Trainee Evaluations of Preparedness for Clinical Trials in Medical Oncology—A National Questionnaire
Abstract
1. Introduction
2. Materials and Methods
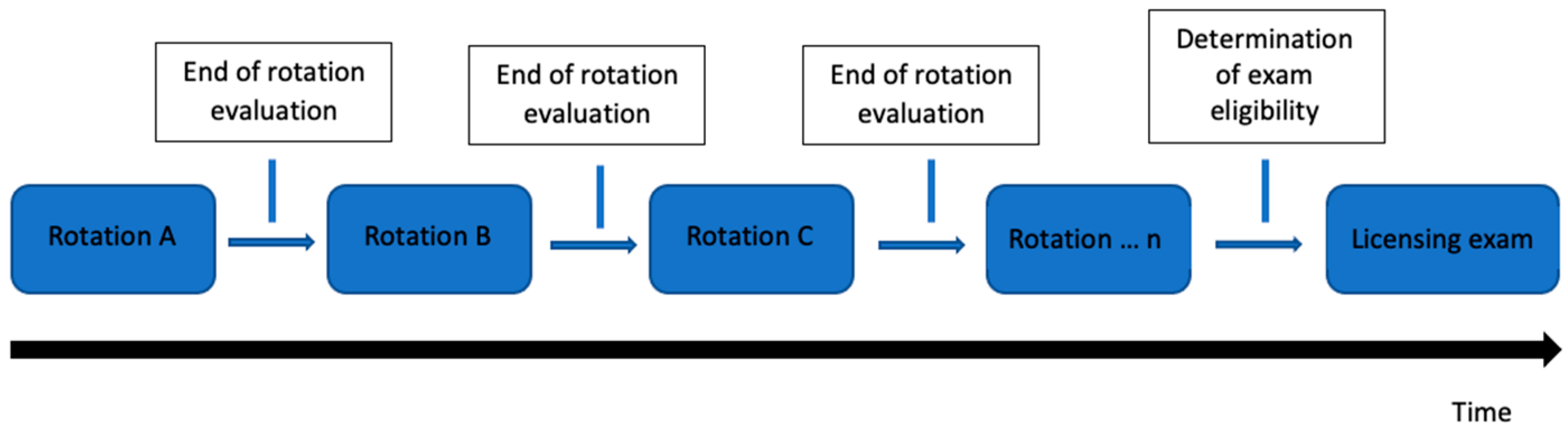
2.1. Study Design
2.2. Questionnaire Development
2.3. Questionnaire Distribution
2.4. Outcomes and Data Analysis
3. Results
