Real-Time Ultrasound-Computed Tomography Fusion with Volume Navigation to Assess Pancreatic Cystic Lesions
Abstract
:1. Introduction
2. Materials and Methods
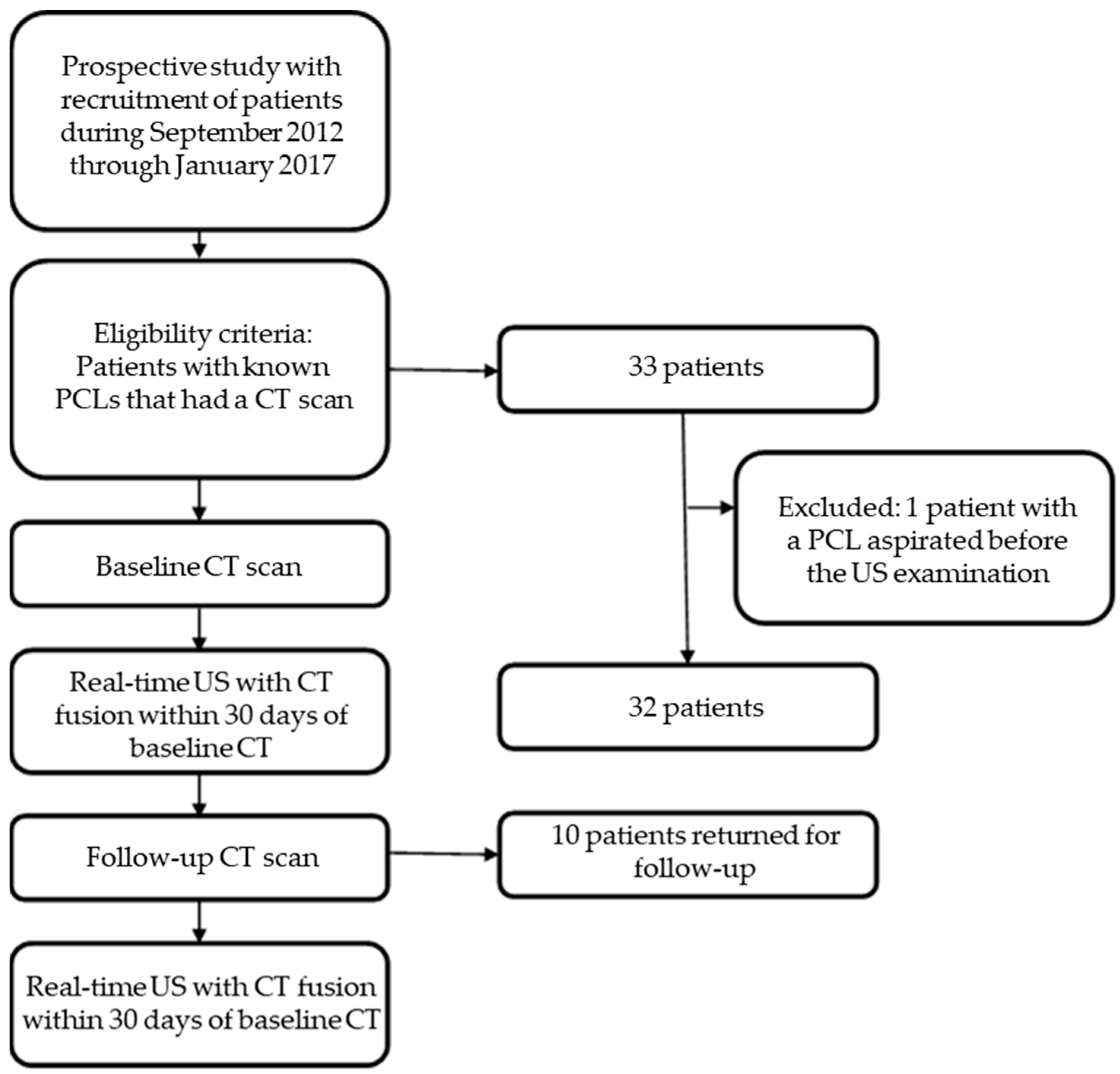
2.1. Patients
2.2. Study Design
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Study Population and PCL Characteristics
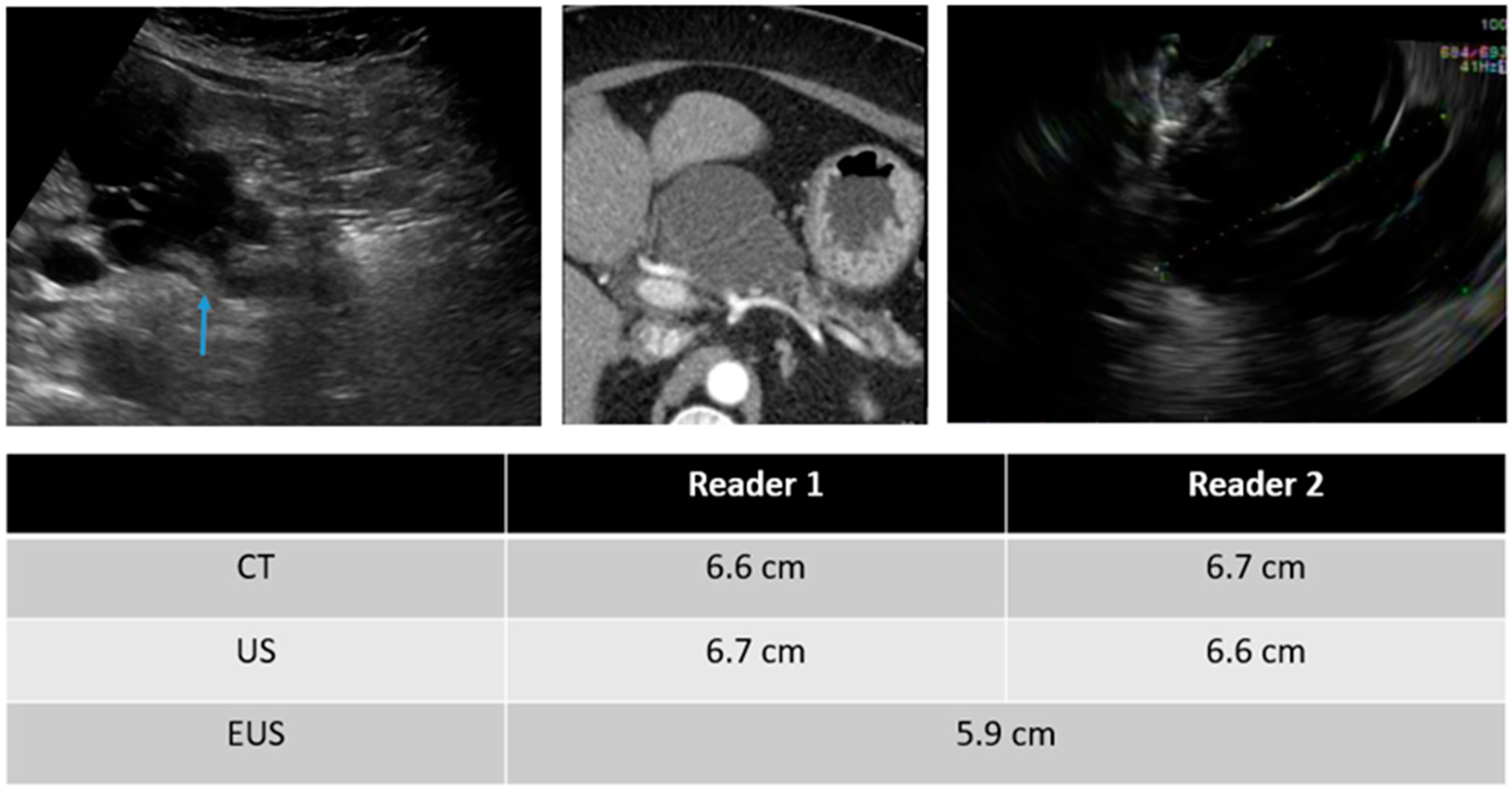
3.2. Detection of the Lesions
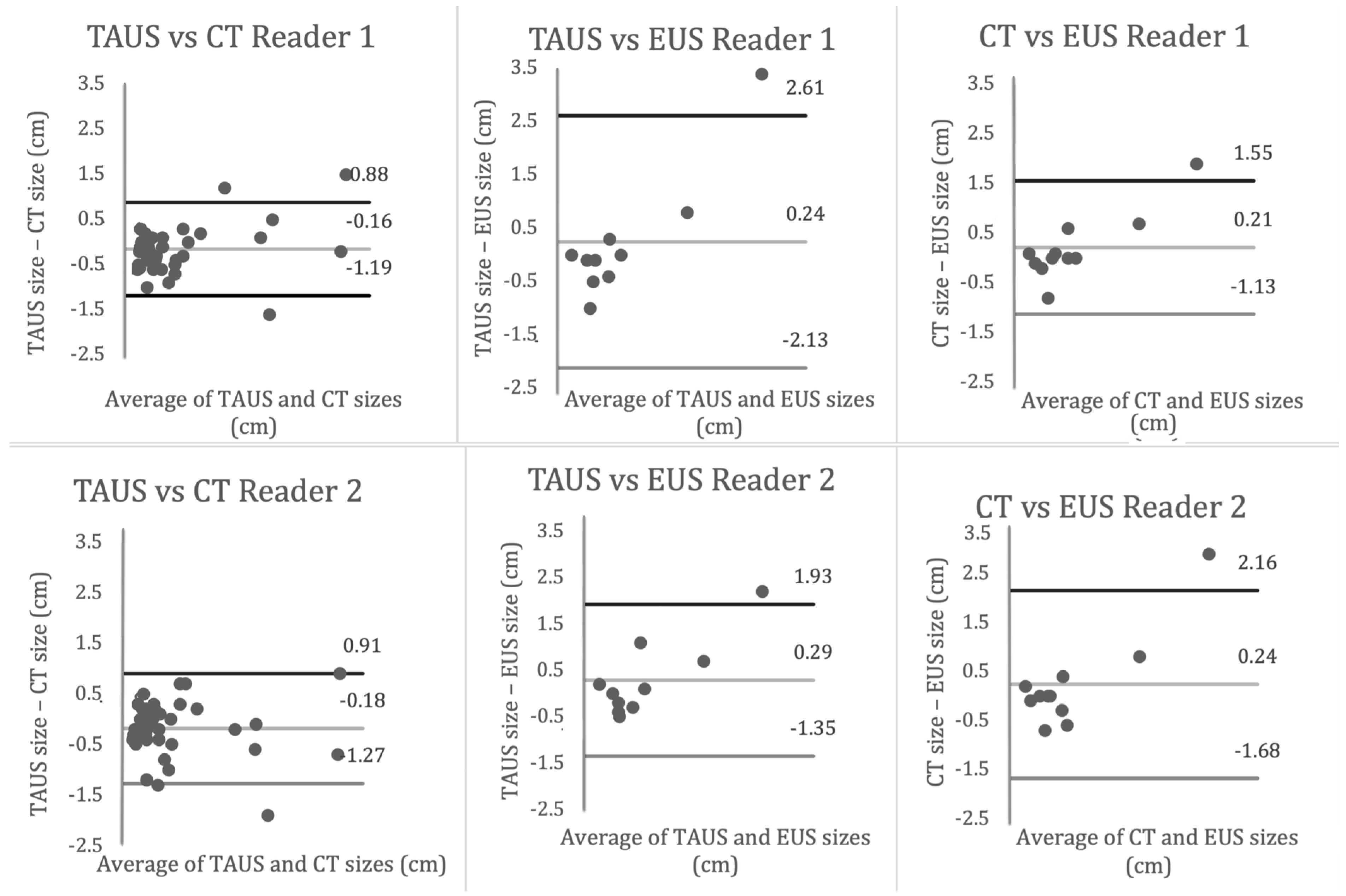
3.3. Inter-Reader Variability
3.4. Inter-Modality Variability
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gardner, T.B.; Glass, L.M.; Smith, K.D.; Ripple, G.H.; Barth, R.J.; Klibansky, D.A.; Colacchio, T.A.; Tsapakos, M.J.; Suriawinata, A.A.; Tsongalis, G.J.; et al. Pancreatic Cyst Prevalence and the Risk of Mucin-Producing Adenocarcinoma in US Adults. Am. J. Gastroenterol. 2013, 108, 1546–1550. [Google Scholar] [CrossRef] [PubMed]
- Zerboni, G.; Signoretti, M.; Crippa, S.; Falconi, M.; Arcidiacono, P.G.; Capurso, G. Systematic review and meta-analysis: Prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology 2018, 19, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Moris, M.; Bridges, M.D.; Pooley, R.A.; Raimondo, M.; Woodward, T.A.; Stauffer, J.A.; Asbun, H.J.; Wallace, M.B. Association Between Advances in High-Resolution Cross-Section Imaging Technologies and Increase in Prevalence of Pancreatic Cysts From 2005 to 2014. Clin. Gastroenterol. Hepatol. 2015, 14, 585–593.e3. