Longitudinal Assessment of FT3 to FT4 Conversion Ratio in Predicting the Efficacy of First-Line Pembrolizumab-Based Therapy in Advanced Non-Small Cell Lung Cancer: A Propensity-Score Matching Analysis of Data from the National Drug Monitoring Agency
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Collection and Assessment of Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
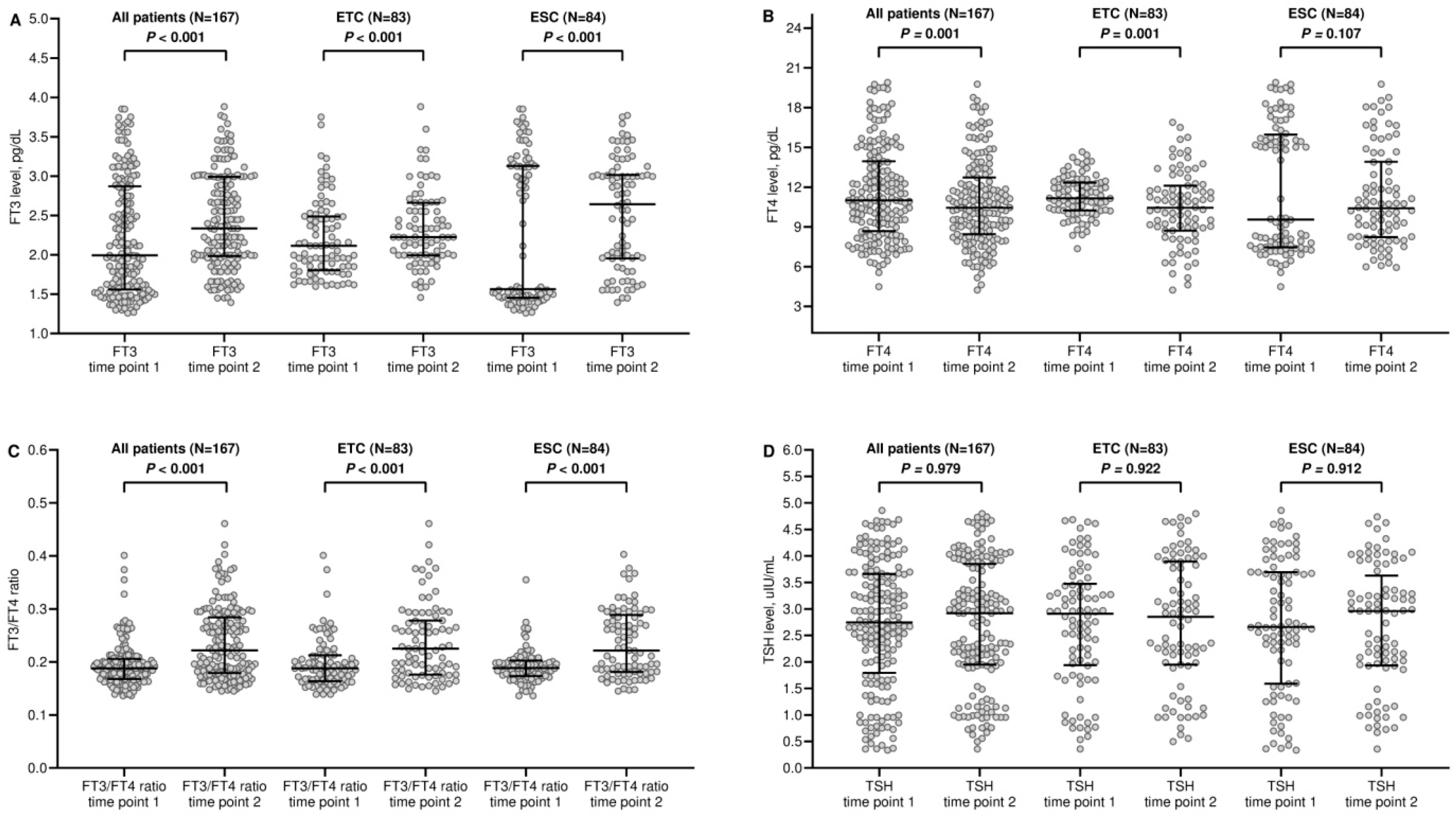
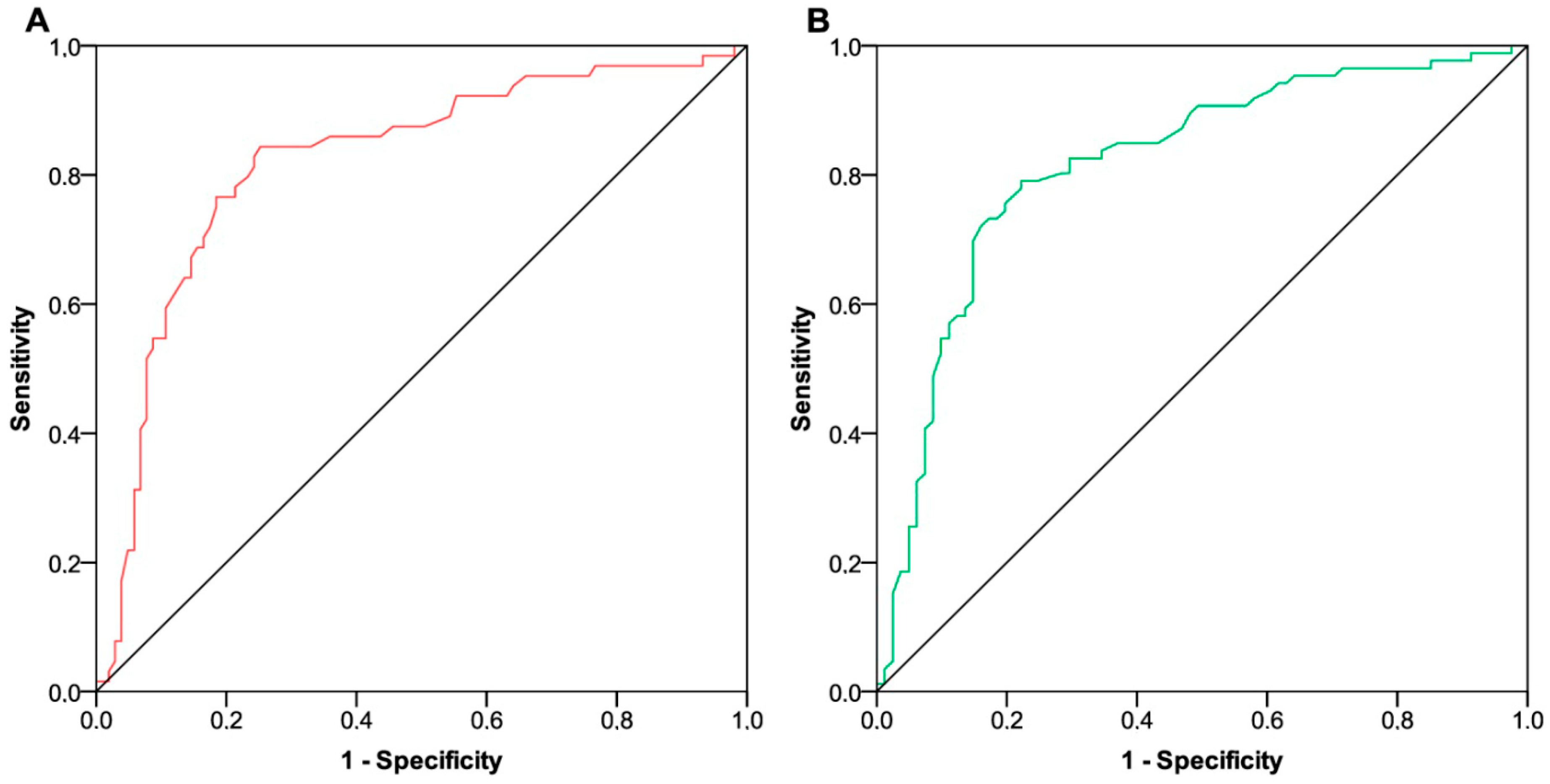
3.2. Dynamic Changes in Thyroid Function
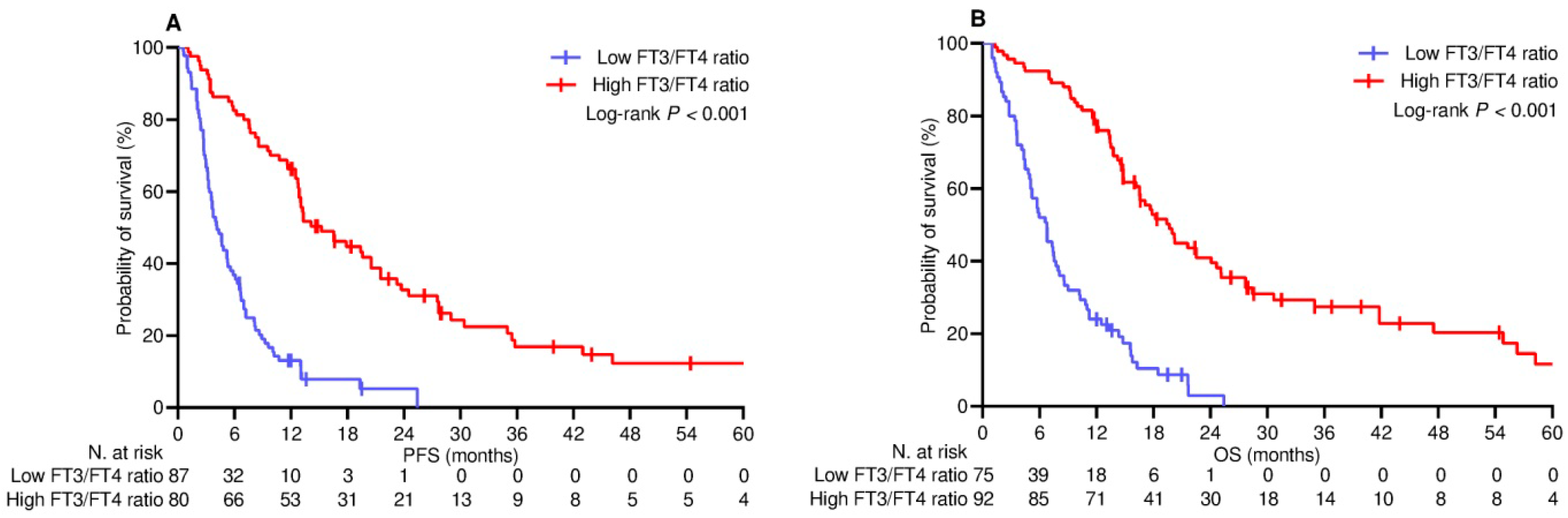
3.3. Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rui, R.; Zhou, L.; He, S. Cancer immunotherapies: Advances and bottlenecks. Front. Immunol. 2023, 14, 1212476. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.C.; Baines, A.C.; Gong, Y.; Moore, R., Jr.; Pamuk, G.E.; Saber, H.; Subedee, A.; Thompson, M.D.; Xiao, W.; Pazdur, R.; et al. Trends in the approval of cancer therapies by the FDA in the twenty-first century. Nat. Rev. Drug Discov. 2023, 22, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Riely, G.J.; Wood, D.E.; Ettinger, D.S.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R. Non-Small Cell Lung Cancer, Version 4.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 249–274. [Google Scholar] [CrossRef] [PubMed]
- Novello, S.; Kowalski, D.M.; Luft, A.; Gümüş, M.; Vicente, D.; Mazières, J.; Rodríguez-Cid, J.; Tafreshi, A.; Cheng, Y.; Lee, K.H.; et al. Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J. Clin. Oncol. 2023, 41, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Gadgeel, S.; Speranza, G.; Felip, E.; Esteban, E.; Dómine, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.G.; Peled, N.; et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes from the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lee, J.-S.; Ciuleanu, T.-E.; Caro, R.B.; Nishio, M.; Urban, L.; Audigier-Valette, C.; Lupinacci, L.; Sangha, R.; Pluzanski, A.; et al. Five-Year Survival Outcomes with Nivolumab Plus Ipilimumab versus Chemotherapy as First-Line Treatment for Metastatic Non-Small-Cell Lung Cancer in CheckMate 227. J. Clin. Oncol. 2023, 41, 1200–1212. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Lei, Y.; Li, X.; Huang, Q.; Zheng, X.; Liu, M. Progress and Challenges of Predictive Biomarkers for Immune Checkpoint Blockade. Front. Oncol. 2021, 11, 617335. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Z.; Zhang, W.; Zhang, W.; Buzdin, A.; Mu, X.; Yan, Q.; Zhao, X.; Chang, H.H.; Duhon, M.; et al. FDA-Approved and Emerging Next Generation Predictive Biomarkers for Immune Checkpoint Inhibitors in Cancer Patients. Front. Oncol. 2021, 11, 683419. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Fu, Y.; Zeng, Y.; Liu, X.; Peng, Y.; Hu, C.; Deng, C.; Qiu, Z.; Zou, J.; Liu, Y.; et al. The key to immunotherapy: How to choose better therapeutic biomarkers for patients with non-small cell lung cancer. Biomark. Res. 2022, 10, 9. [Google Scholar] [CrossRef]
