Abstract
Immuno-oncology (IO) combination therapy is the first-line treatment for advanced renal cell carcinoma (RCC). However, biomarkers for predicting the response to IO combination therapy are lacking. Here, we investigated the association between the expression of soluble immune checkpoint molecules and the therapeutic efficacy of IO combination therapy in advanced RCC. The expression of soluble programmed cell death-1 (sPD-1), soluble programmed cell death ligand-1 (sPD-L1), soluble PD-L2 (sPD-L2), and lymphocyte activation gene-3 (sLAG-3) was assessed in plasma samples from 42 patients with advanced RCC who received first-line IO combination therapy. All IMDC risk classifications were represented among the patients, including 14.3, 57.1, and 28.6% with favorable, intermediate, and poor risk, respectively. Univariate analysis revealed that prior nephrectomy, sPD-L2 levels, and sLAG-3 levels were significant factors affecting progression-free survival (PFS), whereas multivariate analyses suggested that sPD-L2 and sLAG-3 levels were independent prognostic factors for PFS. In a univariate analysis of the overall survival, prior nephrectomy and sPD-L2 levels were significant factors; no significant differences were observed in the multivariate analysis. No significant correlation was observed between the sPD-L2 and sLAG-3 levels and PD-L2 and LAG-3 expression via immunohistochemistry. In conclusion, sPD-L2 and sLAG-3 expression may serve as a potential biomarker for predicting IO combination therapy efficacy.
1. Introduction
Immune checkpoint inhibitors (ICIs) targeting programmed cell death-1 (PD-1), programmed cell death ligand-1 (PD-L1), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) are a major class of immuno-oncology therapeutics that have significantly improved the prognosis of advanced renal cell carcinoma (RCC) [1]. Following clinical trials, immuno-oncology (IO) combination therapies such as IO + IO therapy or IO therapy + tyrosine kinase inhibitors (TKIs) have become the standard first-line treatment for advanced RCC in Japan [2,3,4,5].
In recent years, a variety of biomarkers have been investigated to reflect the effects of ICIs, including PD-L1 expression in tumor cells, the tumor mutation burden, the neoantigen burden, polybromo1 gene mutation, immune cell infiltration, and the gut microbiota. However, biomarkers for the efficacy of IO combination therapy remain elusive. Therefore, the search for biomarkers that predict the effect of IO combination therapy is required for patients with advanced RCC.
Several studies have reported that PD-L1 expression in RCC tumor cells is associated with prognosis and the therapeutic response to TKIs [6,7]. In contrast, high PD-L1 expression has been shown to be associated with better therapeutic effects than low PD-L1 expression in patients with RCC treated with immune checkpoint inhibitors [1]. Furthermore, several studies have indicated that PD-L1 expression, programmed cell death-ligand 2 (PD-L2), and lymphocyte activation gene-3 (LAG-3) are associated with the therapeutic efficacy of ICI treatment for advanced RCC [7,8,9,10]. However, these reports are controversial, indicating the limited utility of PD-L1 as a predictive biomarker in RCC and that treatment decisions in RCC should not depend on PD-L1 expression levels [11].
In recent years, soluble immune checkpoint molecules released into the blood after the cleavage of the extracellular domain of tumor cells have attracted attention [12,13]. Transmembrane PD-L1 has a soluble form that is produced by tumor cells or activated mature dendritic cells and is associated with diversity in the composition and function of the PD-1/PD-L1 signaling pathway [14]. Soluble PD-L1 (sPD-L1) appears to decrease interferon-gamma secretion by T cells and may be involved in systemic antitumor immunomodulation by targeting T lymphocytes in secondary lymphoid organs [15]. The relationship between the expression of sPD-L1 and the prognosis of advanced RCC is unclear. In addition, there are few reports on the clinical role of soluble immune checkpoint molecules such as sPD-L2 and sLAG-3 in advanced RCC.
Here, we investigated the potential roles of new plasma biomarkers, including soluble PD-1 (sPD-1), sPD-L1, soluble PD-L2 (sPD-L2), and soluble LAG-3 (sLAG-3), as putative predictive biomarkers for determining the efficacy of IO combination therapy in advanced RCC. In addition, we also examined the association between the soluble immune checkpoint molecule levels and the expression of immune checkpoint molecules in tumors.
