Dysregulation of Cholesterol Homeostasis in Ovarian Cancer
Abstract
:1. Introduction
2. Physiology of Cholesterol Metabolism
3. Cholesterol Metabolism Dysregulation in Cancer
3.1. The Role of Aberrant Cholesterol Metabolism in Ovarian Cancer Proliferation
3.2. The Role of Aberrant Cholesterol Metabolism in Ovarian Cancer Metastasis
3.3. The Role of Aberrant Cholesterol Metabolism in Ovarian Cancer Drug Resistance
4. Potential Target Pathways in Cholesterol Metabolism
5. Repurposing Hypocholesterolemic Drugs as Anti-Cancer Agents in Ovarian Cancer
6. The Impact of Aberrant Lipid Metabolism on Steroid Hormones in Ovarian Cancer
7. The Role of Aberrant Synthesis of Bile Acids in Ovarian Cancer
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Devouassoux-Shisheboran, M.; Genestie, C. Pathobiology of Ovarian Carcinomas. Chin. J. Cancer 2015, 34, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian Cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shan, X.; Dong, H.; Li, M.; Yue, Y. Prediction for 2-Year Mortality of Metastatic Ovarian Cancer Patients Based on Surveillance, Epidemiology, and End Results Database. Front. Surg. 2022, 9, 974536. [Google Scholar] [CrossRef] [PubMed]
- Desai, A. Epithelial Ovarian Cancer: An Overview. World J. Transl. Med. 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Barnes, B.M.; Nelson, L.; Tighe, A.; Burghel, G.J.; Lin, I.-H.; Desai, S.; McGrail, J.C.; Morgan, R.D.; Taylor, S.S. Distinct Transcriptional Programs Stratify Ovarian Cancer Cell Lines into the Five Major Histological Subtypes. Genome Med. 2021, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Lisio, M.-A.; Fu, L.; Goyeneche, A.; Gao, Z.-H.; Telleria, C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef] [PubMed]
- Tania, M.; Khan, M.A.; Song, Y. Association of Lipid Metabolism with Ovarian Cancer. Curr. Oncol. 2010, 17, 6–11. [Google Scholar] [CrossRef]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid Metabolism in Cancer Progression and Therapeutic Strategies. MedComm 2021, 2, 27–59. [Google Scholar] [CrossRef]
- Chaudhry, S.; Thomas, S.N.; Simmons, G.E., Jr. Targeting Lipid Metabolism in the Treatment of Ovarian Cancer. Oncotarget 2022, 13, 768–783. [Google Scholar] [CrossRef]
- Rada, M.; Krzywon, L.; Kapelanski-Lamoureux, A.; Petrillo, S.; Reynolds, A.R.; Lazaris, A.; Seidah, N.; Metrakos, P. High Levels of Serum Cholesterol Positively Correlate with the Risk of the Development of Vessel Co-Opting Tumours in Colorectal Cancer Liver Metastases. medRxiv 2022. [Google Scholar] [CrossRef]
- Cox, R.A.; García-Palmieri, M.R. Cholesterol, Triglycerides, and Associated Lipoproteins. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H., Ed.; Butterworth: Boston, MA, USA, 1990; ISBN 040990077X. [Google Scholar]
- Huff, T.; Boyd, B.; Jialal, I. Physiology, Cholesterol; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Cedó, L.; Metso, J.; Santos, D.; García-León, A.; Plana, N.; Sabate-Soler, S.; Rotllan, N.; Rivas-Urbina, A.; Méndez-Lara, K.A.; Tondo, M.; et al. LDL Receptor Regulates the Reverse Transport of Macrophage-Derived Unesterified Cholesterol via Concerted Action of the HDL-LDL Axis: Insight from Mouse Models. Circ. Res. 2020, 127, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, J.; Bull, C.J.; Vincent, E.E.; Robinson, J.; Walther, A.; Smith, G.D.; Lewis, S.J.; Relton, C.L.; Martin, R.M. Association Between Genetically Proxied Inhibition of HMG-CoA Reductase and Epithelial Ovarian Cancer. JAMA 2020, 323, 646. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhang, W.; Li, S.; Yang, H. The Role of Cholesterol Metabolism in Cancer. Am. J. Cancer Res. 2019, 9, 219–227. [Google Scholar] [PubMed]
- Halimi, H.; Farjadian, S. Cholesterol: An Important Actor on the Cancer Immune Scene. Front. Immunol. 2022, 13, 1057546. [Google Scholar] [CrossRef]
