Planned Abscopal Effect with Concurrent Pembrolizumab and Ablative Radiotherapy to Pulmonary Metastasis: A Case Report and Review of the Literature
Abstract
1. Introduction
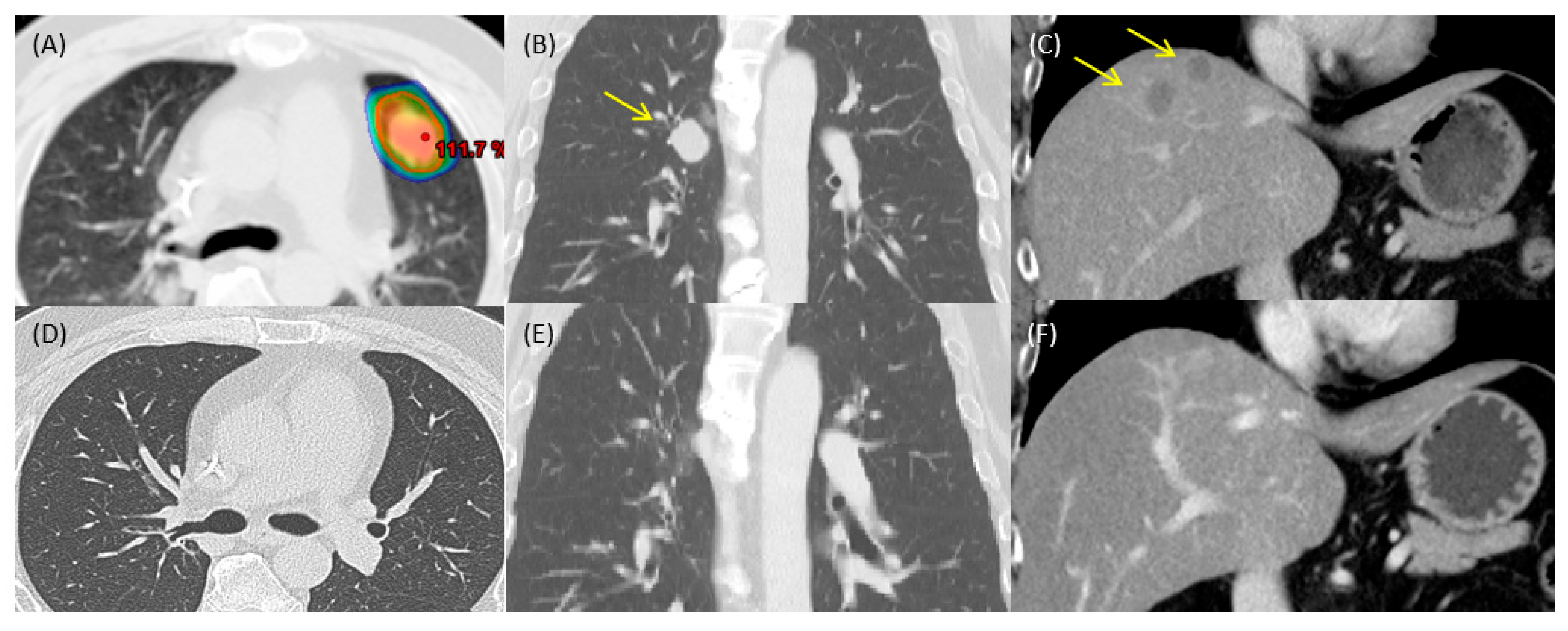
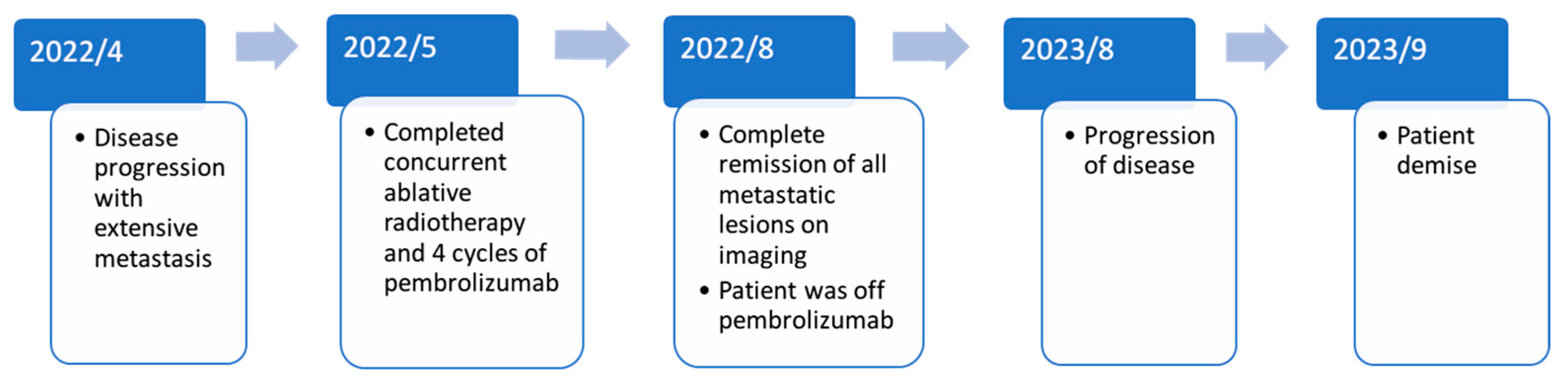
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statements
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-C.; Chen, Y.-L.; Lin, H.-W.; Chiang, Y.-C.; Chang, C.-F.; Hsieh, S.-F.; Chen, C.-A.; Sun, W.-Z.; Cheng, W.-F. Irradiation Enhances Abscopal Anti-tumor Effects of Antigen-Specific Immunotherapy through Regulating Tumor Microenvironment. Mol. Ther. 2018, 26, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, M.E.; Vanpouille-Box, C.; Melero, I.; Formenti, S.C.; Demaria, S. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol. 2018, 39, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Abuodeh, Y.; Venkat, P.; Kim, S. Systematic review of case reports on the abscopal effect. Curr. Probl. Cancer 2016, 40, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Theelen, W.S.M.; Chen, D.; Verma, V.; Hobbs, B.P.; Peulen, H.M.U.; Aerts, J.G.J.V.; Bahce, I.; Niemeijer, A.L.N.; Chang, J.Y.; de Groot, P.M.; et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Respir. Med. 2021, 9, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, Y.; Kong, L.; Shi, F.; Zhu, H.; Yu, J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Daro-Faye, M.; Kassouf, W.; Souhami, L.; Marcq, G.; Cury, F.; Niazi, T.; Sargos, P. Combined radiotherapy and immunotherapy in urothelial bladder cancer: Harnessing the full potential of the anti-tumor immune response. World J. Urol. 2021, 39, 1331–1343. [Google Scholar] [CrossRef]
- Kono, S.; Hashimoto, Y.; Shirai, Y.; Kunihiro, Y.; Ohmatsu, K.; Kawanishi, M.; Kuribayashi, S.; Karasawa, K. A long-term survival case of bladder cancer with distant metastases: Abscopal effect of brain metastases after stereotactic radiotherapy with immune checkpoint blockade therapy to lung metastases. Int. Cancer Conf. J. 2023, 12, 205–209. [Google Scholar] [CrossRef]
