed]
- Ercan, Ş.; Kaymaz, Ö.; Yücel, N.; Orçun, A. Serum Concentrations of CA 125, CA 15-3, CA 19-9 and CEA in Normal Pregnancy: A Longitudinal Study. Arch. Gynecol. Obstet. 2012, 285, 579–584. [Google Scholar] [CrossRef]
- Mazze, R.I.; Kallén, B. Reproductive Outcome after Anesthesia and Operation during Pregnancy: A Registry Study of 5405 Cases. Am. J. Obstet. Gynecol. 1989, 161, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Berveiller, P.; Boere, I.A.; Cardonick, E.; Fruscio, R.; Fumagalli, M.; Halaska, M.J.; Hasenburg, A.; Johansson, A.L.V.; Lambertini, M.; et al. Gynecologic Cancers in Pregnancy: Guidelines Based on a Third International Consensus Meeting. Ann. Oncol. 2019, 30, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Weisz, B.; Meirow, D.; Schiff, E.; Lishner, M. Impact and Treatment of Cancer during Pregnancy. Expert Rev. Anticancer. Ther. 2004, 4, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Ngu, S.-F.; Ngan, H.Y.S. Chemotherapy in Pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 33, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Uzan, C.; Gouy, S.; Verschraegen, C.; Haie-Meder, C. Gynaecological Cancers in Pregnancy. Lancet 2012, 379, 558–569. [Google Scholar] [CrossRef]
- Guidi, S.; Berghella, V.; Scambia, G.; Fagotti, A.; Vidiri, A.; Restaino, S.; Vizzielli, G.; Inzani, F.; Cavaliere, A.F. Adult Granulosa Cell Tumor in Pregnancy: A New Case and a Review of the Literature. Healthcare 2021, 9, 1455. [Google Scholar] [CrossRef]
- Grigoriadis, C.; Eleftheriades, M.; Panoskaltsis, T.; Bacanu, A.M.; Vitoratos, N.; Kondi-Pafiti, A.; Tsangkas, A.; Tympa, A.; Hassiakos, D. Ovarian Cancer Diagnosed during Pregnancy: Clinicopathological Characteristics and Management. G Chir 2014, 35, 69–72. [Google Scholar] [PubMed]
- Behtash, N.; Karimi Zarchi, M.; Modares Gilani, M.; Ghaemmaghami, F.; Mousavi, A.; Ghotbizadeh, F. Ovarian Carcinoma Associated with Pregnancy: A Clinicopathologic Analysis of 23 Cases and Review of the Literature. BMC Pregnancy Childbirth 2008, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Blake, E.A.; De Zoysa, M.Y.; Morocco, E.B.; Kaiser, S.B.; Kodama, M.; Grubbs, B.H.; Matsuo, K. Teenage Pregnancy Complicated by Primary Invasive Ovarian Cancer: Association for Oncologic Outcome. J. Gynecol. Oncol. 2018, 29, e79. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Moschetta, M.; Tatsi, K.; Tsiouris, A.K.; Pavlidis, N. A Review on Pregnancy Complicated by Ovarian Epithelial and Non-Epithelial Malignant Tumors: Diagnostic and Therapeutic Perspectives. J. Adv. Res. 2018, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Ito, T.; Hamaguchi, F.; Kasuga, M.; Mikami, T.; Hino, M.; Yokoyama, R.; Yamamura, S.; Sakata, H.; Minamiguchi, S.; et al. Primary and Recurrent Serous Borderline Tumors during Pregnancy: A Case Report and Literature Review. Int. Cancer Conf. J. 2021, 10, 160–169. [Google Scholar] [CrossRef]
- Pei, Y.; Gou, Y.; Li, N.; Yang, X.; Han, X.; Huiling, L. Efficacy and Safety of Platinum-Based Chemotherapy for Ovarian Cancer During Pregnancy: A Systematic Review and Meta-Analysis. Oncol. Ther. 2022, 10, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO–ESGO Consensus Conference Recommendations on Ovarian Cancer: Pathology and Molecular Biology, Early and Advanced Stages, Borderline Tumours and Recurrent Disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- King, L.A.; Nevin, P.C.; Williams, P.P.; Carson, L.F. Treatment of Advanced Epithelial Ovarian Carcinoma in Pregnancy with Cisplatin-Based Chemotherapy. Gynecol. Oncol. 1991, 41, 78–80. [Google Scholar] [CrossRef]