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Sekhar, A.; Rofsky, N.M.; Pedrosa, I. Prevalence of Incidental Pancreatic Cysts in the Adult Population on MR Imaging. Am. J. Gastroenterol. 2010, 105, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Van Huijgevoort, N.C.M.; del Chiaro, M.; Wolfgang, C.L.; van Hooft, J.E.; Besselink, M.G. Diagnosis and management of pancreatic cystic neoplasms: Current evidence and guidelines. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Postlewait, L.M.; Ethun, C.G.; McInnis, M.R.; Merchant, N.; Parikh, A.; Idrees, K.; Isom, C.A.; Hawkins, W.; Fields, R.C.; Strand, M.; et al. Association of Preoperative Risk Factors with Malignancy in Pancreatic Mucinous Cystic Neoplasms. JAMA Surg. 2017, 152, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Munigala, S.; Gelrud, A.; Agarwal, B. Risk of pancreatic cancer in patients with pancreatic cyst. Gastrointest. Endosc. 2015, 84, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Megibow, A.J.; Baker, M.E.; Morgan, D.E.; Kamel, I.R.; Sahani, D.V.; Newman, E.; Brugge, W.R.; Berland, L.L.; Pandharipande, P.V. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J. Am. Coll. Radiol. 2017, 14, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Kim, J.H.; Joo, I.; Lee, S.; Choi, S.-Y.; Han, J.K. Transabdominal Ultrasound Detection of Pancreatic Cysts Incidentally Detected at CT, MRI, or Endoscopic Ultrasound. Am. J. Roentgenol. 