Evaluating the Efficacy of Immunotherapy in Fragile Hospitalized Patients
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Patient Enrollment
2.3. Data Collection
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment Characteristics
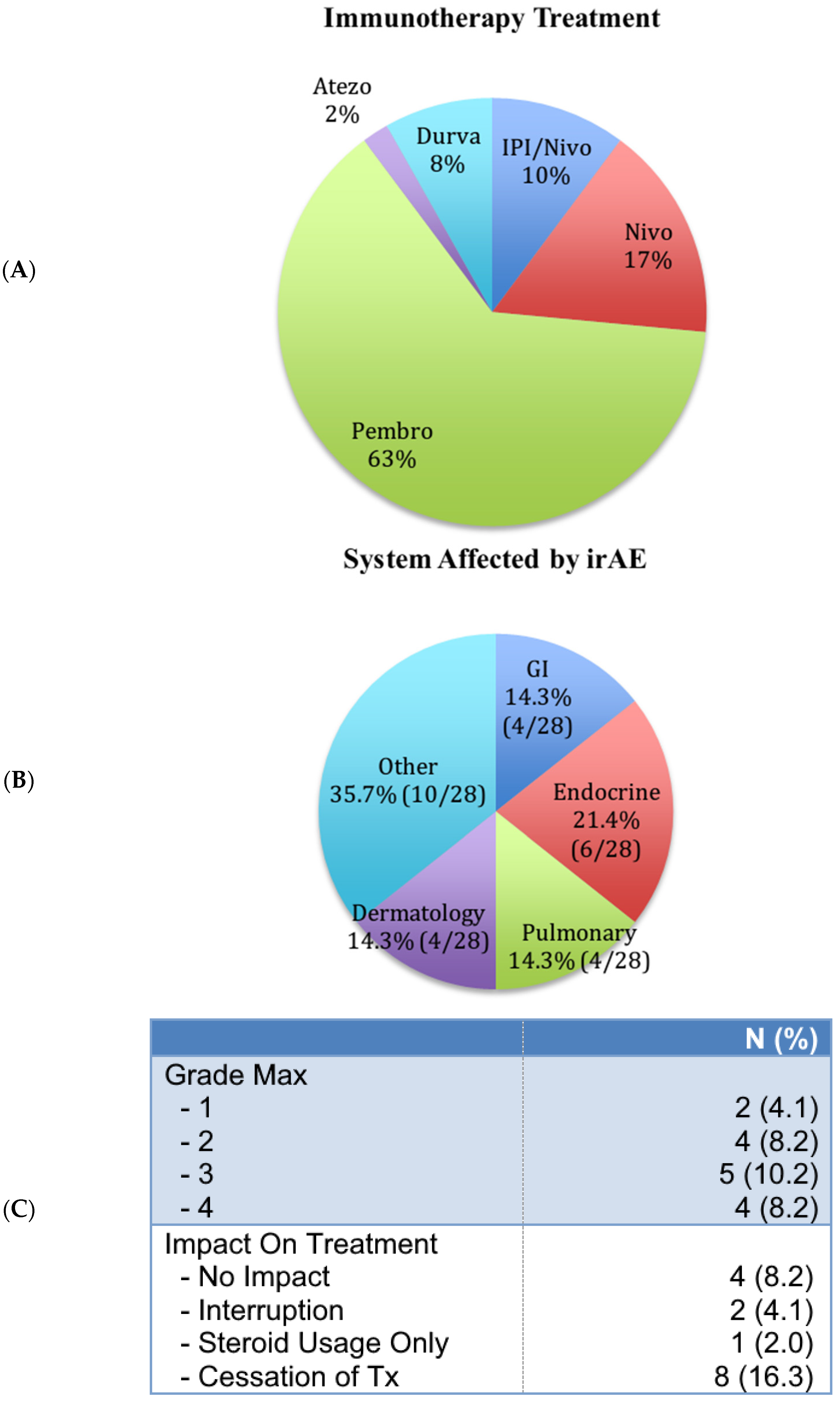
3.3. ICI-Related Adverse Events
3.4. Effectiveness of Immunotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Mollica, V.; Santoni, M.; Massari, F. Cancer Immunotherapy: Current and Future Perspectives on a Therapeutic Revolution. J. Clin. Med. 2021, 10, 5246. [Google Scholar] [CrossRef] [PubMed]
- Korman, A.J.; Garrett-Thomson, S.C.; Lonberg, N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat. Rev. Drug Discov. 2022, 21, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.C.; Baines, A.C.; Gong, Y.; Moore, R., Jr.; Pamuk, G.E.; Saber, H.; Subedee, A.; Thompson, M.D.; Xiao, W.; Pazdur, R.; et al. Trends in the approval of cancer therapies by the FDA in the twenty-first century. Nat. Rev. Drug Discov. 2023, 22, 625–640. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Iranzo, P.; Callejo, A.; Assaf, J.D.; Molina, G.; Lopez, D.E.; Garcia-Illescas, D.; Pardo, N.; Navarro, A.; Martinez-Marti, A.; Cedres, S.; et al. Overview of Checkpoint Inhibitors Mechanism of Action: Role of Immune-Related Adverse Events and Their Treatment on Progression of Underlying Cancer. Front. Med. 2022, 9, 875974. [Google Scholar] [CrossRef]
- Waldmann, T.A. Immunotherapy: Past, present and future. Nat. Med. 2003, 9, 269–277. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomized, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Toribio-Vazquez, C.; Gomez Rivas, J.; Yebes, A.; Carrion, D.M.; Quesada-Olarte, J.; Trelles, C.R.; Alvarez-Maestro, M.; van der Poel, H.; Martinez-Pineiro, L. Immunotherapy toxicity. Diagnosis and treatment. Arch. Esp. Urol. 2020, 73, 906–917. [Google Scholar] [PubMed]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef]
- Sosa, A.; Lopez Cadena, E.; Simon Olive, C.; Karachaliou, N.; Rosell, R. Clinical assessment of immune-related adverse events. Ther. Adv. Med. Oncol. 2018, 10, 1758835918764628. [Google Scholar] [CrossRef] [PubMed]
- Pierro, M.; Baldini, C.; Auclin, E.; Vincent, H.; Varga, A.; Martin Romano, P.; Vuagnat, P.; Besse, B.; Planchard, D.; Hollebecque, A.; et al. Predicting Immunotherapy Outcomes in Older Patients with Solid Tumors Using the LIPI Score. Cancers 2022, 14, 5078. [Google Scholar] [CrossRef] [PubMed]
- Muchnik, E.; Loh, K.P.; Strawderman, M.; Magnuson, A.; Mohile, S.G.; Estrah, V.; Maggiore, R.J. Immune Checkpoint Inhibitors in Real-World Treatment of Older Adults with Non-Small Cell Lung Cancer. J. Am. Geriatr. Soc. 2019, 67, 905–912. [Google Scholar] [CrossRef]