2. Materials and Methods
2.1. Patients
This study included 42 patients who had received IO combination therapies such as nivolumab plus ipilimumab (NIVO + IPI), axitinib plus avelumab (AXI + AVEL), and lenvatinib plus pembrolizumab (LEN + PEMB) as first-line treatment at the Kurume University Hospital from July 2020 to August 2022. Pretreatment assessments of the patients’ clinical characteristics and blood data were performed immediately before the initiation of IO combination therapy. This study was conducted in full compliance with the Declaration of Helsinki of the World Medical Association and was approved by the Ethics Review Committee of the Kurume University School of Medicine (approval number: 20118). Written informed consent was obtained from all patients before their participation in this study. The clinical information of these patients was obtained from their medical records and retrospectively reviewed and analyzed.
2.2. ELISA
Following enrollment, peripheral blood samples were collected from the patients in tubes containing heparin as an anticoagulant before the initiation of first-line treatment. The sPD-1, sPD-L1, sPD-L2, and sLAG-3 concentrations were calculated using an enzyme-linked immunosorbent assay (ELISA). ELISA tests were performed using commercial kits (ab252360 H9uman PD-1 ELISA Kit, Abcam; ab214565 Human PD-L1 (clone 28-8) ELISA Kit, Abcam, Cambridge, UK; ab231928 Human PD-L2 ELISA Kit, Abcam, Cambridge, UK; and ab193707 Human LAG-3 ELISA Kit, Abcam, Cambridge, UK) according to the manufacturer’s instructions. To generate standard curves, the recombinant protein corresponding to each test was used at a prespecified concentration. The results were obtained using a spectrophotometer (absorbance at 450 nm). Additionally, the concentrations were calculated based on standard curves. All samples, standards, and negative controls were analyzed in duplicate.
2.3. Immunohistochemical Analysis
Paraffin-embedded tissue samples were cut to a thickness of 4 µm, placed on a glass slide for examination, and labeled with either anti-PD-L2 antibodies (1:1000; clone 176611, R&D Systems, Minneapolis, MN, USA) or anti-LAG-3 antibodies (1:100; clone 17B4, Novus Biologicals, CO, USA) using a BenchMark ULTRA (Ventana Automated Systems, Inc., Tucson, AZ, USA). As previously described, PD-L2 expression in tumors was considered positive if ≧5% of tumor cells were present [7]. Similarly, LAG-3 expression in tumors was positive if ≧1% of tumor cells were present [16].
2.4. Statistical Analysis
Progression-free survival (PFS) for first-line IO combination therapy and overall survival (OS) from the initiation of IO combination therapy to the date of mortality was determined using the Kaplan–Meier method, and analyzed using the log-rank test. A comparison of PFS and OS between and among cohorts was achieved via a log-rank test. Univariate and multivariate analyses using the Cox proportional hazards model were performed to identify the risk factors for PFS and OS based on the calculation of hazard ratios (HR) with 95% confidence intervals (CI). The median values of sPD-1, sPD-L1, sPD-L2, and sLAG-3 were used as the cutoff values. All statistical analyses were performed using JMP version 17 (SAS Institute, Inc., Cary, NC, USA) and a value of p < 0.05 was considered.
3. Results
3.1. Patient Characteristics
The patient characteristics are summarized in Table 1. The median age of the participants was 69.5 years (range, 42–80 years), and the majority of patients were male (81.0%). All international metastatic renal cell carcinoma database consortium (IMDC) risk groups were represented among the patients, with 14.3, 57.1, and 28.6% of the patients presenting a favorable, intermediate, and poor risk, respectively. The percentage of patients who underwent nephrectomy before IO combination therapy was 45.2%. The majority of the patients were diagnosed with advanced RCC with clear cell histology (81.0%). Most patients received NIVO + IPI (61.9%) or AXI + AVEL (31.0%) as first-line IO combination therapy. The remaining patients were treated with LEN + PEMB.

Table 1.
Clinical and pathological features of patients with advanced renal cell carcinoma treated with immuno-oncology combination therapy.