- Otton, G.; Higgins, S.; Phillips, K.A.; Quinn, M. A Case of Early-Stage Epithelial Ovarian Cancer in Pregnancy. Int. J. Gynecol. Cancer 2001, 11, 413–417. [Google Scholar] [CrossRef]
- Sood, A.K.; Shahin, M.S.; Sorosky, J.I. Paclitaxel and Platinum Chemotherapy for Ovarian Carcinoma during Pregnancy. Gynecol. Oncol. 2001, 83, 599–600. [Google Scholar] [CrossRef]
- Ferrandina, G.; Distefano, M.; Testa, A.; De Vincenzo, R.; Scambia, G. Management of an Advanced Ovarian Cancer at 15 Weeks of Gestation: Case Report and Literature Review. Gynecol. Oncol. 2005, 97, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, G.; Gramignano, G.; Mais, V.; Melis, G.B.; Parodo, G.; Carrucciu, G.M. Use of Chemotherapy for Ovarian Cancer during Human Pregnancy: Case Report and Literature Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 131, 238–239. [Google Scholar] [CrossRef]
- Modares Gilani, M.; Karimi Zarchi, M.; Behtash, N.; Ghaemmaghami, F.; Mousavi, A.S.; Behnamfar, F. Preservation of Pregnancy in a Patient with Advanced Ovarian Cancer at 20 Weeks of Gestation: Case Report and Literature Review. Int. J. Gynecol. Cancer 2007, 17, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Rouzi, A.A.; Sahly, N.N.; Sahly, N.F.; Alahwal, M.S. Cisplatinum and Docetaxel for Ovarian Cancer in Pregnancy. Arch. Gynecol. Obstet. 2009, 280, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Borowsky, M.E.; Jain, V.D. Intraperitoneal Chemotherapy in a Pregnant Woman with Ovarian Cancer. Obstet. Gynecol. 2013, 122, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, L.; Jia, Y.; Jia, Z.; Li, Z.; Cui, S.; Cui, M. Long-Term Multidisciplinary Integrative Therapy Management Resulted in Favorable Outcomes for Ovarian Cancer during Pregnancy: A Case Report and Literature Review. J. Ovarian Res. 2019, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Bacalbaşa, N.; Bălescu, I.; Vîlcu, M.; Dima, S.; Iliescu, L.; Brezean, I. Cytoreductive Surgery for Advanced Stage Ovarian Cancer in the Second Trimester of Pregnancy—A Case Report and Literature Review. Medicine 2020, 99, e21127. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, E.; Pina, A.; Avon-Després, C.; Mercier, F.; Cormier, B. Hyperthermic Intraperitoneal Chemotherapy and Interval Debulking Surgery in Conjunction With Elective Cesarean Delivery. Obstet. Gynecol. 2023, 141, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Maringe, C.; Walters, S.; Butler, J.; Coleman, M.P.; Hacker, N.; Hanna, L.; Mosgaard, B.J.; Nordin, A.; Rosen, B.; Engholm, G.; et al. Stage at Diagnosis and Ovarian Cancer Survival: Evidence from the International Cancer Benchmarking Partnership. Gynecol. Oncol. 2012, 127, 75–82. [Google Scholar] [CrossRef]
- Matz, M.; Coleman, M.P.; Carreira, H.; Salmerón, D.; Chirlaque, M.D.; Allemani, C.; Bouzbid, S.; Hamdi-Chérif, M.; Zaidi, Z.; Bah, E.; et al. Worldwide Comparison of Ovarian Cancer Survival: Histological Group and Stage at Diagnosis (CONCORD-2). Gynecol. Oncol. 2017, 144, 396–404. [Google Scholar] [CrossRef]