- Nie, N.F.; Liu, Z.L.; Feng, M.X.; Liu, L.; Luo, N.; Li, L.; He, Y. Lazarus type response to immunotherapy in three patients with poor performance status and locally advanced NSCLC: A case series and literature review. Ann. Palliat. Med. 2021, 10, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Magee, D.E.; Hird, A.E.; Klaassen, Z.; Sridhar, S.S.; Nam, R.K.; Wallis, C.J.D.; Kulkarni, G.S. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: A systematic review and meta-analysis of randomized clinical trials. Ann. Oncol. 2020, 31, 50–60. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Loupakis, F.; Randon, G.; Raimondi, A.; Salati, M.; Trapani, D.; Pagani, F.; Depetris, I.; Maddalena, G.; Morano, F.; et al. Efficacy and Safety of Immune Checkpoint Inhibitors in Patients with Microsatellite Instability-High End-Stage Cancers and Poor Performance Status Related to High Disease Burden. Oncologist 2020, 25, 803–809. [Google Scholar] [CrossRef]
- Blum, S.M.; Rouhani, S.J.; Sullivan, R.J. Effects of immune-related adverse events (irAEs) and their treatment on antitumor immune responses. Immunol. Rev. 2023, 318, 167–178. [Google Scholar] [CrossRef]
- Ao, G.; de Miguel, M.; Gomes, A.; Liu, R.; Boni, V.; Moreno, I.; Cardenas, J.M.; Cubillo, A.; Ugidos, L.; Calvo, E. Toxicity and antitumor activity of novel agents in elderly patients with cancer included in phase 1 studies. Investig. New Drugs 2021, 39, 1694–1701. [Google Scholar] [CrossRef]
- Marron, T.U.; Ryan, A.E.; Reddy, S.M.; Kaczanowska, S.; Younis, R.H.; Thakkar, D.; Zhang, J.; Bartkowiak, T.; Howard, R.; Anderson, K.G.; et al. Considerations for treatment duration in responders to immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e001901. [Google Scholar] [CrossRef]
- Brands, X.; Haak, B.W.; Klarenbeek, A.M.; Otto, N.A.; Faber, D.R.; Lutter, R.; Scicluna, B.P.; Wiersinga, W.J.; van der Poll, T. Concurrent Immune Suppression and Hyperinflammation in Patients With Community-Acquired Pneumonia. Front. Immunol. 2020, 11, 796. [Google Scholar] [CrossRef]
- Yende, S.; Kellum, J.A.; Talisa, V.B.; Peck Palmer, O.M.; Chang, C.H.; Filbin, M.R.; Shapiro, N.I.; Hou, P.C.; Venkat, A.; LoVecchio, F.; et al. Long-term Host Immune Response Trajectories Among Hospitalized Patients with Sepsis. JAMA Netw. Open 2019, 2, e198686. [Google Scholar] [CrossRef]
- Yende, S.; D’Angelo, G.; Kellum, J.A.; Weissfeld, L.; Fine, J.; Welch, R.D.; Kong, L.; Carter, M.; Angus, D.C.; Gen, I.M.S.I. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am. J. Respir. Crit. Care Med. 2008, 177, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litiere, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Latif, M.F.; Farooq, A.; Tirmazy, S.H.; AlShahrani, S.; Bashir, S.; Bukhari, N. Performance Status Assessment by Using ECOG (Eastern Cooperative Oncology Group) Score for Cancer Patients by Oncology Healthcare Professionals. Case Rep. Oncol. 2019, 12, 728–736. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute (U.S.). Common Terminology Criteria for Adverse Events (CTCAE); U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute: Bethesda, MD, USA, 2009; 194p. [Google Scholar]