- Jung, S.; Kang, D.; Guallar, E.; Yu, J.; Lee, J.; Kim, S.; Nam, S.; Cho, J.; Lee, S. Impact of Serum Lipid on Breast Cancer Recurrence. J. Clin. Med. 2020, 9, 2846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-L.; Zhu, W.-W.; Wang, S.-H.; Gao, C.; Pan, J.-J.; Du, Z.-G.; Lu, L.; Jia, H.-L.; Dong, Q.-Z.; Chen, J.-H.; et al. Organ-Specific Cholesterol Metabolic Aberration Fuels Liver Metastasis of Colorectal Cancer. Theranostics 2021, 11, 6560–6572. [Google Scholar] [CrossRef]
- Xia, L.; Ding, S.; Wang, X.; Zhang, X.; Zhu, L.; Zhang, H.; Li, H. Advances in Ovarian Cancer Treatment Using a Combination of Statins with Other Drugs. Front. Pharmacol. 2023, 13, 1048484. [Google Scholar] [CrossRef]
- Tulloch, J.C.; Antczak, A.A.; Wilkes, J.W. The application of decision analysis to evaluate the need for extraction of asymptomatic third molars. J. Oral Maxillofac. Surg. 1987, 45, 855–863. [Google Scholar] [CrossRef]
- Wang, K.-H.; Liu, C.-H.; Ding, D.-C. Statins as Repurposed Drugs in Gynecological Cancer: A Review. Int. J. Mol. Sci. 2022, 23, 13937. [Google Scholar] [CrossRef]
- He, J.; Siu, M.K.Y.; Ngan, H.Y.S.; Chan, K.K.L. Aberrant Cholesterol Metabolism in Ovarian Cancer: Identification of Novel Therapeutic Targets. Front. Oncol. 2021, 11, 738177. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, L.; Lu, Y.; Jiang, F.; Yang, X.-A. SREBP2 Contributes to Cisplatin Resistance in Ovarian Cancer Cells. Exp. Biol. Med. 2018, 243, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Alford, S.H.; Rattan, R.; Diaz, M.; Munkarah, A.R. Association of High-Density Lipoprotein Cholesterol with Ovarian Cancer Diagnosis. Gynecol. Oncol. 2015, 137, 182. [Google Scholar] [CrossRef]
- Froelich, W. The Role of Lipophilic Statins in Reducing Epithelial Ovarian Cancer. Oncol. Times 2020, 42, 34. [Google Scholar] [CrossRef]
- Craig, M.; Yarrarapu, S.N.S.; Dimri, M. Biochemistry, Cholesterol; StatePearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Giacomini, I.; Gianfanti, F.; Desbats, M.A.; Orso, G.; Berretta, M.; Prayer-Galetti, T.; Ragazzi, E.; Cocetta, V. Cholesterol Metabolic Reprogramming in Cancer and Its Pharmacological Modulation as Therapeutic Strategy. Front. Oncol. 2021, 11, 682911. [Google Scholar] [CrossRef]
- Brody, T. (Ed.) Lipids. In Nutritional Biochemistry; Academic Press: San Diego, CA, USA, 1999; pp. 311–378. [Google Scholar]
- Kenneth, R.; Feingold, M. Introduction to Lipids and Lipoproteins. Feingold, K.R., Anawalt, B., Blackman, M.R., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK305896/ (accessed on 14 August 2023).
- Rahmany, S.; Jialal, I. Biochemistry, Chylomicron; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Guerra, B.; Recio, C.; Aranda-Tavío, H.; Guerra-Rodríguez, M.; García-Castellano, J.M.; Fernández-Pérez, L. The Mevalonate Pathway, a Metabolic Target in Cancer Therapy. Front. Oncol. 2021, 11, 626971. [Google Scholar] [CrossRef] [PubMed]
- Sitaula, S.; Burris, T.P. Cholesterol and Other Steroids. In Encyclopedia of Cell Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 173–179. [Google Scholar]
- Shelness, G.S.; Sellers, J.A. Very-Low-Density Lipoprotein Assembly and Secretion. Curr. Opin. Lipidol. 2001, 12, 151–157. [Google Scholar] [CrossRef]
- Xue, L.; Qi, H.; Zhang, H.; Ding, L.; Huang, Q.; Zhao, D.; Wu, B.J.; Li, X. Targeting SREBP-2-Regulated Mevalonate Metabolism for Cancer Therapy. Front. Oncol. 2020, 10, 1510. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.-H.; Im, S.-S. The Cellular Function of SCAP in Metabolic Signaling. Exp. Mol. Med. 2020, 52, 724–729. [Google Scholar] [CrossRef]
- Wróblewska, M. The origin and metabolism of a nascent pre-β high density lipoprotein involved in cellular cholesterol efflux. Acta Biochim. Pol. 2011, 58, 275–285. [Google Scholar] [CrossRef]