- Ancel, J.; Dormoy, V.; Raby, B.N.; Dalstein, V.; Durlach, A.; Dewolf, M.; Gilles, C.; Polette, M.; Deslée, G. Soluble biomarkers to predict clinical outcomes in non-small cell lung cancer treated by immune checkpoints inhibitors. Front. Immunol. 2023, 14, 1171649. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, A.; Okuma, Y. Perspective on immune oncology with liquid biopsy, peripheral blood mononuclear cells, and microbiome with non-invasive biomarkers in cancer patients. Clin. Transl. Oncol. 2018, 20, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Hsu, J.M.; Sun, L.; Wang, S.C.; Hung, M.C. Advances and prospects of biomarkers for immune checkpoint inhibitors. Cell Rep. Med. 2024, 5, 101621. [Google Scholar] [CrossRef] [PubMed]
- Schirripa, M.; Pasqualetti, G.; Giampieri, R.; Scartozzi, M.; Lonardi, S.; Rumanò, L.; Bergamo, F.; Stragliotto, S.; Murgioni, S.; Alberti, G.; et al. Prognostic value of thyroid hormone ratios in patients with advanced metastatic colorectal cancer treated with regorafenib: The TOREADOR study. Clin. Color. Cancer 2018, 17, e601–e615. [Google Scholar] [CrossRef] [PubMed]
- Pasqualetti, G.; Schirripa, M.; Dochy, E.; Fassan, M.; Ziranu, P.; Puzzoni, M.; Scartozzi, M.; Alberti, G.; Lonardi, S.; Zagonel, V.; et al. Thyroid hormones ratio is a major prognostic marker in advanced metastatic colorectal cancer: Results from the phase III randomised CORRECT trial. Eur. J. Cancer 2020, 133, 66–73. [Google Scholar] [CrossRef]
- Maruzzo, M.; Verzoni, E.; Vitale, M.G.; Dionese, M.; Buti, S.; Galli, L.; Zivi, A.; Watutantrige-Fernando, S.; Zielli, T.; Zanardi, E.; et al. Prognostic value of thyroid hormone ratio in patients with advanced metastatic renal cell carcinoma: Results from the threefour study (Meet-URO 14). Front. Oncol. 2021, 11, 787835. [Google Scholar] [CrossRef]
- Pierantoni, F.; Dionese, M.; Basso, U.; Lai, E.; Cavasin, N.; Erbetta, E.; Mattana, A.; Bimbatti, D.; Zagonel, V.; Lonardi, S.; et al. The prognostic Value of Thyroid Hormone Levels in Immunotherapy-Treated Patients with Metastatic Urothelial Carcinoma. Clin. Genitourin. Cancer 2023, 21, e378–e385. [Google Scholar] [CrossRef]
- Warner, M.H.; Beckett, G.J. Mechanism Behind the non-Thyroidal Illness Syndrome: An Update. J. Endocrinol. 2010, 205, 1–13. [Google Scholar] [CrossRef]