The levels of soluble immune checkpoint molecules before the initiation of IO combination therapy are shown in Table 1. The median sPD-1, sPD-L1, sPD-L2, and sLAG-3 concentrations were 291.3 pg/mL (149.4–1649.9), 39.8 pg/mL (8.0–395.8), 9291.6 (1726.1–27,900.8) pg/mL, and 19.4 ng/mL (15.2–41.1), respectively. The median pretreatment C-reactive protein and neutrophil to lymphocyte ratio were 0.71 mg/dL and 3.28, respectively.
3.2. Baseline Soluble Immune Checkpoint Molecules as Predictive Biomarkers of IO Combination Therapy Outcome in Advanced RCC
Using the cutoff values determined from the median values, we classified patients with low and high levels of each immune checkpoint molecule. Figure 1 and Figure 2 show the estimated PFS and OS curves of patients with advanced RCC treated with IO combination therapy according to their sPD-1, sPD-L1, sPD-L2, and sLAG-3 levels, respectively. A high sPD-L2 level was a predictor of significantly worse PFS (p = 0.0027) and OS (p = 0.0363) than a low sPD-L2 level. On the other hand, a low sLAG-3 level was a predictor of significantly worse PFS (p = 0.0030) than a high sLAG-3 level. However, no significant difference in OS was observed between the two groups. In contrast, no significant differences in PFS and OS were observed between patients with low and high sPD-1 and sPD-L1 levels.
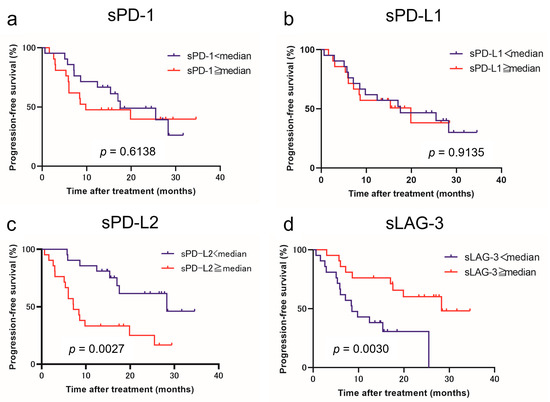
Figure 1.
Progression-free survival (PFS) in patients with advanced renal cell carcinoma treated with immuno-oncology combination therapy according to the pretreatment soluble immune checkpoint molecules. Survival curve PFS with respect to sPD-1 (a), sPD-L1 (b), sPD-L2 (c), and sLAG-3 (d) levels.
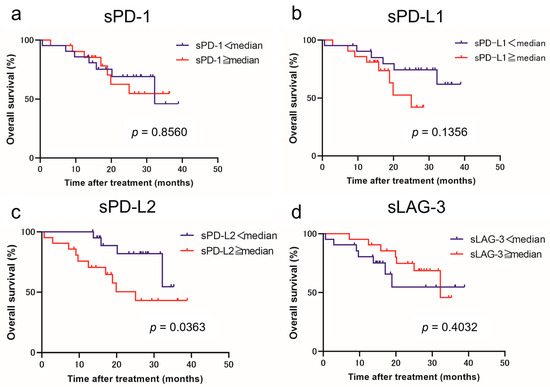
Figure 2.
Overall survival (OS) in patients with advanced renal cell carcinoma treated with immuno-oncology combination therapy according to the pretreatment soluble immune checkpoint molecule levels. Survival curve OS with respect to sPD-1 (a), sPD-L1 (b), sPD-L2 (c), and sLAG-3 (d) levels.
3.3. Soluble Immune Checkpoint Molecules Expression and Clinical Course
To identify the pretreatment prognostic factors before IO combination therapy associated with PFS and OS, we performed univariate and multivariate analyses using the Cox proportional hazards model (Table 2 and Table 3). The univariate analysis revealed that prior nephrectomy (HR = 2.659, 95% CI = 1.102–6.412, p = 0.0295), sPD-L2 levels (HR = 3.455, 95% CI = 1.463–8.158, p = 0.0047), and sLAG-3 levels (HR = 0.270, 95% CI = 0.108–0.675, p = 0.0051) were significant factors affecting PFS. Multivariate analyses suggested that sPD-L2 (HR = 2.918, 95% CI = 1.201–7.085, p = 0.0180) and sLAG-3 (HR = 0.387, 95% CI = 0.154–0.974, p = 0.0438) levels had independent prognostic effects on PFS. In the univariate analysis related to OS, prior nephrectomy (HR = 3.768, 95% CI = 1.049–13.533, p = 0.0420) and sPD-L2 levels (HR = 3.241, 95% CI = 1.012–10.375, p = 0.0477) were the significant factors affecting OS. However, the multivariate analysis showed a trend for prior nephrectomy and sPD-L2, but did not identify an independent factor for OS.