- Duval, C.; Touche, V.; Tailleux, A.; Fruchart, J.-C.; Fievet, C.; Clavey, V.; Staels, B.; Lestavel, S. Niemann–Pick C1 like 1 gene expression is down-regulated by LXR activators in the intestine. Biochem. Biophys. Res. Commun. 2006, 340, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Widenmaier, S.B.; Snyder, N.A.; Nguyen, T.B.; Arduini, A.; Lee, G.Y.; Arruda, A.P.; Saksi, J.; Bartelt, A.; Hotamisligil, G.S. NRF1 Is an ER Membrane Sensor That Is Central to Cholesterol Homeostasis. Cell 2017, 171, 1094–1109.e15. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bao, X.; Hu, M.; Chang, H.; Jiao, M.; Cheng, J.; Xie, L.; Huang, Q.; Li, F.; Li, C.Y. Inhibition of PCSK9 Potentiates Immune Checkpoint Therapy for Cancer. Nature 2020, 588, 693–698. [Google Scholar] [CrossRef]
- Pobezinskaya, Y.L.; Liu, Z. The role of TRADD in death receptor signaling. Cell Cycle 2012, 11, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Coradini, D. De novo cholesterol biosynthesis: An additional therapeutic target for the treatment of postmenopausal breast cancer with excessive adipose tissue. Explor. Target. Anti-Tumor Ther. 2022, 3, 841–852. [Google Scholar] [CrossRef]
- Maione, F.; Oliaro-Bosso, S.; Meda, C.; Di Nicolantonio, F.; Bussolino, F.; Balliano, G.; Viola, F.; Giraudo, E. The Cholesterol Biosynthesis Enzyme Oxidosqualene Cyclase Is a New Target to Impair Tumour Angiogenesis and Metastasis Dissemination. Sci. Rep. 2015, 5, 9054. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Tu, R.; Liu, H.; Qing, G. Regulation of Cancer Cell Metabolism: Oncogenic MYC in the Driver’s Seat. Signal Transduct. Target. Ther. 2020, 5, 124. [Google Scholar] [CrossRef]
- Zhong, C.; Fan, L.; Yao, F.; Shi, J.; Fang, W.; Zhao, H. HMGCR Is Necessary for the Tumorigenecity of Esophageal Squamous Cell Carcinoma and Is Regulated by Myc. Tumor Biol. 2014, 35, 4123–4129. [Google Scholar] [CrossRef]
- Cheng, C.; Geng, F.; Cheng, X.; Guo, D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun. 2018, 38, 27. [Google Scholar] [CrossRef]
- Haskins, J.W.; Zhang, S.; Means, R.E.; Kelleher, J.K.; Cline, G.W.; Canfrán-Duque, A.; Suárez, Y.; Stern, D.F.; Shi, L.; Kidder, K.; et al. Neuregulin-activated ERBB4 induces the SREBP-2 cholesterol biosynthetic pathway and increases low-density lipoprotein uptake. Sci. Signal. 2015, 8, ra111. [Google Scholar] [CrossRef]
- Bakiri, L.; Hamacher, R.; Graña, O.; Guío-Carrión, A.; Campos-Olivas, R.; Martinez, L.; Dienes, H.P.; Thomsen, M.K.; Hasenfuss, S.C.; Wagner, E.F. Liver carcinogenesis by FOS-dependent inflammation and cholesterol dysregulation. J. Exp. Med. 2017, 214, 1387–1409. [Google Scholar] [CrossRef]
- Luchetti, G.; Sircar, R.; Kong, J.H.; Nachtergaele, S.; Sagner, A.; Byrne, E.F.; Covey, D.F.; Siebold, C.; Rohatgi, R. Cholesterol Activates the G-Protein Coupled Receptor Smoothened to Promote Hedgehog Signaling. eLife 2016, 5, e20304. [Google Scholar] [CrossRef] [PubMed]
- Nallanthighal, S.; Rada, M.; Heiserman, J.P.; Cha, J.; Sage, J.; Zhou, B.; Yang, W.; Hu, Y.; Korgaonkar, C.; Hanos, C.T.; et al. Inhibition of Collagen XI Alpha 1-Induced Fatty Acid Oxidation Triggers Apoptotic Cell Death in Cisplatin-Resistant Ovarian Cancer. Cell Death Dis. 2020, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, V. Omentum a Powerful Biological Source in Regenerative Surgery. Regen. Ther. 2019, 11, 182–191. [Google Scholar] [CrossRef]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid Composition of the Cancer Cell Membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained Proliferation in Cancer: Mechanisms and Novel Therapeutic Targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef] [PubMed]