- van der Spek, A.H.; Fliers, E.; Boelen, A. Thyroid Hormone and Deiodination in Innate Immune Cells. Endocrinology 2021, 162, bqaa200. [Google Scholar] [CrossRef]
- Rubingh, J.; van der Spek, A.; Fliers, E.; Boelen, A. The Role of Thyroid Hormone in the Innate and Adaptive Immune Response during Infection. Compr. Physiol. 2020, 10, 1277–1287. [Google Scholar]
- Karaviti, D.; Kani, E.R.; Karaviti, E.; Gerontiti, E.; Michalopoulou, O.; Stefanaki, K.; Kazakou, P.; Vasileiou, V.; Psaltopoulou, T.; Paschou, S.A. Thyroid disorders induced by immune checkpoint inhibitors. Endocrine 2024, 85, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Registri Farmaci Sottoposti a Monitoraggio. Available online: https://www.aifa.gov.it/registri-farmaci-sottoposti-a-monitoraggio (accessed on 30 September 2024).
- STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE). Available online: https://www.strobe-statement.org (accessed on 30 September 2024).
- PD-L1 IHC 22C3 Pharmdx. Available online: https://www.agilent.com/en/product/pharmdx/pd-l1-ihc-22c3-pharmdx (accessed on 30 September 2024).
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [PubMed]
- R Website. The R Project for Statistical Computing. Available online: https://www.r-project.org (accessed on 30 September 2024).
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-world evidence—What is it and what can it tell us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef] [PubMed]
- Brookhart, M.A.; Schneeweiss, S.; Rothman, K.J.; Glynn, R.J.; Avorn, J.; Stürmer, T. Variable selection for propensity score models. Am. J. Epidemiol. 2006, 163, 1149–1156. [Google Scholar] [CrossRef]
- Luongo, C.; Dentice, M.; Salvatore, D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nat. Rev. Endocrinol. 2019, 15, 479–488. [Google Scholar] [CrossRef]
- Iervasi, G.; Pingitore, A.; Landi, P.; Raciti, M.; Ripoli, A.; Scarlattini, M.; L’Abbate, A.; Donato, L. Low-T3 syndrome: A strong prognostic predictor of death in patients with heart disease. Circulation 2003, 107, 708–713. [Google Scholar] [CrossRef]
- Scoscia, E.; Baglioni, S.; Eslami, A.; Iervasi, G.; Monti, S.; Todisco, T. Low triiodothyronine (T3) state: A predictor of outcome in respiratory failure? Results of a clinical pilot study. Eur. J. Endocrinol. 2004, 151, 557–560. [Google Scholar] [CrossRef]
- Xiong, H.; Yan, P.; Huang, Q.; Shuai, T.; Liu, J.; Zhu, L.; Lu, J.; Shi, X.; Yang, K.; Liu, J. A prognostic role for non-thyroidal illness syndrome in chronic renal failure: A systematic review and meta-analysis. Int. J. Surg. 2019, 70, 44–52. [Google Scholar] [CrossRef]