- Fortner, R.T.; Trewin-Nybråten, C.B.; Paulsen, T.; Langseth, H. Characterization of Ovarian Cancer Survival by Histotype and Stage: A Nationwide Study in Norway. Int. J. Cancer 2023, 153, 969–978. [Google Scholar] [CrossRef]
- Aslam, N.; Ong, C.; Woelfer, B.; Nicolaides, K.; Jurkovic, D. Serum CA125 at 11–14 Weeks of Gestation in Women with Morphologically Normal Ovaries. BJOG Int. J. Obstet. Gynaecol. 2000, 107, 689–690. [Google Scholar] [CrossRef]
- Amampai, R.; Suprasert, P. Cancer Antigen 125 during Pregnancy in Women without Ovarian Tumor Is Not Often Rising. Obstet. Gynecol. Int. 2018, 2018, e8141583. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.R.T.; Sarani, B.; Bhanot, P.; Feldman, E. Surgery in Pregnancy. Curr. Probl. Surg. 2012, 49, 333–388. [Google Scholar] [CrossRef]
- Rasmussen, A.S.; Christiansen, C.F.; Uldbjerg, N.; Nørgaard, M. Obstetric and Non-Obstetric Surgery during Pregnancy: A 20-Year Danish Population-Based Prevalence Study. BMJ Open 2019, 9, e028136. [Google Scholar] [CrossRef] [PubMed]
- Cagino, K.; Li, X.; Thomas, C.; Delgado, D.; Christos, P.; Acholonu, U. Surgical Management of Adnexal Masses in Pregnancy: A Systematic Review and Meta-Analysis. J. Minim. Invasive Gynecol. 2021, 28, 1171–1182. [Google Scholar] [CrossRef]
- Haggerty, E.; Daly, J. Anaesthesia and Non-Obstetric Surgery in Pregnancy. BJA Educ. 2021, 21, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.E.; Sakhel, K.; Chauhan, S.P.; Abuhamad, A.Z. Adnexal Mass during Pregnancy: A Review. Am. J. Perinatol. 2015, 32, 1010–1016. [Google Scholar] [CrossRef]
- Shigemi, D.; Aso, S.; Matsui, H.; Fushimi, K.; Yasunaga, H. Safety of Laparoscopic Surgery for Benign Diseases during Pregnancy: A Nationwide Retrospective Cohort Study. J. Minim. Invasive Gynecol. 2019, 26, 501–506. [Google Scholar] [CrossRef]
- Bunyavejchevin, S.; Phupong, V. Laparoscopic Surgery for Presumed Benign Ovarian Tumor during Pregnancy. Cochrane Database Syst. Rev. 2013, 2013, CD005459. [Google Scholar] [CrossRef]
- Bouchelion, A.; Bauer, A.M.; Phillis, M.; Hackney, D.; Armstrong, A. Outcomes of Oophorectomy in Pregnancy: A Retrospective Cohort [28N]. Obstet. Gynecol. 2020, 135, 153S. [Google Scholar] [CrossRef]
- Mancari, R.; Tomasi-Cont, N.; Sarno, M.A.; Azim, H.A., Jr.; Franchi, D.; Carinelli, S.; Biglia, N.; Colombo, N.; Peccatori, F.A. Treatment Options for Pregnant Women with Ovarian Tumors. Int. J. Gynecol. Cancer 2014, 24, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Van Calsteren, K.; Verbesselt, R.; Ottevanger, N.; Halaska, M.; Heyns, L.; Van Bree, R.; de Bruijn, E.; Chai, D.; Delforge, M.; Noens, L.; et al. Pharmacokinetics of Chemotherapeutic Agents in Pregnancy: A Preclinical and Clinical Study. Acta Obstet. Et Gynecol. Scand. 2010, 89, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.W.; Wing, H.T.; Pak, C.N.; Mok, T.S.K.; Tam, B.; Tze, K.L. Microphthalmos Associated with Dartmouth Combination Chemotherapy in Pregnancy: A Case Report. J. Reprod. Med. Obstet. Gynecol. 2007, 52, 575–576. [Google Scholar]