- Raftopulos, N.L.; Washaya, T.C.; Niederprüm, A.; Egert, A.; Hakeem-Sanni, M.F.; Varney, B.; Aishah, A.; Georgieva, M.L.; Olsson, E.; dos Santos, D.Z.; et al. Prostate Cancer Cell Proliferation Is Influenced by LDL-Cholesterol Availability and Cholesteryl Ester Turnover. Cancer Metab. 2022, 10, 1. [Google Scholar] [CrossRef]
- Dobbin, Z.; Landen, C. The Importance of the PI3K/AKT/MTOR Pathway in the Progression of Ovarian Cancer. Int. J. Mol. Sci. 2013, 14, 8213–8227. [Google Scholar] [CrossRef]
- Long, J.; Zhang, C.-J.; Zhu, N.; Du, K.; Yin, Y.-F.; Tan, X.; Liao, D.-F.; Qin, L. Lipid Metabolism and Carcinogenesis, Cancer Development. Am. J. Cancer Res. 2018, 8, 778–791. [Google Scholar]
- Paplomata, E.; O’Regan, R. The PI3K/AKT/MTOR Pathway in Breast Cancer: Targets, Trials and Biomarkers. Ther. Adv. Med. Oncol. 2014, 6, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Ayyagari, V.; Li, M.; Pasman, Z.; Wang, X.; Louis, S.; Diaz-Sylvester, P.; Groesch, K.; Wilson, T.; Brard, L. Assessment of the Diagnostic and Prognostic Relevance of ACAT1 and CE Levels in Plasma, Peritoneal Fluid and Tumor Tissue of Epithelial Ovarian Cancer Patients—A Pilot Study. BMC Cancer 2022, 22, 387. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Song, B.; Xu, C. Cholesterol Metabolism in Cancer: Mechanisms and Therapeutic Opportunities. Nat. Metab. 2020, 2, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Seah, S.; Loh, X.; Chan, C.-W.; Hartman, M.; Goh, B.-C.; Lee, S.-C. Simvastatin-Induced Breast Cancer Cell Death and Deactivation of PI3K/Akt and MAPK/ERK Signalling Are Reversed by Metabolic Products of the Mevalonate Pathway. Oncotarget 2016, 7, 2532–2544. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Swamy, S.N.; Devaraj, V.R.; Premalatha, C.S.; Pallavi, V.R.; Sagar, B.C.; Shinde, D.D.; Gawari, R. Metabolic Reprogramming and Lipophagy Mediates Survival of Ascites Derived Metastatic Ovarian Cancer Cells. Asian Pac. J. Cancer Prev. 2022, 23, 1699–1709. [Google Scholar] [CrossRef]
- Zhan, S.; Yung, M.M.H.; Siu, M.K.Y.; Jiao, P.; Ngan, H.Y.S.; Chan, D.W.; Chan, K.K.L. New Insights into Ferroptosis Initiating Therapies (FIT) by Targeting the Rewired Lipid Metabolism in Ovarian Cancer Peritoneal Metastases. Int. J. Mol. Sci. 2022, 23, 15263. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, O.F.; Noory, M.A.; Robertson, G.P. The Role of Cholesterol in Cancer. Cancer Res. 2016, 76, 2063–2070. [Google Scholar] [CrossRef]
- Ulug, E.; Nergiz-Unal, R. Dietary Fatty Acids and CD36-Mediated Cholesterol Homeostasis: Potential Mechanisms. Nutr. Res. Rev. 2021, 34, 64–77. [Google Scholar] [CrossRef]
- Ladanyi, A.; Mukherjee, A.; Kenny, H.A.; Johnson, A.; Mitra, A.K.; Sundaresan, S.; Nieman, K.M.; Pascual, G.; Benitah, S.A.; Montag, A.; et al. Adipocyte-Induced CD36 Expression Drives Ovarian Cancer Progression and Metastasis. Oncogene 2018, 37, 2285–2301. [Google Scholar] [CrossRef]
- Lagace, T.A. PCSK9 and LDLR Degradation. Curr. Opin. Lipidol. 2014, 25, 387–393. [Google Scholar] [CrossRef]
- Jacome Sanz, D.; Raivola, J.; Karvonen, H.; Arjama, M.; Barker, H.; Murumägi, A.; Ungureanu, D. Evaluating Targeted Therapies in Ovarian Cancer Metabolism: Novel Role for PCSK9 and Second Generation MTOR Inhibitors. Cancers 2021, 13, 3727. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Liu, L.; Ma, C.; Li, J.; Du, C.; XU, S.; Han, L.; Li, L.; Wang, X. E-Cadherin Promotes Proliferation of Human Ovarian Cancer Cells in Vitro via Activating MEK/ERK Pathway. Acta Pharmacol. Sin. 2012, 33, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, M.; Dhanasekaran, D.N.; Song, Y.S. Activation of LXRɑ/β by Cholesterol in Malignant Ascites Promotes Chemoresistance in Ovarian Cancer. BMC Cancer 2018, 18, 1232. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, D.; Avolio, R.; Calice, G.; Laezza, C.; Paladino, S.; Navarra, G.; Maddalena, F.; Crispo, F.; Pagano, C.; Bifulco, M.; et al. Cholesterol Homeostasis Modulates Platinum Sensitivity in Human Ovarian Cancer. Cells 2020, 9, 828. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wei, X.; Qiao, S.; Zhang, X.; Li, R.; Hu, S.; Mao, H.; Liu, P. Low Density Lipoprotein Receptor (LDLR) and 3-Hydroxy-3-Methylglutaryl Coenzyme a Reductase (HMGCR) Expression Are Associated with Platinum-Resistance and Prognosis in Ovarian Carcinoma Patients. Cancer Manag. Res. 2021, 13, 9015–9024. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Ma, L.; Baek, A.E.; Vardanyan, A.; Vembar, V.; Chen, J.J.; Nelson, A.T.; Burdette, J.E.; Nelson, E.R. Host CYP27A1 Expression Is Essential for Ovarian Cancer Progression. Endocr. Relat. Cancer 2019, 26, 659–675. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Jia, R. Role of de Novo Cholesterol Synthesis Enzymes in Cancer. J. Cancer 2020, 11, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Madison, B.B. Srebp2: A Master Regulator of Sterol and Fatty Acid Synthesis. J. Lipid Res. 2016, 57, 333–335. [Google Scholar] [CrossRef]
- Liu, S.; Jing, F.; Yu, C.; Gao, L.; Qin, Y.; Zhao, J. AICAR-Induced Activation of AMPK Inhibits TSH/SREBP-2/HMGCR Pathway in Liver. PLoS ONE 2015, 10, e0124951. [Google Scholar] [CrossRef]
- Kostopoulou, F.; Gkretsi, V.; Malizos, K.N.; Iliopoulos, D.; Oikonomou, P.; Poultsides, L.; Tsezou, A. Central Role of SREBP-2 in the Pathogenesis of Osteoarthritis. PLoS ONE 2012, 7, e35753. [Google Scholar] [CrossRef]
- Kumari, A. Cholesterol Synthesis. In Sweet Biochemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 27–31. [Google Scholar]
- Bjarnadottir, O.; Feldt, M.; Inasu, M.; Bendahl, P.-O.; Elebro, K.; Kimbung, S.; Borgquist, S. Statin Use, HMGCR Expression, and Breast Cancer Survival—The Malmö Diet and Cancer Study. Sci. Rep. 2020, 10, 558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, L.; Nguyen, D.; Lu, H. TP53 Mutations in Epithelial Ovarian Cancer. Transl. Cancer Res. 2016, 5, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Betters, J.L.; Yu, L. Niemann-Pick C1-Like 1 (NPC1L1) Protein in Intestinal and Hepatic Cholesterol Transport. Annu. Rev. Physiol. 2011, 73, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zeng, J.; Liu, W.; Meng, J.; Wang, C.; Shi, L.; Yang, S.; Chang, J.; Xing, D. The Role of NPC1L1 in Cancer. Front. Pharmacol. 2022, 13, 956619. [Google Scholar] [CrossRef] [PubMed]
- Lánczky, A.; Győrffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (Kmplot): Development and Implementation. J. Med. Internet Res. 2021, 23, e27633. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Cholesterol Lowering Drugs. In Endotext [Internet]; Feingold, K., Anawalt, B., Blackman, M., Boyce, A., Chrousos, G., Corpas, E., Herder, W., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Wang, Q.; Zhi, Z.; Han, H.; Zhao, Q.; Wang, X.; Cao, S.; Zhao, J. Statin Use Improves the Prognosis of Ovarian Cancer: An Updated and Comprehensive Meta-analysis. Oncol. Lett. 2022, 25, 65. [Google Scholar] [CrossRef] [PubMed]
- Majidi, A.; Na, R.; Jordan, S.J.; De Fazio, A.; Webb, P.M. Statin Use and Survival Following a Diagnosis of Ovarian Cancer: A Prospective Observational Study. Int. J. Cancer 2021, 148, 1608–1615. [Google Scholar] [CrossRef]
- Feng, J.-L.; Dixon-Suen, S.C.; Jordan, S.J.; Webb, P.M. Statin use and survival among women with ovarian cancer: An Australian national data-linkage study. Br. J. Cancer 2021, 125, 766–771. [Google Scholar] [CrossRef]
- Akinwunmi, B.; Vitonis, A.F.; Titus, L.; Terry, K.L.; Cramer, D.W. Statin Therapy and Association with Ovarian Cancer Risk in the New England Case Control (NEC) Study. Int. J. Cancer 2019, 144, 991–1000. [Google Scholar] [CrossRef]