2018, 210, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.R.M.; Strickland, C.D.; Tamjeedi, B.; Brook, A.; Mortele, K.J.; Brook, O.R.; Kane, R.A.; Siewert, B. Utility of transabdominal ultrasound for surveillance of known pancreatic cystic lesions: Prospective evaluation with MRI as reference standard. Abdom. Imaging 2017, 43, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.H.; Kim, J.H.; Kang, H.-J.; Choi, S.-Y.; Park, Y.S.; Lee, E.S.; Park, H.J. Transabdominal Ultrasound for Follow-Up of Incidentally Detected Low-Risk Pancreatic Cysts: A Prospective Multicenter Study. Am. J. Roentgenol. 2021, 216, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Faccioli, N.; Santi, E.; Foti, G.; D’onofrio, M. Cost-effectiveness analysis of including contrast-enhanced ultrasound in management of pancreatic cystic neoplasms. La Radiol. Medica 2022, 127, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Praveen, S.P.; Srinivasu, P.N.; Shafi, J.; Wozniak, M.; Ijaz, M.F. ResNet-32 and FastAI for diagnoses of ductal carcinoma from 2D tissue slides. Sci. Rep. 2022, 12, 20804. [Google Scholar] [CrossRef]
- Ullah, I.; Ali, F.; Shah, B.; El-Sappagh, S.; Abuhmed, T.; Park, S.H. A deep learning based dual encoder–decoder framework for anatomical structure segmentation in chest X-ray images. Sci. Rep. 2023, 13, 791. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.; Ali, K.; Vyas, S.; Dezsi, K.; Strickland, D.; Basinski, T.; Chen, D.