- Pasqualetti, G.; Calsolaro, V.; Bernardini, S.; Linsalata, G.; Bigazzi, R.; Caraccio, N.; Monzani, F. Degree of Peripheral Thyroxin Deiodination, Frailty, and Long-Term Survival in Hospitalized Older Patients. J. Clin. Endocrinol. Metab. 2018, 103, 1867–1876. [Google Scholar] [CrossRef]
- Hartl, L.; Simbrunner, B.; Jachs, M.; Wolf, P.; Bauer, D.J.M.; Scheiner, B.; Balcar, L.; Semmler, G.; Schwarz, M.; Marculescu, R.; et al. Lower free triiodothyronine (fT3) levels in cirrhosis are linked to systemic inflammation, higher risk of acute-on-chronic liver failure, and mortality. JHEP Rep. 2023, 6, 100954. [Google Scholar] [CrossRef]
- Shyh-Chang, N. Metabolic Changes During Cancer Cachexia Pathogenesis. Adv. Exp. Med. Biol. 2017, 1026, 233–249. [Google Scholar] [PubMed]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef] [PubMed]
- Yasar, Z.A.; Kirakli, C.; Yilmaz, U.; Ucar, Z.Z.; Talay, F. Can non-thyroid illness syndrome predict mortality in lung cancer patients? A prospective cohort study. Horm. Cancer 2014, 5, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Fragidis, S.; Sombolos, K.; Thodis, E.; Panagoutsos, S.; Mourvati, E.; Pikilidou, M.; Papagianni, A.; Pasadakis, P.; Vargemezis, V. Low T3 syndrome and long-term mortality in chronic hemodialysis patients. World J. Nephrol. 2015, 4, 415–422. [Google Scholar] [CrossRef]
- Deligiorgi, M.V.; Sagredou, S.; Vakkas, L.; Trafalis, D.T. The Continuum of Thyroid Disorders Related to Immune Checkpoint Inhibitors: Still Many Pending Queries. Cancers 2021, 13, 5277. [Google Scholar] [CrossRef]
- Wenzek, C.; Boelen, A.; Westendorf, A.M.; Engel, D.R.; Moeller, L.C.; Führer, D. The interplay of thyroid hormones and the immune system—Where we stand and why we need to know about it. Eur. J. Endocrinol. 2022, 186, R65–R77. [Google Scholar] [CrossRef]
- De Luca, R.; Davis, P.J.; Lin, H.Y.; Gionfra, F.; Percario, Z.A.; Affabris, E.; Pedersen, J.Z.; Marchese, C.; Trivedi, P.; Anastasiadou, E.; et al. Thyroid Hormones Interaction with Immune Response, Inflammation and Non-thyroidal Illness Syndrome. Front. Cell Dev. Biol. 2021, 8, 614030. [Google Scholar] [CrossRef]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Patients with Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.L.; Yang, X.Y.; Dong, Z.R.; Chen, Z.Q.; Hong, J.G.; Li, T. The Prediction Potential of the Pretreatment Lung Immune Prognostic Index for the Therapeutic Outcomes of Immune Checkpoint Inhibitors in Patients with Solid Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 691002. [Google Scholar] [CrossRef] [PubMed]
- Lasa, M.; Contreras-Jurado, C. Thyroid hormones act as modulators of inflammation through their nuclear receptors. Front. Endocrinol. 2022, 13, 937099. [Google Scholar] [CrossRef]