- Ishiyama, Y.; Takagi, T.; Yoshida, K.; Iizuka, J.; Kakuta, Y.; Okumi, M.; Ishida, H.; Tanabe, K. Possible abscopal effect in urothelial carcinoma of the upper urinary tract after treatment with immune checkpoint inhibitors. IJU Case Rep. 2020, 3, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Mampuya, W.A.; Bouchaab, H.; Schaefer, N.; Kinj, R.; La Rosa, S.; Letovanec, I.; Ozsahin, M.; Bourhis, J.; Coukos, G.; Peters, S.; et al. Abscopal effect in a patient with malignant pleural mesothelioma treated with palliative radiotherapy and pembrolizumab. Clin. Transl. Radiat. Oncol. 2021, 27, 85–88. [Google Scholar] [CrossRef]
- Jagodinsky, J.C.; Morris, Z.S. Priming and Propagating Anti-tumor Immunity: Focal Hypofractionated Radiation for in Situ Vaccination and Systemic Targeted Radionuclide Theranostics for Immunomodulation of Tumor Microenvironments. Semin. Radiat. Oncol. 2020, 30, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Monjazeb, A.M.; Schalper, K.A.; Villarroel-Espindola, F.; Nguyen, A.; Shiao, S.L.; Young, K. Effects of Radiation on the Tumor Microenvironment. Semin. Radiat. Oncol. 2020, 30, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.Y.; O’Hara, M.H.; Mitchell, T.C.; Vonderheide, R.H.; Wherry, E.J.; Minn, A.J.; Maity, A. Combining Radiation with Immunotherapy: The University of Pennsylvania Experience. Semin. Radiat. Oncol. 2020, 30, 173–180. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.S.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, J.P.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef] [PubMed]
- Grivas, P.; Park, S.H.; Voog, E.; Caserta, C.; Gurney, H.; Bellmunt, J.; Kalofonos, H.; Ullén, A.; Loriot, Y.; Sridhar, S.S.; et al. Avelumab First-line Maintenance Therapy for Advanced Urothelial Carcinoma: Comprehensive Clinical Subgroup Analyses from the JAVELIN Bladder 100 Phase 3 Trial. Eur. Urol. 2023, 84, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Dutt, S.; Ahmed, M.M.; Loo, B.W., Jr.; Strober, S. Novel Radiation Therapy Paradigms and Immunomodulation: Heresies and Hope. Semin. Radiat. Oncol. 2020, 30, 194–200. [Google Scholar] [CrossRef] [PubMed]