-T.; Jiang, K.; Centeno, B.; Malafa, M.; et al. Comparison of imaging modalities for measuring the diameter of intraductal papillary mucinous neoplasms of the pancreas. Pancreatology 2020, 20, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Maimone, S.; Agrawal, D.; Pollack, M.J.; Wong, R.C.; Willis, J.; Faulx, A.L.; Isenberg, G.A.; Chak, A. Variability in measurements of pancreatic cyst size among EUS, CT, and magnetic resonance imaging modalities. Gastrointest. Endosc. 2010, 71, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Leeds, J.; Nayar, M.; Dawwas, M.; Scott, J.; Anderson, K.; Haugk, B.; Oppong, K. Comparison of endoscopic ultrasound and computed tomography in the assessment of pancreatic cyst size using pathology as the gold standard. Pancreatology 2013, 13, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Aghdassi, A.A.; Schauer, B.; Duscha, D.; Ittermann, T.; Pickartz, T.; Budde, C.; Simon, P.; Moskwa, P.; Kromrey, M.L.; Bülow, R.; et al. Comparability of size measurements of the pancreas in magnetic resonance imaging and transabdominal ultrasound. Clin. Anat. 2019, 33, 431–439. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Value |
|---|---|
| Sex | |
| Male | 17/32 |
| Female | 15/32 |
| Age (y) | |
| Median | 67 |
| Range | 46–85 |
| Patients who presented for follow-up | 10 |
| Location of PCL (no. of lesions) | |
| Head | 17/43 |
| Neck | 5/43 |
| Body | 10/43 |
| Tail | 11/43 |
| PCLs detected by TAUS (no. of lesions) | 40/43 |
| Patients with EUS within 6 months of TAUS (no.) | 13 |
| Reader 1 | Reader 2 | |
|---|---|---|
| Size of PCLs | ||
| <1.5 cm | ||
| TAUS | 23 | 19 |
| CT | 22 | 21 |
| 1.5–2.5 cm | ||
| TAUS | 7 | 11 |
| CT | 7 | 10 |
| >2.5 cm | ||