- Nasser, N.J.; Gorenberg, M.; Agbarya, A. First line Immunotherapy for Non-Small Cell Lung Cancer. Pharmaceuticals 2020, 13, 373. [Google Scholar] [CrossRef]
- Grivas, P.; Plimack, E.R.; Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Bellmunt, J.; Powles, T.; Hahn, N.M.; de Wit, R.; Bajorin, D.F.; et al. Pembrolizumab as First-line Therapy in Cisplatin-ineligible Advanced Urothelial Cancer (KEYNOTE-052): Outcomes in Older Patients by Age and Performance Status. Eur. Urol. Oncol. 2020, 3, 351–359. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Mazza, C.; Escudier, B.; Albiges, L. Nivolumab in renal cell carcinoma: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2017, 9, 171–181. [Google Scholar] [CrossRef]
- Sacchi de Camargo Correia, G.; Pai, T.; Li, S.; Connor, D.; Zhao, Y.; Lou, Y.; Manochakian, R. Immune-Related Adverse Events in Patients with Lung Cancer. Curr. Oncol. Rep. 2023, 25, 1259–1275. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zheng, Y.; Wang, P.P.; Ding, Z.Y. Toxicities of Immunotherapy for Small Cell Lung Cancer. Front. Oncol. 2021, 11, 603658. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Y.; Sun, H.; Ye, L.; Zeng, F.; Chen, X.; Deng, G. The Prognostic Significance of Baseline Neutrophil-to-Lymphocyte Ratio in Melanoma Patients Receiving Immunotherapy. J. Immunother. 2022, 45, 43–50. [Google Scholar] [CrossRef]
- Popovic, A.; Petkovic, I.; Dimitrijevic, A.; Jovic, A. Prognostic Value of Lactate Dehydrogenase in Patients with Melanoma Treated with Pembrolizumab. Acta Dermatovenerol. Croat 2023, 31, 86–91. [Google Scholar]
- Wei, Y.; Xu, J.; Huang, X.; Xie, S.; Lin, P.; Wang, C.; Guo, Y.; Zou, S.; Zhao, Z.; Wen, W.; et al. C-reactive protein and lactate dehydrogenase serum levels potentially predict the response to checkpoint inhibitors in patients with advanced non-small cell lung cancer. J. Thorac. Dis. 2023, 15, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, Z.; Zhuang, Q.; Fan, M.; Ding, T.; Lu, H.; He, X. Prognostic Value of Serum Lactate Dehydrogenase in Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166482. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | N (%) |
|---|---|
| Sex | |
| 21 (42.9) |
| 28 (57.1) |
| Age | |
| 64 (37–85) |
| 64.4 ± 10 |
| 37–85 |
| Cancer type | |
| 32 (65.3) |
| 7 (14.3) |
| 5 (10.2) |
| 2 (4.1) |
| 1 (2.0) |