- Elmore, R.G.; Ioffe, Y.; Scoles, D.R.; Karlan, B.Y.; Li, A.J. Impact of statin therapy on survival in epithelial ovarian cancer. Gynecol Oncol. 2008, 111, 102–105. [Google Scholar] [CrossRef]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin Use and Reduced Cancer-Related Mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Lavie, O.; Pinchev, M.; Rennert, H.S.; Segev, Y.; Rennert, G. The effect of statins on risk and survival of gynecological malignancies. Gynecol. Oncol. 2013, 130, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Habis, M.; Wroblewski, K.; Bradaric, M.; Ismail, N.; Yamada, S.D.; Litchfield, L.; Lengyel, E.; Romero, I.L. Statin Therapy Is Associated with Improved Survival in Patients with Non-Serous-Papillary Epithelial Ovarian Cancer: A Retrospective Cohort Analysis. PLoS ONE 2014, 9, e104521. [Google Scholar] [CrossRef]
- Bar, D.; Lavie, O.; Stein, N.; Feferkorn, I.; Shai, A. The effect of metabolic comorbidities and commonly used drugs on the prognosis of patients with ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 207, 227–231. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Wang, Q.; Xu, Q.-H.; Yan, L.; Gao, X.-F.; Lu, Y.-H.; Wang, L. Statin as a Combined Therapy for Advanced-Stage Ovarian Cancer: A Propensity Score Matched Analysis. BioMed Res. Int. 2016, 2016, 9125238. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Aragaki, A.K.; Tang, J.Y.; Kurian, A.W.; Manson, J.E.; Chlebowski, R.T.; Simon, M.; Desai, P.; Wassertheil-Smoller, S.; Liu, S.; et al. Statin use and all-cancer survival: Prospective results from the Women’s Health Initiative. Br. J. Cancer 2016, 115, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Couttenier, A.; Lacroix, O.; Vaes, E.; Cardwell, C.; De Schutter, H.; Robert, A. Statin use is associated with improved survival in ovarian cancer: A retrospective population-based study. PLoS ONE 2017, 12, e0189233. [Google Scholar] [CrossRef]
- Verdoodt, F.; Hansen, M.K.; Kjaer, S.K.; Pottegård, A.; Friis, S.; Dehlendorff, C. Statin use and mortality among ovarian cancer patients: A population-based cohort study. Int. J. Cancer 2017, 141, 279–286. [Google Scholar] [CrossRef]
- Vogel, T.J.; Goodman, M.T.; Li, A.J.; Jeon, C.Y. Statin treatment is associated with survival in a nationally representative population of elderly women with epithelial ovarian cancer. Gynecol. Oncol. 2017, 146, 340–345. [Google Scholar] [CrossRef]
- Urpilainen, E.; Marttila, M.; Hautakoski, A.; Arffman, M.; Sund, R.; Ilanne-Parikka, P.; Arima, R.; Kangaskokko, J.; Puistola, U.; Hinkula, M.; et al. Prognosis of ovarian cancer in women with type 2 diabetes using metformin and other forms of antidiabetic medication or statins: A retrospective cohort study. BMC Cancer 2018, 18, 767. [Google Scholar] [CrossRef]
- Harding, B.N.; Delaney, J.A.; Urban, R.R.; Weiss, N.S. Use of Statin Medications Following Diagnosis in Relation to Survival among Women with Ovarian Cancer. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, W.; Ge, C.; Guan, H.; Yang, H.; Zhang, X. Form-stable oxalic acid dihydrate/glycolic acid-based composite PCMs for thermal energy storage. Renew. Energy 2019, 136, 657–663. [Google Scholar] [CrossRef]
- Yu, L.; Hu, Y.; Xu, J.; Qiao, R.; Zhong, H.; Han, B.; Xia, J.; Zhong, R. Multi-target angiogenesis inhibitor combined with PD-1 inhibitors may benefit advanced non-small cell lung cancer patients in late line after failure of EGFR-TKI therapy. Int. J Cancer. 2023, 153, 635–643. [Google Scholar] [CrossRef] [PubMed]
- McKenna, L.; McKay, G.; Fisher, M. Evolocumab. Pract. Diabetes 2017, 34, 329–330a. [Google Scholar] [CrossRef]