- Marnitz, S.; Köhler, C.; Oppelt, P.; Schmittel, A.; Favero, G.; Hasenbein, K.; Schneider, A.; Markman, M. Cisplatin Application in Pregnancy: First in Vivo Analysis of 7 Patients. Oncology 2010, 79, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Cardonick, E.; Bhat, A.; Gilmandyar, D.; Somer, R. Maternal and Fetal Outcomes of Taxane Chemotherapy in Breast and Ovarian Cancer during Pregnancy: Case Series and Review of the Literature. Ann. Oncol. 2012, 23, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Løhaugen, G.C.C.; Gramstad, A.; Evensen, K.A.I.; Martinussen, M.; Lindqvist, S.; Indredavik, M.; Vik, T.; Brubakk, A.-M.; Skranes, J. Cognitive Profile in Young Adults Born Preterm at Very Low Birthweight. Dev. Med. Child Neurol. 2010, 52, 1133–1138. [Google Scholar] [CrossRef]
- Amant, F.; Vandenbroucke, T.; Verheecke, M.; Fumagalli, M.; Halaska, M.J.; Boere, I.; Han, S.; Gziri, M.M.; Peccatori, F.; Rob, L.; et al. Pediatric Outcome after Maternal Cancer Diagnosed during Pregnancy. N. Engl. J. Med. 2015, 373, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Zanetti-Dällenbach, R.; Tschudin, S.; Lapaire, O.; Holzgreve, W.; Wight, E.; Bitzer, J. Psychological Management of Pregnancy-Related Breast Cancer. Breast 2006, 15, S53–S59. [Google Scholar] [CrossRef]
- Vandenbroucke, T.; Han, S.N.; Van Calsteren, K.; Wilderjans, T.F.; Van den Bergh, B.R.H.; Claes, L.; Amant, F. Psychological Distress and Cognitive Coping in Pregnant Women Diagnosed with Cancer and Their Partners. Psycho-Oncology 2017, 26, 1215–1221. [Google Scholar] [CrossRef]
- Vannier, S.A.; Rosen, N.O. Sexual Distress and Sexual Problems During Pregnancy: Associations With Sexual and Relationship Satisfaction. J. Sex. Med. 2017, 14, 387–395. [Google Scholar] [CrossRef] [PubMed]
| First Author | Year | Patient Age | Tumor Localization | Histology | FIGO Stage |
|---|---|---|---|---|---|
| King [28] | 1990 | 24 | Right ovary | HGSC | IIIC |
| Otton [29] | 2001 | 31 | Right ovary | HGSC | IC |
| Sood [30] | 2001 | 33 | Right ovary | HGSC | IIIC |
| Ferrandina [31] | 2005 | 40 | Bilateral ovaries | HGSC | IIIC |
| Mantovani [32] | 2007 | 34 | Right ovary | HGSC | IIIC |
| Modares Gilani [33] | 2007 | 42 | Right ovary | HGSC | IIIC |
| Rouzi [34] | 2009 | 32 | Left ovary | HGSC | IIIC |
| Smith [35] | 2013 | 36 | Left ovary | HGSC | IIB |
| Vivod | 2014 | 36 | Bilateral ovaries | HGSC | IIIC |
| Xu [36] | 2019 | 34 | Bilateral ovaries | HGSC | IIIC |
| Bacalbasa [37] | 2020 | 27 | Right ovary | HGSC | IIIC |
| Tremblay [38] | 2023 | 40 | Bilateral ovaries | HGSC | IIIC |
| First Author | Symptoms/Signs | Gestational Age at Diagnosis (Weeks) | Radiologic Work-up | Tumor Maximal Diameter (mm) | Ca-125 at Diagnosis (U/mL) |
|---|---|---|---|---|---|
| King [28] | Asymptomatic | 9 | US | 100 | 62 |
| Otton [29] | Abdominal pain | 6 | US | 80 | 562 |
| Sood [30] | Abdominal pain | 27 | US | 100 | 568 |
| Ferrandina [31] | Asymptomatic | 14 | US | 180 | 1240 |
| Mantovani [32] | Asymptomatic | 9 | US | 51 | 751 |
| Modares Gilani [33] | Asymptomatic | 18 | US | 80 | 1000 |
| Rouzi [34] | Abdominal pain | 18 | US + MR | 150 | 580 |
| Smith [35] | Asymptomatic | 9 | US | 110 | 183 |
| Vivod | Abdominal pain | 28 | US + MR | 140 | 801 |
| Xu [36] | Abdominal pain | 18 | US | 150 | 125 |
| Bacalbasa [37] | Pelvic pain | 9 | US + MR | 40 | NS |
| Tremblay [38] | Asymptomatic | 27 | US + MR | 100 | 769 |
| First Author | Week at Surgery | Surgery in Pregnancy | Surgery at Delivery | Chemotherapy during Pregnancy |
|---|---|---|---|---|
| King [28] | 16 | Exploratory laparotomy, RSO, left ovarian biopsy, partial omentectomy, small bowel nodule biopsy | No | Six cycles of cisplatin and cyclophosphamid |