Table 2.
Univariate and multivariate analyses of progression-free survival in patients with advanced renal cell carcinoma, treated with immuno-oncology combination therapy.

Table 3.
Univariate and multivariate analyses of overall survival in patients with advanced renal cell carcinoma, treated with immuno-oncology combination therapy.
3.4. Association between Soluble Immune Checkpoint Molecule Levels and the Expression of Immune Checkpoint Molecules in Tumors
The PD-L2 and LAG-3 expression in tumors was evaluated to investigate their correlation with sPD-L2 and sLAG-3 levels before the initiation of IO combination therapy (Figure 3). No correlation was observed between the sPD-L2 levels and PD-L2 expression in the tumors (p = 0.6820). Similarly, no correlation was found between the sLAG-3 levels and LAG-3 expression in tumors (p = 0.8762). A survival comparison of the PD-L2 and LAG-3 expression in tumors showed no correlation between expression and PFS or OS (Figure 4 and Figure 5).
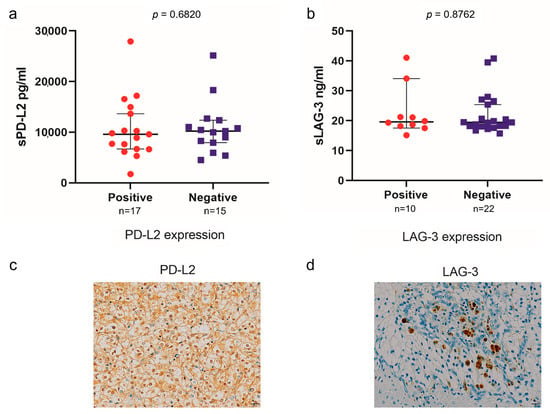
Figure 3.
Association between sPD-L2 and PD-L2 expression in tumors (a). Association between sLAG-3 and LAG-3 expression in tumors (b). The representative immunohistochemical picture of PD-L2 expression in tumors (c). The representative immunohistochemical picture of LAG-3 expression in tumors (d).
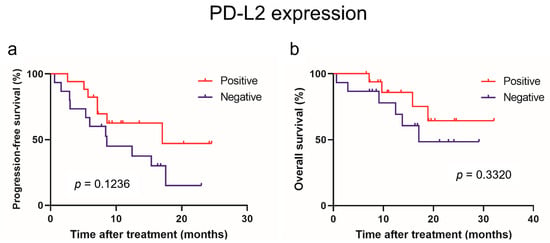
Figure 4.
Progression-free survival (a) and overall survival (b) in patients with advanced renal cell carcinoma treated with mmune-oncology combination therapy based on immunohistochemical expression of PD-L2.
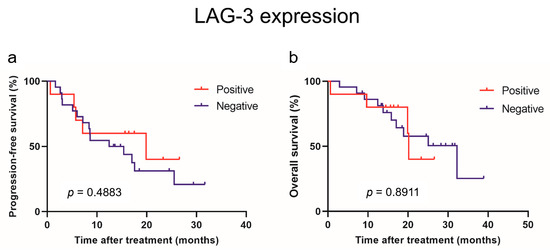
Figure 5.
Progression-free survival (a) and overall survival (b) in patients with advanced renal cell carcinoma treated with mmune-oncology combination therapy based on immunohistochemical expression of LAG-3.
4. Discussion
PD-1 and its ligands have been reported to exist in soluble forms that can be released by immune and tumor cells and measured in body fluids, such as plasma [17]. There have been several reports on the soluble immune checkpoint molecules in patients with cancer treated with ICIs. However, the clinical roles of these proteins remain controversial.
In recent years, sPD-L1 has also been used as a prognostic biomarker for various cancers [15,18]. It has been reported that sPD-L1 acts as a decoy and attenuates the ICI effects. Furthermore, previous reports have shown that sPD-L1 in the plasma of patients with non-small cell lung cancer (NSCLC) binds to PD-1 and that sPD-L1 may compete with ICIs by binding the PD-1 expressed on the surface of T cells, thereby promoting the exhaustion of activated T cells. Therefore, high sPD-L1 levels may be associated with ICI ineffectiveness. Several studies have shown that high sPD-L1 levels are closely associated with a poor prognosis [13].