| Variable | Unadjusted Population | PSM-Adjusted Population | ||||||
|---|---|---|---|---|---|---|---|---|
| All Patients (n = 258) | ETC (n = 170) | ESC (n = 88) | p Value | All Patients (n = 176) | ETC (n = 88) | ESC (n = 88) | p Value | |
| Age | ||||||||
| - Mean (SD), years | 70.0 (8.4) | 70.0 (7.9) | 69.5 (9.0) | 0.332 | 68.4 (8.7) | 68.3 (8.7) | 68.6 (8.8) | 0.689 |
| - ≥70 years | 136 (52.7%) | 91 (53.5%) | 45 (51.1%) | 0.715 | 88 (50.0%) | 43 (48.9%) | 45 (51.1%) | 0.763 |
| Sex | 0.69 | 0.744 | ||||||
| - Female | 78 (30.2%) | 50 (29.4%) | 28 (31.8%) | 54 (30.7%) | 26 (29.5%) | 28 (31.8%) | ||
| - Male | 180 (69.8%) | 120 (70.6%) | 60 (68.2%) | 122 (69.3%) | 62 (70.5%) | 60 (68.2%) | ||
| ECOG PS | 0.986 | 0.579 | ||||||
| - 0 or 1 | 208 (80.6%) | 137 (80.6%) | 71 (80.7%) | 139 (79.0%) | 68 (77.3%) | 71 (80.7%) | ||
| - 2 | 50 (19.4%) | 33 (19.4%) | 17 (19.3%) | 37 (21.0%) | 20 (22.7%) | 17 (19.3%) | ||
| Histologic subtype | 0.545 | 0.604 | ||||||
| - Nonsquamous | 202 (78.3%) | 135 (79.4%) | 67 (76.1%) | 131 (74.4%) | 64 (72.7%) | 67 (76.1%) | ||
| - Squamous | 56 (21.7%) | 35 (20.6%) | 21 (23.9%) | 45 (25.6%) | 24 (27.3%) | 21 (23.9%) | ||
| No. of metastatic sites | 0.611 | 0.444 | ||||||
| - ≤2 | 138 (53.5%) | 89 (52.4%) | 49 (55.7%) | 103 (58.5%) | 54 (61.4%) | 49 (47.6%) | ||
| - >2 | 120 (46.5%) | 81 (47.6%) | 39 (44.3%) | 73 (41.5%) | 34 (38.6%) | 39 (44.3%) | ||
| Bone metastasis | 53 (20.5%) | 35 (20.6%) | 18 (20.5%) | 0.98 | 31 (17.6%) | 13 (14.8%) | 18 (20.5%) | 0.322 |
| Brain metastasis | 58 (22.5%) | 37 (21.8%) | 21 (23.9%) | 0.702 | 43 (24.4%) | 22 (25.0%) | 21 (23.9%) | 0.861 |
| Liver metastasis | 28 (10.9%) | 19 (11.2%) | 9 (10.2%) | 0.816 | 16 (9.1%) | 7 (8.0%) | 9 (10.2%) | 0.6 |
| PD-L1 TPS | 0.116 | 0.127 | ||||||
| - <1% | 61 (23.6%) | 46 (27.0%) | 15 (17.0%) | 48 (27.3%) | 30 (34.1%) | 18 (20.4%) | ||
| - ≥1% and ≤49% | 41 (15.9%) | 23 (13.5%) | 18 (20.4%) | 19 (10.8%) | 7 (7.9%) | 12 (13.6%) | ||
| - ≥50% | 156 (60.4%) | 101 (59.4%) | 55 (62.5%) | 109 (61.9%) | 54 (61.4%) | 55 (62.5%) | ||
| BMI | ||||||||
| - Mean (SD), kg/m2 | 25.1 (4.80) | 24.8 (5.0) | 25.6 (4.5) | 0.635 | 26.0 (4.8) | 26.2 (5.2) | 25.8 (4.4) | 0.776 |
| - ≥25 kg/m2 | 130 (50.4%) | 83 (48.8%) | 47 (53.4%) | 0.485 | 92 (52.3%) | 45 (51.1%) | 47 (53.4%) | 0.763 |
| Smoking habits | 0.943 | 0.799 | ||||||
| - Never | 23 (8.9%) | 15 (8.8%) | 8 (9.1%) | 17 (9.7%) | 9 (10.2%) | 8 (9.1%) | ||
| - Ever | 235 (91.1%) | 155 (91.2%) | 80 (90.9%) | 159 (90.3%) | 79 (89.8%) | 80 (90.9%) | ||
| Previous thoracic RT | 35 (13.6%) | 22 (12.9%) | 13 (14.8%) | 0.684 | 26 (14.8%) | 13 (14.8%) | 13 (14.8%) | 1 |