| Otton [29] | 16 | Exploratory laparotomy, ovarian cystectomy | Hysterectomy, BSO, omentectomy, right pelvic and para-aortic LND, no clinical evidence of macroscopic disease | Four cycles of cisplatin |
| Sood [30] | 28 | Exploratory laparotomy, RSO, infracolic omentectomy, optimal cytoreduction | Exploratory laparotomy, hysterectomy, LSO, suboptimal cytoreduction | Three cycles of cisplatin and paclitaxel |
| Ferrandina [31] | 15 | Exploratory laparotomy, BSO, infracolic omentectomy, appendectomy, multiple biopsies | Exploratory laparotomy, hysterectomy, multiple biopsies | Six cycles of cisplatin |
| Mantovani [32] | 17 | Exploratory laparotomy, RSO, left ovarian biopsy, omental biopsy, removal of two pelvic metastasis | Exploratory laparotomy, hysterectomy, LSO, removal of the superior third of the vagina, omentectomy, pelvic LND, appendectomy, multiple biopsies | Five cycles of paclitaxel |
| Modares Gilani [33] | 20 | Exploratory laparotomy RSO, partial omentectomy, lymph node sampling, multiple biopsies | Exploratory laparotomy, hysterectomy, LSO, omentectomy | Four cycles of carboplatin and paclitaxel |
| Rouzi [34] | 20 | Exploratory laparotomy LSO, omental biopsy | Exploratory laparotomy, hysterectomy, RSO, omentectomy, multiple biopsies | Four cycles of cisplatin and docetaxel |
| Smith [35] | 12 | Exploratory laparotomy, LSO, para-aortic and left pelvic LND, omentectomy, multiple biopsies, appendectomy | No | Four cycles of intraperitoneal carboplatin and IV paclitaxel |
| Vivod | 28 | Laparoscopic exploration, multiple peritoneal biopsies | Hysterectomy, BSO, omentectomy, resection of the rectosigmoid colon with anastomosis, pelvic peritonectomy + intraperitoneal chemotherapy (cisplatin) | No |
| Xu [36] | 21 | Exploratory laparotomy, BSO, infra-colic omentectomy | Hysterectomy, pelvic and para-aortic LND, omentectomy, appendectomy, partial sigmoidectomy, all apparent independent cancer nodules were resected | Four cycles of cisplatin and docetaxel |
| Bacalbasa [37] | 10 | Laparoscopic exploration, cystectomy, no pathological aspect was revealed | At 14 weeks termination of pregnancy, hysterectomy, BSO, omentectomy, pelvic and para-aortic LND, multiple biopsies | No |
| Tremblay [38] | 27 | No surgery US-guided tumor biopsy | Hysterectomy, BSO, omentectomy, all apparent independent cancer nodules were resected + HIPEC (cisplatin) | Three cycles of cisplatin and paclitaxel |
| First Author | Gestational Age at Delivery (Weeks) | Mode of Delivery | Pregnancy Outcome | Birth Baby Weight (Grams) | Baby Outcome |
|---|---|---|---|---|---|
| King [28] | 37 | Vaginal | Live birth | 3060 | Normal growth and development at 24 months of age |
| Otton [29] | 31 | Cesarean section | Live birth | 1740 | Normal growth and development at 12 months of age |
| Sood [30] | 37 | Cesarean section | Live birth | 2800 | Normal growth and development at 30 months of age |
| Ferrandina [31] | 36 | Cesarean section | Live birth | 3000 | Normal growth and development at 42 months of age |
| Mantovani [32] | 38 | Cesarean section | Live birth | 2490 | Normal growth and development at 16 months of age |
| Modares Gilani [33] | 35 | Cesarean section | Live birth | 2600 | Normal growth and development at 6 months of age |