Montemagno et al. reported that a high sPD-L1 level is an independent prognostic factor in patients with metastatic RCC treated with sunitinib [19]. Wakita et al. also showed that elevated levels of sPD-L1 may indicate a worse treatment response to nivolumab in metastatic RCC treated with ICIs [20]. However, they showed that patients with low levels of sPD-L1 had a poorer OS than those with high levels of sPD-L1, whereas there was no significant difference in the PFS upon nivolumab treatment. In this study, there was no significant difference in the PFS and OS after IO combination therapy based on sPD-L1 expression. Previous reports have also shown no prognostic value for advanced RCC treated with ICIs based on sPD-L1 levels, similar to our findings. Therefore, further studies are required to better understand the role of sPD-L1 in advanced RCC.
In one study, immunohistochemical analysis revealed significantly lower PD-L2 expression in RCC patients than in patients without PD-L2 expression [7]. In contrast, Shin et al. showed that the expression of PD-L2 in tumors was not associated with VEGF-TKI responsiveness or patient outcomes [21]. Thus, the role of PD-L2 in RCC remains unclear. sPD-L2 is a splice variant of membrane-bound PD-L2 that retains the ability to bind to membrane-bound PD-1 receptors for immune regulation. Few studies have examined the clinical role of sPD-L2 in cancer. Wang et al. showed that elevated preoperative serum sPD-L2 levels are associated with the risk of recurrence in clear cell RCC [22]. Research on patients with NSCLC treated with nivolumab has also shown that low sPD-L2 levels are associated with grade 3–4 toxicities [23]. Recently, several studies have shown that the presence of immune-related adverse events (irAEs) during ICI treatment is a predictor of therapeutic effects [24]. Although there have been no previous reports evaluating sPD-L2 levels and the treatment response to ICIs in patients with advanced RCC, the results of this study suggest that low sPD-L2 levels might be a biomarker able to predict a favorable response in patients treated with ICIs. In our study, univariate analysis showed that prior nephrectomy, sPD-L2 levels, and sLAG-3 levels were significant factors affecting PFS. Multivariate analyses suggested that the sPD-L2 level had an independent prognostic effect on PFS. In the univariate analysis of OS, prior nephrectomy and sPD-L2 levels were significant factors. However, the multivariate analysis did not identify sPD-L2 as an independent predictor of OS.
LAG-3 is a transmembrane protein that is structurally similar to CD4 and is expressed on the surface of activated T cells [25]. Although the role of LAG-3 has not been clarified, it is believed to be involved in the inhibition of T cell proliferation and activation, and is expressed on tumor-infiltrating T cells, T cells exhausted by chronic infection, and regulatory T cells [26]. It is believed to play an important role as an immunosuppressive factor in cancer immunity, infection immunity, autoimmune diseases, and immune checkpoint molecules, such as PD-1 and CTLA-4. In studies on NSCLC, low levels of s-LAG3 were reported to be associated with locally advanced or metastatic disease spread [27]. In breast and gastric cancers, detectable levels of sLAG-3 were associated with a favorable prognosis [28,29]. In contrast, Botticelli et al. demonstrated that sLAG-3 is associated with poor prognosis in head and neck squamous cell carcinoma [30]. Previous reports have not clarified the relationship between sLAG-3 and cancer prognosis. On the other hand, high sLAG-3 levels showed better PFS in this study, while no correlation with OS was observed. Thus, further studies are required to clarify these contradictory findings.
In this study, no correlation was found between sPD-L2 and sLAG-3 concentrations and PD-L2 and LAG-3 expression in the immunohistochemical analysis of renal tumor tissue. Previous reports have shown that the expression of soluble immune checkpoint molecules in the plasma reflects heterogeneity across tumors, whereas the expression of PD-L1 in tumors is heterogeneous, not only within a single tumor site but also between various tumor sites [31,32]. Furthermore, soluble immune checkpoint molecules can be secreted by cells other than tumor cells, such as immune cells [33]. The difference between soluble immune checkpoint molecules’ concentrations and immunohistochemical expression could be explained by the fact that plasma reflects the entire tumor heterogeneity, whereas the expression of immune checkpoint molecules in immunohistochemistry can be heterogeneous within one tumor site as well as between different tumor sites. In our study, there was no significant correlation between the PD-L2 or LAG-3 expression in tumors and the PFS or OS in patients with advanced RCC treated with IO combination therapy.