| LIPI score | 0.999 | 0.953 | ||||||
| - 0 | 100 (38.8%) | 66 (38.8%) | 34 (38.6%) | 70 (39.8%) | 36 (40.9%) | 34 (38.6%) | ||
| - 1 | 88 (34.1%) | 58 (34.1%) | 30 (34.1%) | 59 (33.5%) | 29 (33.0%) | 30 (34.1%) | ||
| - 2 | 70 (27.1%) | 46 (27.1%) | 24 (27.3%) | 47 (26.7%) | 23 (26.1%) | 24 (27.3%) | ||
| First-line therapy | 0.887 | 0.958 | ||||||
| - Only pembrolizumab | 156 (60.5%) | 101 (59.4%) | 55 (62.5%) | 109 (61.9%) | 54 (61.4%) | 55 (62.5%) | ||
| - Pemetrexed-based | 84 (32.6%) | 57 (33.5%) | 27 (30.7%) | 54 (30.7%) | 27 (30.7%) | 27 (30.7%) | ||
| - Paclitaxel-based | 18 (7.0%) | 12 (7.1%) | 6 (6.8%) | 13 (7.4%) | 7 (8.0%) | 6 (6.7%) | ||
| Corticosteroids a | 103 (39.9%) | 66 (38.8%) | 37 (42.0%) | 0.616 | 72 (40.9%) | 35 (39.8%) | 37 (42.0%) | 0.759 |
| APAP b | 101 (39.1%) | 74 (43.5%) | 27 (30.6%) | 0.045 | 53 (28.7%) | 26 (29.5%) | 27 (30.7%) | 0.869 |
| Systemic antibiotics c | 58 (22.5%) | 40 (23.5%) | 18 (20.5%) | 0.575 | 33 (18.8%) | 15 (17.0%) | 18 (20.5%) | 0.562 |
| PPI d | 98 (38.0%) | 72 (42.3%) | 26 (29.5%) | 0.044 | 46 (26.1%) | 20 (22.7%) | 26 (29.5%) | 0.303 |
| FT3, pg/dL | 0.091 | 0.096 | ||||||
| - Median (IQR) | 2.05 (1.57–2.93) | 2.16 (1.85–2.61) | 1.59 (1.47–3.05) | 1.99 (1.56–2.87) | 2.11 (1.80–2.48) | 1.56 (1.45–3.12) | ||
| FT4, pg/dL | 0.451 | 0.519 | ||||||
| - Median (IQR) | 10.97 (8.53–13.75) | 11.04 (10.27–12.48) | 9.46 (7.27–16.11) | 11.01 (8.67–13.94) | 11.15 (10.33–12.33) | 9.55 (7.46–16.23) | ||
| TSH, uIU/mL | ||||||||
| - Median (IQR) | 2.76 (1.74–3.72) | 2.82 (1.87–3.61) | 2.61 (1.56–3.72) | 0.897 | 2.74 (1.79–3.66) | 2.90 (1.93–3.47) | 2.66 (1.58–3.69) | 0.866 |
| Time Point 1 | Time Point 2 | p Value | |
|---|---|---|---|
| PSM-adjusted population (n = 167) | |||
| FT3, pg/dL - median (IQR) | 2.05 (1.55–2.87) | 2.33 (1.98–2.99) | <0.001 |
| FT4, pg/dL - median (IQR) | 11.12 (8.64–13.94) | 10.44 (8.45–12.72) | 0.001 |
| TSH, uIU/mL - median (IQR) | 2.75 (1.72–3.66) | 2.91 (1.94–3.84) | 0.979 |
| FT3/FT4 ratio | 0.18 (0.16–0.20) | 0.22 (0.17–0.28) | <0.001 |
| ETC (n = 83) | |||
| FT3, pg/dL - median (IQR) | 2.11 (1.85–2.51) | 2.22 (1.99–2.66) | <0.001 |
| FT4, pg/dL - median (IQR) | 11.25 (10.24–12.42) | 10.44 (8.72–12.11) | 0.001 |
| TSH, uIU/mL - median (IQR) | 2.94 (1.74–3.58) | 2.85 (1.94–3.89) | 0.922 |
| FT3/FT4 ratio | 0.18 (0.16–0.21) | 0.22 (0.17–0.27) | <0.001 |
| ESC (n = 84) | |||
| FT3, pg/dL - median (IQR) | 1.56 (1.45–3.15) | 2.64 (1.95–3.01) | <0.001 |
| FT4, pg/dL - median (IQR) | 9.55 (7.45–16.23) | 10.41 (8.23–13.90) | 0.107 |
| TSH, uIU/mL - median (IQR) | 2.66 (1.54–3.69) | 2.95 (1.93–3.62) | 0.912 |