| TAUS | 14 | 10 |
| CT | 10 | 11 |
| Mean size of detected PCLs (cm) | ||
| TAUS | 2.40 ± 2.65 | 2.41 ± 2.56 |
| CT | 2.45 ± 2.43 | 2.48 ± 2.53 |
| EUS * | 2.73 ± 2.10 | |
| Intra-reader variability in measured size | Reader 1 vs. Reader 2 | |
| TAUS | −0.01 ± 0.39 | |
| CT | −0.03 ± 0.43 | |
| Comparison | Soft-Tissue Component | MPD Dilation | MPD Communication |
|---|---|---|---|
| Inter-reader variability | |||
| TAUS | 0.58 | 0.47 | 0.23 |
| CT | 0.23 | 0.39 | 0.58 |
| Inter-modality variability | |||
| TAUS vs. CT | |||
| Reader 1 | 0.89 | 0.89 | 0.85 |
| Reader 2 | 0.70 | 0.66 | 0.60 |
| TAUS vs. EUS | |||
| Reader 1 | 0.44 | −0.20 | 0.17 |
| Reader 2 | 0.07 | 0.55 | 0 |
| CT vs. EUS | |||
| Reader 1 | 0.62 | −0.19 | 0.27 |
| Reader 2 | 0.13 | 0.21 | −0.10 |
| TAUS vs. CT | TAUS vs. EUS | CT vs. EUS | |
| All PCLs (cm) | |||
| Reader 1 | −0.16 ± 0.53 * | 0.13 ± 1.14 | 0.15 ± 0.65 * |
| Reader 2 | −0.19 ± 0.55 * | 0.18 ± 0.81 | 0.22 ± 0.90 |
| By location (cm) | |||
| Head | |||
| Reader 1 | −0.16 ± 0.62 * | 0.07 ± 0.49 * | 0.14 ± 0.37 * |
| Reader 2 | −0.17 ± 0.60 * | 0.20 ± 0.63 | 0.11 ± 0.46 |
| Neck | |||
| Reader 1 | −0.04 ± 0.18 * | NA | NA |
| Reader 2 | −0.28 ± 0.57 | NA | NA |
| Body | |||
| Reader 1 | −0.37 ± 0.37 | 0.65 ± 1.84 | 0.43 ± 1.00 |
| Reader 2 | −0.21 ± 0.59 | 0.48 ± 1.17 | 0.70 ± 1.48 |
| Tail | |||
| Reader 1 | −0.37 ± 0.23 * | −0.75 ± 0.35 | −0.35 ± 0.64 |
| Reader 2 | −0.13 ± 0.38 * | −0.45 ± 0.07 | −0.35 ± 0.50 |
| By size category (cm) | |||
| <1.5 cm | |||