| Rouzi [34] | 34 | Cesarean section | Live birth | 2245 | The baby died 5 days after delivery because of multiple congenital anomalies which were diagnosed before starting chemotherapy |
| Smith [35] | 37 | Cesarean section | Live birth | 2126 | Normal growth and development at 7 months of age |
| Vivod | 31 | Cesarean section | Live birth | 1360 | Normal growth and development at 9 years of age |
| Xu [36] | 35 | Cesarean section | Live birth | 2100 | Normal growth and development at 33 months of age |
| Bacalbasa [37] | Abortion | / | / | / | / |
| Tremblay [38] | 36 | Cesarean section | Live birth | 2965 | Normal growth and development 22 months of age. |
| First Author | Surgery after Pregnancy | Chemotherapy after Pregnancy | Maternal Oncologic Outcome |
|---|---|---|---|
| King [28] | Six weeks postpartum laparotomy, hysterectomy, LSO, omentectomy | Eight courses of cisplatin and etoposide, Intraperitoneal chromic phosphate | Twenty-four months after delivery WSOD |
| Otton [29] | No | Two cycles of carboplatin and paclitaxel | Twelve months after delivery WSOD |
| Sood [30] | No | Three cycles of cisplatin and paclitaxel | The patient died of recurrent ovarian cancer 29 months after diagnosis |
| Ferrandina [31] | Twenty-four months after delivery recurrence of ovarian cancer and removal of nodules in pelvis | Six cycles of carboplatin and paclitaxel 24 months after delivery | Forty-two months after delivery WSOD |
| Mantovani [32] | No | Six cycles of carboplatin and paclitaxel | Sixteen months after delivery WSOD |
| Modares Gilani [33] | No | Three cycles of carboplatin | Six months after delivery WSOD |
| Rouzi [34] | No | Two cycles of cisplatin and docetaxel | Eight months after delivery WSOD |
| Smith [35] | Twelve weeks after delivery, robotic-assisted hysterectomy, RSO, peritoneal biopsies, para-aortic LND | Two cycles of intraperitoneal carboplatin and IV paclitaxel | Seven months after delivery WSOD |
| Vivod | No | Six cycles of carboplatin and paclitaxel | Nine years after delivery WSOD |
| Xu [36] | No | Four cycles of cisplatin and docetaxel for recurrence 24 months after delivery—one cycle of cisplatin and docaxel, six cycles of pegylated liposomal doxorubicin and bevacizumab | Recurrence 24 months after delivery positive pelvic lymph nodes, right supraclavicular lymph node) 33 months after delivery WSOD |
| Bacalbasa [37] | No | NS | One week after surgery WSOD |
| Tremblay [38] | No | Three cycles of cisplatin and paclitaxel | Twenty-two months after delivery WSOD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivod, G.; Merlo, S.; Kovacevic, N. High-Grade Serous Ovarian Cancer during Pregnancy: From Diagnosis to Treatment. Curr. Oncol. 2024, 31, 1920-1935. https://doi.org/10.3390/curroncol31040144
Vivod G, Merlo S, Kovacevic N. High-Grade Serous Ovarian Cancer during Pregnancy: From Diagnosis to Treatment. Current Oncology. 2024; 31(4):1920-1935. https://doi.org/10.3390/curroncol31040144
Chicago/Turabian StyleVivod, Gregor, Sebastjan Merlo, and Nina Kovacevic. 2024. "High-Grade Serous Ovarian Cancer during Pregnancy: From Diagnosis to Treatment" Current Oncology 31, no. 4: 1920-1935. https://doi.org/10.3390/curroncol31040144
APA StyleVivod, G., Merlo, S., & Kovacevic, N. (2024). High-Grade Serous Ovarian Cancer during Pregnancy: From Diagnosis to Treatment. Current Oncology, 31(4), 1920-1935. https://doi.org/10.3390/curroncol31040144