| FT3/FT4 ratio | 0.18 (0.17–0.20) | 0.22 (0.18–0.28) | <0.001 |
| Covariate | Progression-Free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | ||||
| - <70 years | 1 | - | 1 | - |
| - ≥70 years | 0.82 (0.59–1.14) | 0.246 | 0.81 (0.58–1.14) | 0.241 |
| Sex | ||||
| - Female | 1 | - | 1 | - |
| - Male | 0.91 (0.64–1.31) | 0.628 | 0.94 (0.65–1.35) | 0.742 |
| ECOG PS | ||||
| - 0–1 | 1 | - | 1 | - |
| - 2 | 1.48 (0.98–2.22) | 0.057 | 1.46 (0.96–2.23) | 0.074 |
| Histologic subtype | ||||
| - Nonsquamous | 1 | - | 1 | - |
| - Squamous | 0.82 (0.56–1.20) | 0.321 | 0.93 (0.63–1.38) | 0.747 |
| No. of metastatic sites | ||||
| - ≤2 | 1 | - | 1 | - |
| - >2 | 1.22 (0.87–1.71) | 0.233 | 1.23 (0.87–1.75) | 0.229 |
| Bone metastasis | ||||
| - No | 1 | - | 1 | - |
| - Yes | 1.74 (1.15–2.65) | 0.009 | 1.46 (0.93–2.28) | 0.093 |
| Brain metastasis | ||||
| - No | 1 | - | 1 | - |
| - Yes | 0.89 (0.60–1.32) | 0.583 | 0.88 (0.58–1.33) | 0.559 |
| Liver metastasis | ||||
| - No | 1 | - | 1 | - |
| - Yes | 1.44 (0.84–2.47) | 0.179 | 1.27 (0.72–2.27) | 0.402 |
| PD-L1 TPS | ||||
| - ≥50% | 1 | - | 1 | - |
| - <50% | 1.06 (0.75–1.50) | 0.726 | 0.94 (0.65–1.35) | 0.758 |
| BMI | ||||
| - ≥25 kg/m2 | 1 | - | 1 | - |
| - <25 kg/m2 | 1.47 (1.05–2.05) | 0.022 | 1.49 (1.06–2.10) | 0.022 |
| Smoking habits | ||||
| - Ever | 1 | - | 1 | - |
| - Never | 1.95 (1.10–3.48) | 0.022 | 1.39 (0.78–2.47) | 0.257 |
| Previous thoracic RT | ||||
| - No | 1 | - | 1 | - |
| - Yes | 0.81 (0.50–1.30) | 0.389 | 0.74 (0.45–1.21) | 0.239 |
| LIPI score | ||||
| - 0 | 1 | - | 1 | - |
| - 1 | 4.63 (2.90–7.40) | <0.001 | 7.67 (4.43–13.25) | <0.001 |
| - 2 | 14.96 (8.87–25.29) | <0.001 | 32.00 (17.01–60.22) | <0.001 |
| First-line therapy | ||||
| - Only pembrolizumab | 1 | - | 1 | - |
| - Pemetrexed-based | 1.07 (0.74–1.55) | 0.703 | 0.91 (0.61–1.34) | 0.642 |
| - Paclitaxel-based | 1.02 (0.52–1.97) | 0.954 | 1.09 (0.56–2.13) | 0.782 |
| Corticosteroids a | ||||
| - No | 1 | - | 1 | - |
| - Yes | 2.22 (1.58–3.12) | <0.001 | 2.15 (1.52–3.05) | <0.001 |
| APAP b | ||||
| - No | 1 | - | 1 | - |
| - Yes | 1.29 (0.91–1.83) | 0.149 | 1.20 (0.83–1.73) | 0.312 |
| Systemic antibiotics c | ||||
| - No | 1 | - | 1 | - |
| - Yes | 2.04 (1.35–3.07) | 0.001 | 2.08 (1.37–3.16) | 0.001 |
| PPI d | ||||
| - No | 1 | - | 1 | - |
| - Yes | 0.91 (0.62–1.32) | 0.626 | 0.91 (0.61–1.34) | 0.644 |
| On treatment FT3/FT4 ratio | ||||
| - High | 1 | - | 1 | - |
| - Low | 4.25 (2.86–6.17) | <0.001 | 4.36 (2.98–6.39) | <0.001 |
| Covariate | Progression-Free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Bone metastasis | - | - | ||
| - No | 1 | - | ||