- Rada, M.; Reynolds, A.R.; Lazaris, A.; Seidah, N.; Metrakos, P. Inhibition of Proprotein Convertase Subtilisin-like Kexin Type 9 (PCSK9) Potentiates Anti-Angiogenic Therapy in Colorectal Cancer Liver Metastases. bioRxiv 2023. [Google Scholar] [CrossRef]
- Bruckert, E.; Caprio, S.; Wiegman, A.; Charng, M.-J.; Zárate-Morales, C.A.; Baccara-Dinet, M.T.; Manvelian, G.; Ourliac, A.; Scemama, M.; Daniels, S.R. Efficacy and Safety of Alirocumab in Children and Adolescents with Homozygous Familial Hypercholesterolemia: Phase 3, Multinational Open-Label Study. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhou, Z.-S.; Li, N.; Bian, Y.; Wang, Y.-J.; Wang, L.-J.; Li, B.-L.; Song, B.-L. Ezetimibe Blocks the Internalization of NPC1L1 and Cholesterol in Mouse Small Intestine. J. Lipid Res. 2012, 53, 2092–2101. [Google Scholar] [CrossRef]
- Gu, J.; Zhu, N.; Li, H.-F.; Zhang, C.-J.; Gong, Y.-Z.; Liao, D.-F.; Qin, L. Ezetimibe and Cancer: Is There a Connection? Front. Pharmacol. 2022, 13, 831657. [Google Scholar] [CrossRef]
- Solomon, K.R.; Pelton, K.; Boucher, K.; Joo, J.; Tully, C.; Zurakowski, D.; Schaffner, C.P.; Kim, J.; Freeman, M.R. Ezetimibe Is an Inhibitor of Tumor Angiogenesis. Am. J. Pathol. 2009, 174, 1017–1026. [Google Scholar] [CrossRef]
- Miura, K.; Ohnishi, H.; Morimoto, N.; Minami, S.; Ishioka, M.; Watanabe, S.; Tsukui, M.; Takaoka, Y.; Nomoto, H.; Isoda, N.; et al. Ezetimibe Suppresses Development of Liver Tumors by Inhibiting Angiogenesis in Mice Fed a High-Fat Diet. Cancer Sci. 2019, 110, 771–783. [Google Scholar] [CrossRef]
- Nicolle, R.; Blum, Y.; Marisa, L.; Loncle, C.; Gayet, O.; Moutardier, V.; Turrini, O.; Giovannini, M.; Bian, B.; Bigonnet, M.; et al. Pancreatic Adenocarcinoma Therapeutic Targets Revealed by Tumor-Stroma Cross-Talk Analyses in Patient-Derived Xenografts. Cell Rep. 2017, 21, 2458–2470. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sun, J.; Li, M.; Long, Y.; Zhang, D.; Guo, H.; Huang, R.; Yan, J. Oxidized Low-Density Lipoprotein Links Hypercholesterolemia and Bladder Cancer Aggressiveness by Promoting Cancer Stemness. Cancer Res. 2021, 81, 5720–5732. [Google Scholar] [CrossRef] [PubMed]
- Pelton, K.; Coticchia, C.M.; Curatolo, A.S.; Schaffner, C.P.; Zurakowski, D.; Solomon, K.R.; Moses, M.A. Hypercholesterolemia Induces Angiogenesis and Accelerates Growth of Breast Tumors in Vivo. Am. J. Pathol. 2014, 184, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Shin, H.; Wei, X.; Kadegowda, A.K.G.; Chen, R.; Xie, S.K. NPC1L1 Knockout Protects against Colitis-Associated Tumorigenesis in Mice. BMC Cancer 2015, 15, 189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; You, S.; Su, S.; Yeon, A.; Lo, E.M.; Kim, S.; Mohler, J.L.; Freeman, M.R.; Kim, H.L. Cholesterol-Lowering Intervention Decreases MTOR Complex 2 Signaling and Enhances Antitumor Immunity. Clin. Cancer Res. 2022, 28, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Esan, O.; Wierzbicki, A.S. Volanesorsen in the Treatment of Familial Chylomicronemia Syndrome or Hypertriglyceridaemia: Design, Development and Place in Therapy. Drug Des. Devel. Ther. 2020, 14, 2623–2636. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Prado, R.; Perez-Gomez, M.V.; Ortiz, A. Pelacarsen for Lowering Lipoprotein(a): Implications for Patients with Chronic Kidney Disease. Clin. Kidney J. 2020, 13, 753–757. [Google Scholar] [CrossRef]
- Kim, K.; Ginsberg, H.N.; Choi, S.H. New, Novel Lipid-Lowering Agents for Reducing Cardiovascular Risk: Beyond Statins. Diabetes Metab. J. 2022, 46, 517–532. [Google Scholar] [CrossRef]
- Gómora, M.J.; Morales-Vásquez, F.; Pedernera, E.; Perez-Montiel, D.; López-Basave, H.; Villa, A.R.; Hernández-Martínez, A.; Mena, E.; Mendez, C. Sexual Steroid Hormone Receptors Profiles of Ovarian Carcinoma in Mexican Women. Endocr. Connect. 2018, 7, 1006–1012. [Google Scholar] [CrossRef]