| Reader 1 | −0.13 ± 0.23 * | −0.38 ± 0.45 | −0.26 ± 0.34 |
| Reader 2 | −0.13 ± 0.38 * | −0.13 ± 0.30 * | −0.06 ± 0.39 * |
| 1.5–2.5 cm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathew, M.; Virarkar, M.; Sun, J.; Thai, K.; Saleh, M.; Menendez-Santos, M.; Bedi, D.; Lee, J.E.; Katz, M.; Kundra, V.; et al. Real-Time Ultrasound-Computed Tomography Fusion with Volume Navigation to Assess Pancreatic Cystic Lesions. Curr. Oncol. 2023, 30, 8375-8385. https://doi.org/10.3390/curroncol30090608
Mathew M, Virarkar M, Sun J, Thai K, Saleh M, Menendez-Santos M, Bedi D, Lee JE, Katz M, Kundra V, et al. Real-Time Ultrasound-Computed Tomography Fusion with Volume Navigation to Assess Pancreatic Cystic Lesions. Current Oncology. 2023; 30(9):8375-8385. https://doi.org/10.3390/curroncol30090608
Chicago/Turabian StyleMathew, Manoj, Mayur Virarkar, Jia Sun, Khoan Thai, Mohammed Saleh, Manuel Menendez-Santos, Deepak Bedi, Jeffrey E. Lee, Matthew Katz, Vikas Kundra, and et al. 2023. "Real-Time Ultrasound-Computed Tomography Fusion with Volume Navigation to Assess Pancreatic Cystic Lesions" Current Oncology 30, no. 9: 8375-8385. https://doi.org/10.3390/curroncol30090608
APA StyleMathew, M., Virarkar, M., Sun, J., Thai, K., Saleh, M., Menendez-Santos, M., Bedi, D., Lee, J. E., Katz, M., Kundra, V., & Bhosale, P. (2023). Real-Time Ultrasound-Computed Tomography Fusion with Volume Navigation to Assess Pancreatic Cystic Lesions. Current Oncology, 30(9), 8375-8385. https://doi.org/10.3390/curroncol30090608