Our study had several limitations, including its single-institute retrospective design and small sample size. In addition, a standardized cutoff value for the expression of soluble immune checkpoint molecules has not yet been clearly established. Furthermore, our study included patients treated with IO + IO and IO + TKIs as first-line treatments. IO combination therapies (IO + IO and IO + TKI) comprise a combination of different types of agents with different mechanisms of action, and it is debatable whether it is correct to compare these regimens in parallel. Finally, the expressions of PD-L2 and LAG-3 were assessed by radical nephrectomy or renal biopsy. Several reports have shown that, given the significant tumor heterogeneity in RCC, nephrectomy specimens may not accurately reflect the biology of the metastatic sites.
5. Conclusions
In conclusion, sPD-L2 and sLAG-3 expression may prove to be a potential biomarker for predicting the therapeutic efficacy of IO combination therapy in advanced RCC. Additionally, sPD-L2 expression may be a prognostic factor for advanced RCC in the era of IO combination therapy. The evaluation of immune checkpoint molecules is minimally invasive and repeatable using blood samples, and thus immune checkpoint molecules could be useful biomarkers for patients with advanced RCC starting first-line treatment. In this scenario, we expect that further research will help to clarify the potential role of soluble immune checkpoint molecules in advanced renal cell carcinoma and assist in the ongoing process of discovering and validating new biomarkers to better predict the response of patients to IO combination therapy. Therefore, additional studies focusing on improving patient survival and the efficacy of IO combination therapy are required in order to validate our findings.
Author Contributions
Conceptualization, K.U. (Kosuke Ueda), K.U. (Keiichiro Uemura) and T.I.; methodology, K.U. (Kosuke Ueda), S.O. (Satoshi Ohnishi), H.K., T.H., K.C., S.O. (Sachiko Ogasawara) and T.I.; validation, K.U. (Kosuke Ueda), K.U. (Keiichiro Uemura), K.N., S.S. and T.I.; formal analysis, K.U. (Kosuke Ueda), M.N. and S.S.; investigation, K.U. (Kosuke Ueda), H.S., T.H., S.O. (Sachiko Ogasawara), S.S. and T.I.; resources, K.U. (Kosuke Ueda), N.I., Y.S., H.K., T.H., K.C., K.N., S.S. and T.I.; data curation, K.U. (Kosuke Ueda), N.I., Y.S., S.O. (Satoshi Ohnishi), H.S. and K.C.; writing—original draft preparation, K.U. (Kosuke Ueda) and K.U. (Keiichiro Uemura); writing—review and editing, M.N., S.S., H.Y. and T.I.; visualization, K.N. and M.N.; supervision, S.S., H.Y. and T.I. Funding Acquisition, K.U. (Kosuke Ueda). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the JPSS KAKENHI (grant number 23K08749).
Institutional Review Board Statement
The study was approved by the Ethics Review Committee of the Kurume University School of Medicine (approval number: 20118).