| - Yes | 0.99 (0.64–1.52) | 0.972 | ||
| BMI | ||||
| - ≥25 kg/m2 | 1 | - | 1 | - |
| - <25 kg/m2 | 1.29 (0.90–1.84) | 0.156 | 1.41 (0.98–2.02) | 0.06 |
| Smoking habits | - | - | ||
| - Ever | 1 | - | ||
| - Never | 2.22 (1.21–4.05) | 0.009 | ||
| LIPI score | ||||
| - 0 | 1 | - | 1 | - |
| - 1 | 3.50 (2.15–5.71) | <0.001 | 5.72 (3.29–9.93) | <0.001 |
| - 2 | 11.50 (6.52–20.31) | <0.001 | 21.71 (10.94–43.18) | <0.001 |
| Corticosteroids a | ||||
| - No | 1 | - | 1 | - |
| - Yes | 1.72 (1.18–2.51) | 0.005 | 1.70 (1.17–2.47) | 0.005 |
| Systemic antibiotics b | ||||
| - No | 1 | - | 1 | - |
| - Yes | 1.31 (0.84–2.03) | 0.23 | 1.21 (0.77–1.90) | 0.401 |
| On treatment FT3/FT4 ratio | ||||
| - High | 1 | - | 1 | - |
| - Low | 2.51 (1.66–3.78) | <0.001 | 2.18 (1.43–3.34) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelli, F.; Ruggeri, E.M.; Schirripa, M.; Virtuoso, A.; Giannarelli, D.; Raso, A.; Remotti, D.; Fabbri, A. Longitudinal Assessment of FT3 to FT4 Conversion Ratio in Predicting the Efficacy of First-Line Pembrolizumab-Based Therapy in Advanced Non-Small Cell Lung Cancer: A Propensity-Score Matching Analysis of Data from the National Drug Monitoring Agency. Curr. Oncol. 2024, 31, 7647-7662. https://doi.org/10.3390/curroncol31120564
Nelli F, Ruggeri EM, Schirripa M, Virtuoso A, Giannarelli D, Raso A, Remotti D, Fabbri A. Longitudinal Assessment of FT3 to FT4 Conversion Ratio in Predicting the Efficacy of First-Line Pembrolizumab-Based Therapy in Advanced Non-Small Cell Lung Cancer: A Propensity-Score Matching Analysis of Data from the National Drug Monitoring Agency. Current Oncology. 2024; 31(12):7647-7662. https://doi.org/10.3390/curroncol31120564
Chicago/Turabian StyleNelli, Fabrizio, Enzo Maria Ruggeri, Marta Schirripa, Antonella Virtuoso, Diana Giannarelli, Armando Raso, Daniele Remotti, and Agnese Fabbri. 2024. "Longitudinal Assessment of FT3 to FT4 Conversion Ratio in Predicting the Efficacy of First-Line Pembrolizumab-Based Therapy in Advanced Non-Small Cell Lung Cancer: A Propensity-Score Matching Analysis of Data from the National Drug Monitoring Agency" Current Oncology 31, no. 12: 7647-7662. https://doi.org/10.3390/curroncol31120564
APA StyleNelli, F., Ruggeri, E. M., Schirripa, M., Virtuoso, A., Giannarelli, D., Raso, A., Remotti, D., & Fabbri, A. (2024). Longitudinal Assessment of FT3 to FT4 Conversion Ratio in Predicting the Efficacy of First-Line Pembrolizumab-Based Therapy in Advanced Non-Small Cell Lung Cancer: A Propensity-Score Matching Analysis of Data from the National Drug Monitoring Agency. Current Oncology, 31(12), 7647-7662. https://doi.org/10.3390/curroncol31120564