- de Almeida Chuffa, L.G.; Lupi-Júnior, L.A.; Costa, A.B.; de Arruda Amorim, J.P.; Seiva, F.R.F. The Role of Sex Hormones and Steroid Receptors on Female Reproductive Cancers. Steroids 2017, 118, 93–108. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Stafford, J.M. Role of Estrogens in the Regulation of Liver Lipid Metabolism. Adv. Exp. Med. Biol. 2017, 1043, 227–256. [Google Scholar] [PubMed]
- Osaku, D.; Oishi, T.; Kawamura, N.; Iida, Y.; Komatsu, H.; Kudoh, A.; Chikumi, J.; Sato, S.; Harada, T. Differential Expression of Estrogen Receptor Subtypes in Ovarian High-grade Serous Carcinoma and Clear Cell Carcinoma. Reprod. Med. Biol. 2021, 20, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shen, Y.; Li, R. Estrogen Synthesis and Signaling Pathways during Aging: From Periphery to Brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Režen, T.; Rozman, D.; Kovács, T.; Kovács, P.; Sipos, A.; Bai, P.; Mikó, E. The Role of Bile Acids in Carcinogenesis. Cell. Mol. Life Sci. 2022, 79, 243. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, M.; Xu, W.; Yu, S. Research Progress of Bile Acids in Cancer. Front. Oncol. 2022, 11, 778258. [Google Scholar] [CrossRef] [PubMed]
- Sipos, A.; Ujlaki, G.; Mikó, E.; Maka, E.; Szabó, J.; Uray, K.; Krasznai, Z.; Bai, P. The Role of the Microbiome in Ovarian Cancer: Mechanistic Insights into Oncobiosis and to Bacterial Metabolite Signaling. Mol. Med. 2021, 27, 33. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, N.; Hua, J.; Powell, M.; Gibb, R.; Mutch, D.; Herzog, T. Novel Cytotoxic Agents from an Unexpected Source: Bile Acids and Ovarian Tumor Apoptosis. Gynecol. Oncol. 2007, 107, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Noel, O.; Nguyen, M.; Sam, L.; Gerhard, G.S. Bile Acids Upregulate BRCA1 and Downregulate Estrogen Receptor 1 Gene Expression in Ovarian Cancer Cells. Eur. J. Cancer Prev. 2018, 27, 553–556. [Google Scholar] [CrossRef]
- Chen, S.; Dai, X.; Gao, Y.; Shen, F.; Ding, J.; Chen, Q. The Positivity of Estrogen Receptor and Progesterone Receptor May Not Be Associated with Metastasis and Recurrence in Epithelial Ovarian Cancer. Sci. Rep. 2017, 7, 16922. [Google Scholar] [CrossRef]
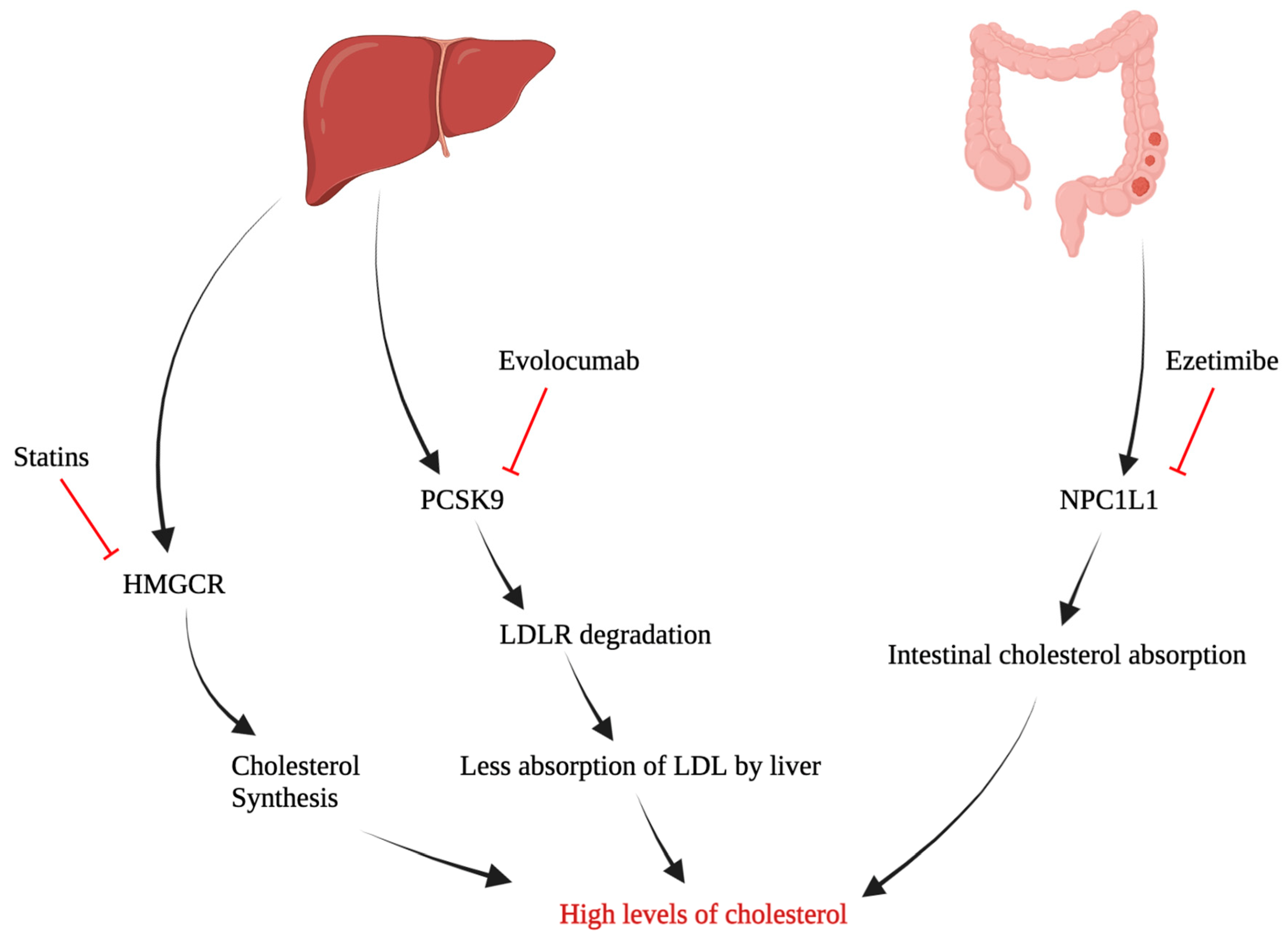

| Drug | Mechanism of Action | Studies | Studies Type | Observation |
|---|---|---|---|---|
| Statins | Blocking hepatic HMGCR, rate limiting step in Cholesterol synthesis | Elmore RG (2008) [89] Nielsen SF (2012) [90] Lavie O (2013) [91] Habis M (2014) [92] Bar D (2016) [93] Chen HY (2016) [94] Wang A (2016) [95] Couttenier A (2017) [96] Verdoodt F (2017) [97] Vogel TJ (2017) [98] Urpilainen E (2018) [99] Harding BN (2019) [100] Feng JL (2021) [87] Hanley GE (2021) [101] Kim DS (2021) [102] Majidi A (2021) [86] | Clinical | Improve Overall Survival time. (HR, 0.79; 95% CI, 0.73–0.85; p < 0.00001) |
| PCSK9 Inhibitor | Inhibit PCSK9 enzyme, ↓ LDLR | Sanz DJ (2021) [68] | Preclinical (OC cell lines) | Impairs cancer cell growth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qusairy, Z.; Gangloff, A.; Leung, S.O.A. Dysregulation of Cholesterol Homeostasis in Ovarian Cancer. Curr. Oncol. 2023, 30, 8386-8400. https://doi.org/10.3390/curroncol30090609
Qusairy Z, Gangloff A, Leung SOA. Dysregulation of Cholesterol Homeostasis in Ovarian Cancer. Current Oncology. 2023; 30(9):8386-8400. https://doi.org/10.3390/curroncol30090609
Chicago/Turabian StyleQusairy, Zahraa, Anne Gangloff, and Shuk On Annie Leung. 2023. "Dysregulation of Cholesterol Homeostasis in Ovarian Cancer" Current Oncology 30, no. 9: 8386-8400. https://doi.org/10.3390/curroncol30090609
APA StyleQusairy, Z., Gangloff, A., & Leung, S. O. A. (2023). Dysregulation of Cholesterol Homeostasis in Ovarian Cancer. Current Oncology, 30(9), 8386-8400. https://doi.org/10.3390/curroncol30090609