| 2 (4.1) |
| Stage | |
| 7 (14.3) |
| 42 (85.7) |
| ECOG | |
| 4 (8.2) |
| 17 (34.7) |
| 14 (28.6) |
| 9 (18.4) |
| 1 (2.0) |
| 4 (8.2) |
| BMI | |
| 3 (6.1) |
| 23 (46.9) |
| 14 (28.6) |
| 8 (16.3) |
| 0 (0) |
| 1 (2.0) |
| Weight loss (≥10% in 6 months) | |
| 12 (24.5) |
| 12 (24.5) |
| 25 (51) |
| Prior Treatment | N (%) |
|---|---|
| Chemotherapy | |
| 16 (32.7) |
| 33 (67.3) |
| Radiation | |
| 20 (40.8) |
| 29 (59.2) |
| Surgery | |
| 26 (53.1) |
| 23 (46.9) |
| Variable | HR [IC 95%] | p-Value |
|---|---|---|
| Ratio N/L | 1.035 [0.985–1.088] | 0.168 |
| CRP (mg/L) | 1.002 [0.998–1.007] | 0.302 |
| Albumin (g/L) | 0.917 [0.852–0.987] | 0.021 |
| LDH (U/L) | 2.224 [1.469–3.367] | <0.001 |
| ECOG | ||
| 0 | Ref. | - |
| 1 | 1.057 [0.231–4.836] | 0.943 |
| 2 | 3.766 [0.845–16.787] | 0.082 |
| 3–4 | 5.666 [1.207–26.594] | 0.028 |
| Number of cycles | ||
| 1–3 | Ref. | - |
| 4–6 | 0.335 [0.152–0.738] | 0.007 |
| >6 | 0.040 [0.009–0.186] | <0.001 |
| Line of treatment | ||
| 1 | Ref. | - |
| ≥2 | 2.603 [1.174–5.769] | 0.019 |
| Weight loss (≥10% in 6 months) | 2.007 [0.803–5.014] | 0.136 |
| Variable | HR [IC 95%] | p-Value |
|---|---|---|
| Ratio N/L | 1.070 [1.014–1.128] | 0.013 |
| CRP (mg/L) | 1.002 [0.996–1.009] | 0.442 |
| Albumin (g/L) | 0.971 [0.872–1.082] | 0.598 |
| LDH (U/L) | 19.128 [0.031–11674.733] | 0.367 |
| ECOG | ||
| 0 | Ref. | - |
| 1 | 0.833 [0.230–3.014] | 0.781 |
| 2 | 1.391 [0.307–6.300] | 0.668 |
| 3–4 | 4.136 [0.867–19.733] | 0.075 |
| Number of cycles | ||
| 1–3 | Ref. | - |
| 4–6 | 0.477 [0.193–1.178] | 0.109 |
| >6 | 0.123 [0.039–0.384] | <0.001 |
| Line of treatment | ||
| 1 | Ref. | - |
| ≥2 | 2.486 [0.719–8.595] | 0.150 |
| Weight loss (≥10% within 6 months) | 2.849 [0.865–9.382] | 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajadurai, C.V.; Gagnon, G.; Allard, C.; Malick, M.; Pavic, M. Evaluating the Efficacy of Immunotherapy in Fragile Hospitalized Patients. Curr. Oncol. 2024, 31, 7040-7050. https://doi.org/10.3390/curroncol31110518
Rajadurai CV, Gagnon G, Allard C, Malick M, Pavic M. Evaluating the Efficacy of Immunotherapy in Fragile Hospitalized Patients. Current Oncology. 2024; 31(11):7040-7050. https://doi.org/10.3390/curroncol31110518
Chicago/Turabian StyleRajadurai, Charles Vincent, Guillaume Gagnon, Catherine Allard, Mandy Malick, and Michel Pavic. 2024. "Evaluating the Efficacy of Immunotherapy in Fragile Hospitalized Patients" Current Oncology 31, no. 11: 7040-7050. https://doi.org/10.3390/curroncol31110518
APA StyleRajadurai, C. V., Gagnon, G., Allard, C., Malick, M., & Pavic, M. (2024). Evaluating the Efficacy of Immunotherapy in Fragile Hospitalized Patients. Current Oncology, 31(11), 7040-7050. https://doi.org/10.3390/curroncol31110518