Informed Consent Statement
Written informed consent was obtained from all patients before their participation in this study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Mayu Hirano for technical assistance.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Suekane, S.; Kurose, H.; Chikui, K.; Nakiri, M.; Nishihara, K.; Matsuo, M.; Kawahara, A.; Yano, H.; Igawa, T. Prognostic value of PD-1 and PD-L1 expression in patients with metastatic clear cell renal cell carcinoma. Urol. Oncol. 2018, 36, 499.e9–499.e16. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Miyake, H.; Fujisawa, M. Expression pattern of immune checkpoint-associated molecules in radical nephrectomy specimens as a prognosticator in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Urol. Oncol. 2017, 35, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Abufaraj, M.; Mostafaei, H.; Quhal, F.; Fajkovic, H.; Remzi, M.; Karakiewicz, P.I.; Egawa, S.; Schmidinger, M.; Shariat, S.F.; et al. The predictive value of programmed death ligand 1 in patients with metastatic renal cell carcinoma treated with immune-checkpoint inhibitors: A systematic review and meta-analysis. Eur. Urol. 2021, 79, 783–792. [Google Scholar] [CrossRef]
- Lee, C.H.; Jung, S.J.; Seo, W.I.; Chung, J.I.; Lee, D.S.; Jeong, D.H.; Jeon, Y.; Choi, I. Coexpression of lymphocyte-activation gene 3 and programmed death ligand-1 in tumor infiltrating immune cells predicts worse outcome in renal cell carcinoma. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221125588. [Google Scholar] [CrossRef]
- Klümper, N.; Ralser, D.J.; Bawden, E.G.; Landsberg, J.; Zarbl, R.; Kristiansen, G.; Toma, M.; Ritter, M.; Hölzel, M.; Ellinger, J.; et al. LAG3 (LAG-3, CD223) DNA methylation correlates with LAG3 expression by tumor and immune cells, immune cell infiltration, and overall survival in clear cell renal cell carcinoma. J. Immunother. Cancer 2020, 8, e000552. [Google Scholar] [CrossRef]
- Martin, S.D.; Bhuiyan, I.; Soleimani, M.; Wang, G. Biomarkers for immune checkpoint inhibitors in renal cell carcinoma. J. Clin. Med. 2023, 12, 4987. [Google Scholar] [CrossRef]
- Shin, K.; Kim, J.; Park, S.J.; Lee, M.A.; Park, J.M.; Choi, M.G.; Kang, D.; Song, K.Y.; Lee, H.H.; Seo, H.S.; et al. Prognostic value of soluble PD-L1 and exosomal PD-L1 in advanced gastric cancer patients receiving systemic chemotherapy. Sci. Rep. 2023, 13, 6952. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Mahoney, K.M.; Giobbie-Hurder, A.; Zhao, F.; Lee, S.; Liao, X.; Rodig, S.; Li, J.; Wu, X.; Butterfield, L.H.; et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol. Res. 2017, 5, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lang, J. Soluble PD-1 and PD-L1: Predictive and prognostic significance in cancer. Oncotarget 2017, 8, 97671–97682. [Google Scholar] [CrossRef] [PubMed]
- Scirocchi, F.; Strigari, L.; Di Filippo, A.; Napoletano, C.; Pace, A.; Rahimi, H.; Botticelli, A.; Rughetti, A.; Nuti, M.; Zizzari, I.G. Soluble PD-L1 as a prognostic factor for immunotherapy treatment in solid tumors: Systematic review and meta-analysis. Int. J. Mol. Sci. 2022, 23, 14496. [Google Scholar] [CrossRef] [PubMed]
- Gide, T.N.; Paver, E.C.; Yaseen, Z.; Maher, N.; Adegoke, N.; Menzies, A.M.; Pires da Silva, I.; Wilmott, J.S.; Long, G.V.; Scolyer, R.A. Lag-3 expression and clinical outcomes in metastatic melanoma patients treated with combination anti-lag-3 + anti-PD-1-based immunotherapies. Oncoimmunology 2023, 12, 2261248. [Google Scholar] [CrossRef] [PubMed]
- Muraro, E.; Romanò, R.; Fanetti, G.; Vaccher, E.; Turturici, I.; Lupato, V.; La Torre, F.B.; Polesel, J.; Fratta, E.; Giacomarra, V.; et al. Tissue and circulating PD-L2: Moving from health and immune-mediated diseases to head and neck oncology. Crit. Rev. Oncol. Hematol. 2022, 175, 103707. [Google Scholar] [CrossRef]
- Chmielewska, I.; Grenda, A.; Krawczyk, P.; Frąk, M.; Kuźnar Kamińska, B.; Mitura, W.; Milanowski, J. The influence of plasma sPD-L1 concentration on the effectiveness of immunotherapy in advanced NSCLC patients. Cancer Immunol. Immunother. 2023, 72, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