| Authors | Year of Publication | Number of Cases | Primary Site | Radiotherapy Regimen | Note |
|---|---|---|---|---|---|
| This study | 2024 | 1 | Bladder | 6000 cGy/10 fx | Radiotherapy to asymptomatic site |
| Kawanishi et al. [8] | 2023 | 1 | Bladder | 5200 cGy/8 fx | Complete response to brain metastasis |
| Ishiyama et al. [9] | 2020 | 1 | Right renal pelvis | 3000 cGy/10 fx | Radiotherapy after pembrolizumab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-T.; Chen, C.-C.; Chao, H.; Chang, C.-C.; Lee, C.-Y. Planned Abscopal Effect with Concurrent Pembrolizumab and Ablative Radiotherapy to Pulmonary Metastasis: A Case Report and Review of the Literature. Curr. Oncol. 2024, 31, 7787-7792. https://doi.org/10.3390/curroncol31120573
Lee Y-T, Chen C-C, Chao H, Chang C-C, Lee C-Y. Planned Abscopal Effect with Concurrent Pembrolizumab and Ablative Radiotherapy to Pulmonary Metastasis: A Case Report and Review of the Literature. Current Oncology. 2024; 31(12):7787-7792. https://doi.org/10.3390/curroncol31120573
Chicago/Turabian StyleLee, Yu-Ting, Chien-Chin Chen, Hsiya Chao, Chih-Chia Chang, and Cheng-Yen Lee. 2024. "Planned Abscopal Effect with Concurrent Pembrolizumab and Ablative Radiotherapy to Pulmonary Metastasis: A Case Report and Review of the Literature" Current Oncology 31, no. 12: 7787-7792. https://doi.org/10.3390/curroncol31120573
APA StyleLee, Y.-T., Chen, C.-C., Chao, H., Chang, C.-C., & Lee, C.-Y. (2024). Planned Abscopal Effect with Concurrent Pembrolizumab and Ablative Radiotherapy to Pulmonary Metastasis: A Case Report and Review of the Literature. Current Oncology, 31(12), 7787-7792. https://doi.org/10.3390/curroncol31120573