- Montemagno, C.; Hagege, A.; Borchiellini, D.; Thamphya, B.; Rastoin, O.; Ambrosetti, D.; Iovanna, J.; Rioux-Leclercq, N.; Porta, C.; Negrier, S.; et al. Soluble forms of PD-L1 and PD-1 as prognostic and predictive markers of sunitinib efficacy in patients with metastatic clear cell renal cell carcinoma. Oncoimmunology 2020, 9, 1846901. [Google Scholar] [CrossRef]
- Wakita, N.; Hinata, N.; Bando, Y.; Hara, T.; Terakawa, T.; Furukawa, J.; Nakano, Y.; Fujisawa, M. Prognostic value of serum soluble PD-L1 in metastatic renal cell carcinoma patients treated with nivolumab. Anticancer Res. 2023, 43, 841–847. [Google Scholar] [CrossRef]
- Shin, S.J.; Jeon, Y.K.; Cho, Y.M.; Lee, J.L.; Chung, D.H.; Park, J.Y.; Go, H. The association between PD-L1 expression and the clinical outcomes to vascular endothelial growth factor-targeted therapy in patients with metastatic clear cell renal cell carcinoma. Oncologist 2015, 20, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, J.; Tu, H.; Liang, D.; Chang, D.W.; Ye, Y.; Wu, X. Soluble immune checkpoint-related proteins as predictors of tumor recurrence, survival, and T cell phenotypes in clear cell renal cell carcinoma patients. J. Immunother. Cancer 2019, 7, 334. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Julie, C.; Dumenil, C.; Hélias-Rodzewicz, Z.; Tisserand, J.; Dumoulin, J.; Giraud, V.; Labrune, S.; Chinet, T.; Emile, J.F.; et al. Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. Oncoimmunology 2018, 7, e1452581. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Suekane, S.; Kurose, H.; Ito, N.; Ogasawara, N.; Hiroshige, T.; Chikui, K.; Ejima, K.; Uemura, K.; Nakiri, M.; et al. Immune-related adverse events are clinical biomarkers to predict favorable outcomes in advanced renal cell carcinoma treated with nivolumab plus ipilimumab. Jpn. J. Clin. Oncol. 2022, 52, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Sugiura, D.; Okazaki, I.M.; Okazaki, T. LAG-3: From molecular functions to clinical applications. J. Immunother. Cancer 2020, 8, e001014. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Zhao, S.; Zhao, C.; Zhou, C.; Hirsch, F.R. sLAG-3 in non-small-cell lung cancer patients’ serum. Onco Targets Ther. 2018, 11, 4781–4784. [Google Scholar] [CrossRef]
- Li, N.; Jilisihan, B.; Wang, W.; Tang, Y.; Keyoumu, S. Soluble LAG3 acts as a potential prognostic marker of gastric cancer and its positive correlation with CD8+T cell frequency and secretion of IL-12 and INF-gamma in peripheral blood. Cancer Biomark. 2018, 23, 341–351. [Google Scholar] [CrossRef]
- Triebel, F.; Hacene, K.; Pichon, M.F. A soluble lymphocyte activation gene-3 (sLAG-3) protein as a prognostic factor in human breast cancer expressing estrogen or progesterone receptors. Cancer Lett. 2006, 235, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, A.; Zizzari, I.G.; Scagnoli, S.; Pomati, G.; Strigari, L.; Cirillo, A.; Cerbelli, B.; Di Filippo, A.; Napoletano, C.; Scirocchi, F.; et al. The role of soluble LAG3 and soluble immune checkpoints profile in advanced head and neck cancer: A pilot study. J. Pers. Med. 2021, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Shiner, R.J.; White, S.D.; Black, J.R.; Trivedi, P.; Stebbing, J.; Sharma, R.; Mauri, F.A. Intra-tumoral heterogeneity in the expression of programmed-death (PD) ligands in isogeneic primary and metastatic lung cancer: Implications for immunotherapy. Oncoimmunology 2016, 5, e1213934. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, D.; Clavé, S.; Taus, Á.; Hardy-Werbin, M.; Rocha, P.; Lorenzo, M.; Menéndez, S.; Salido, M.; Albanell, J.; Pijuan, L.; et al. Heterogeneity of tumor and immune cell PD-L1 expression and lymphocyte counts in surgical NSCLC samples. Clin. Lung Cancer 2017, 18, 682–691.e5. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, K.; Igarashi, T.; Kataoka, Y.; Ishida, M.; Hanaoka, J.; Sumimoto, H.; Daigo, Y. Prognostic impact of soluble PD-L1 derived from tumor-associated macrophages in non-small-cell lung cancer. Cancer Immunol. Immunother. 2023, 72, 3755–3764. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).