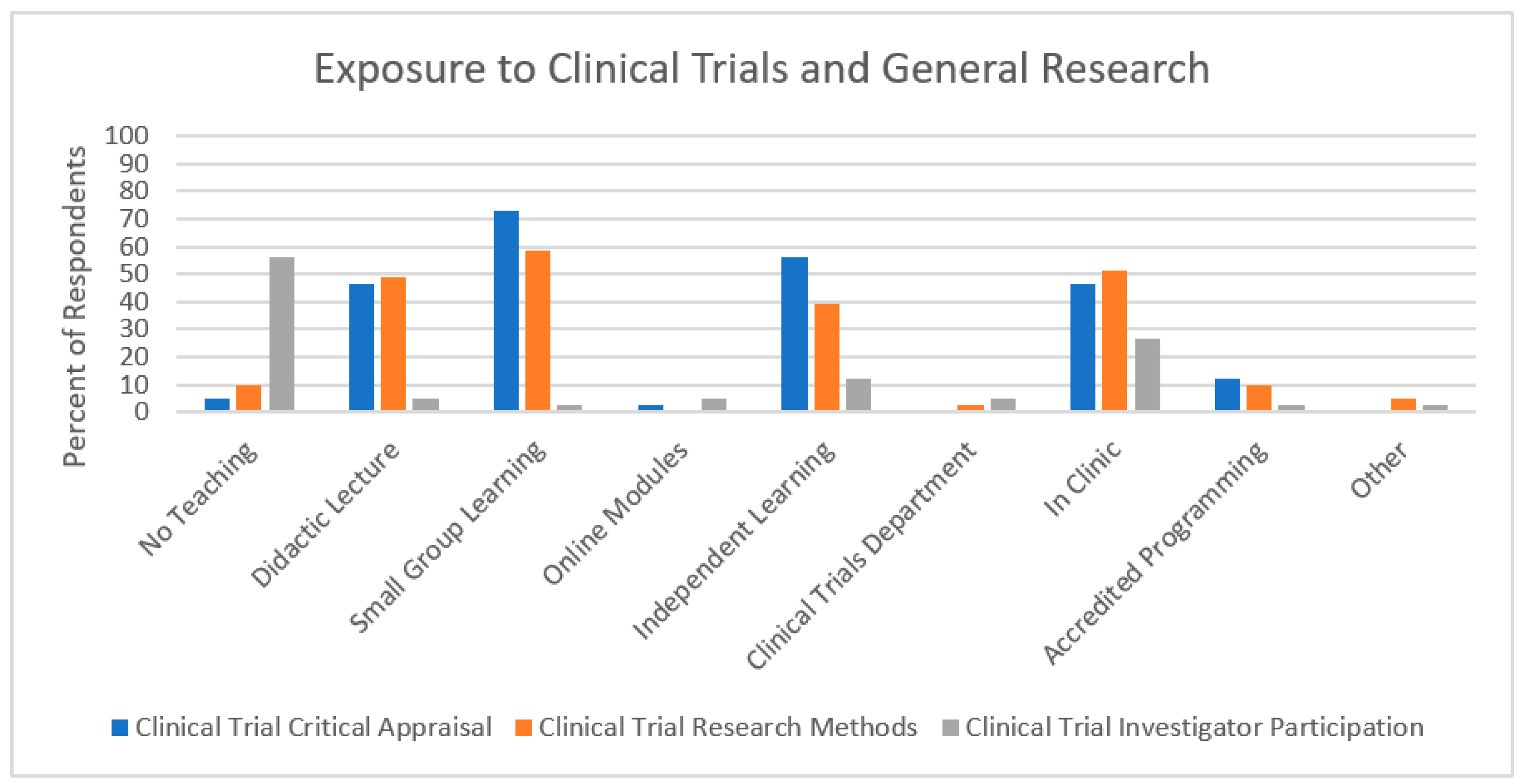
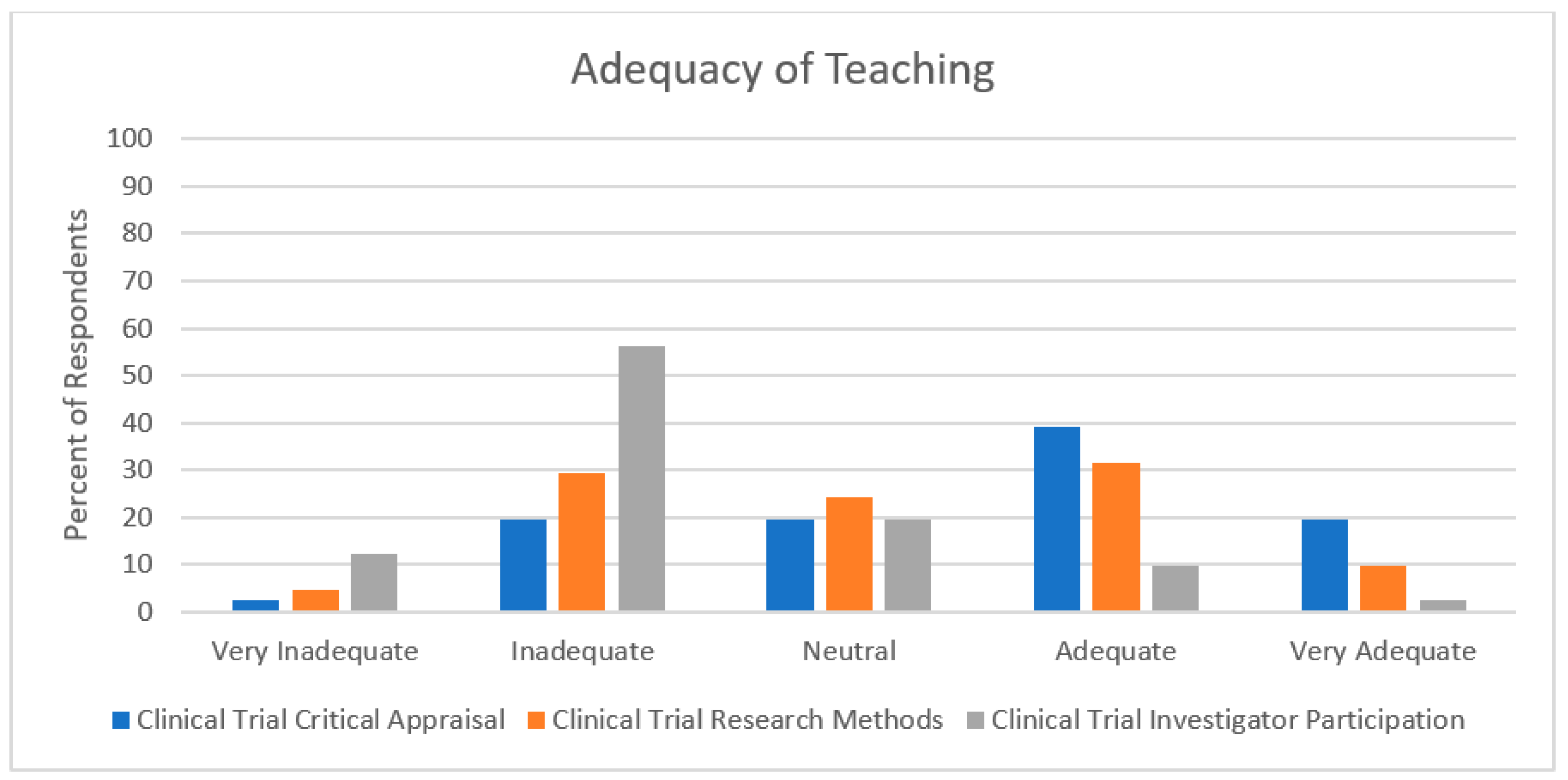
3.1. Exposure to Clinical Trials and General Research
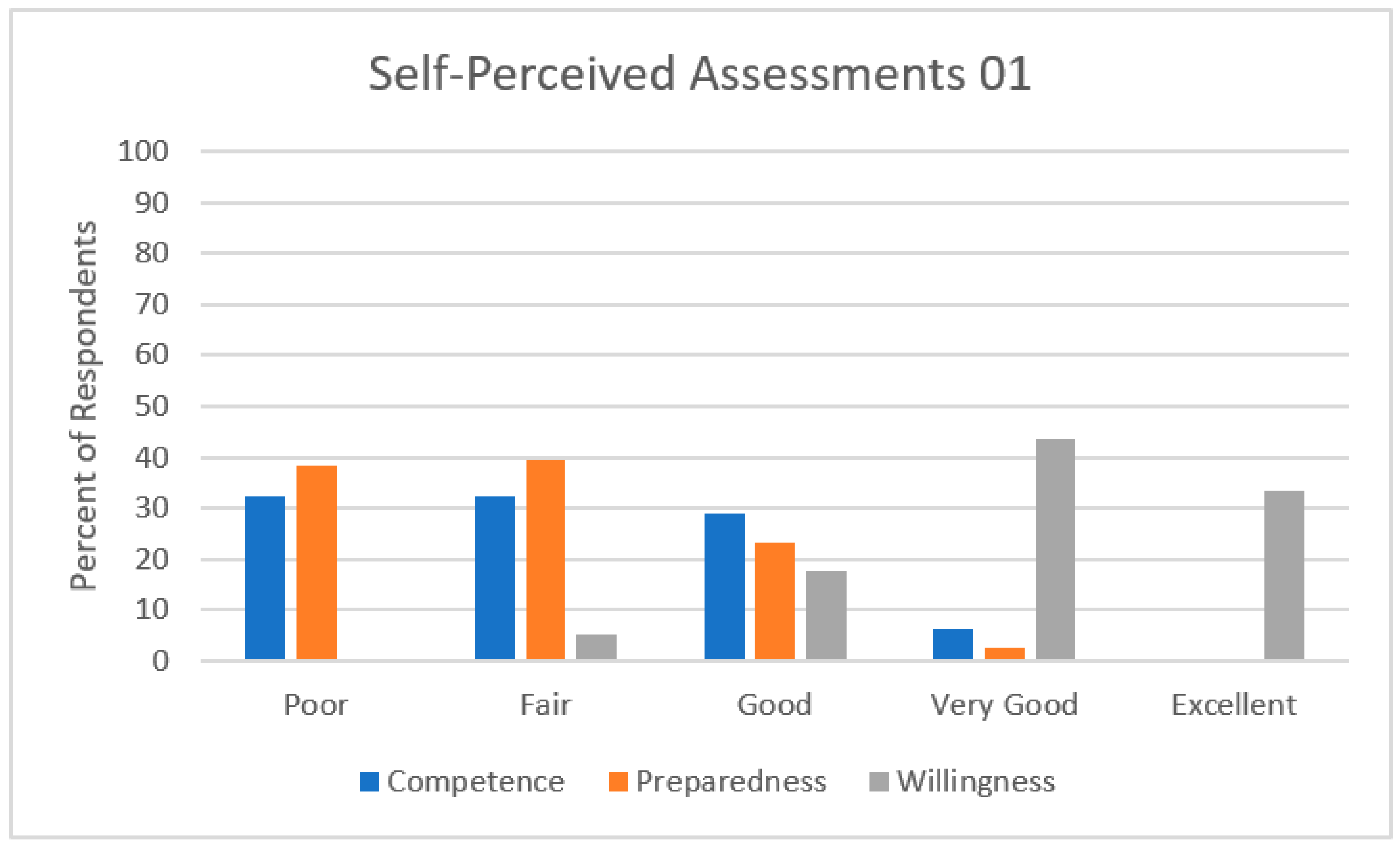
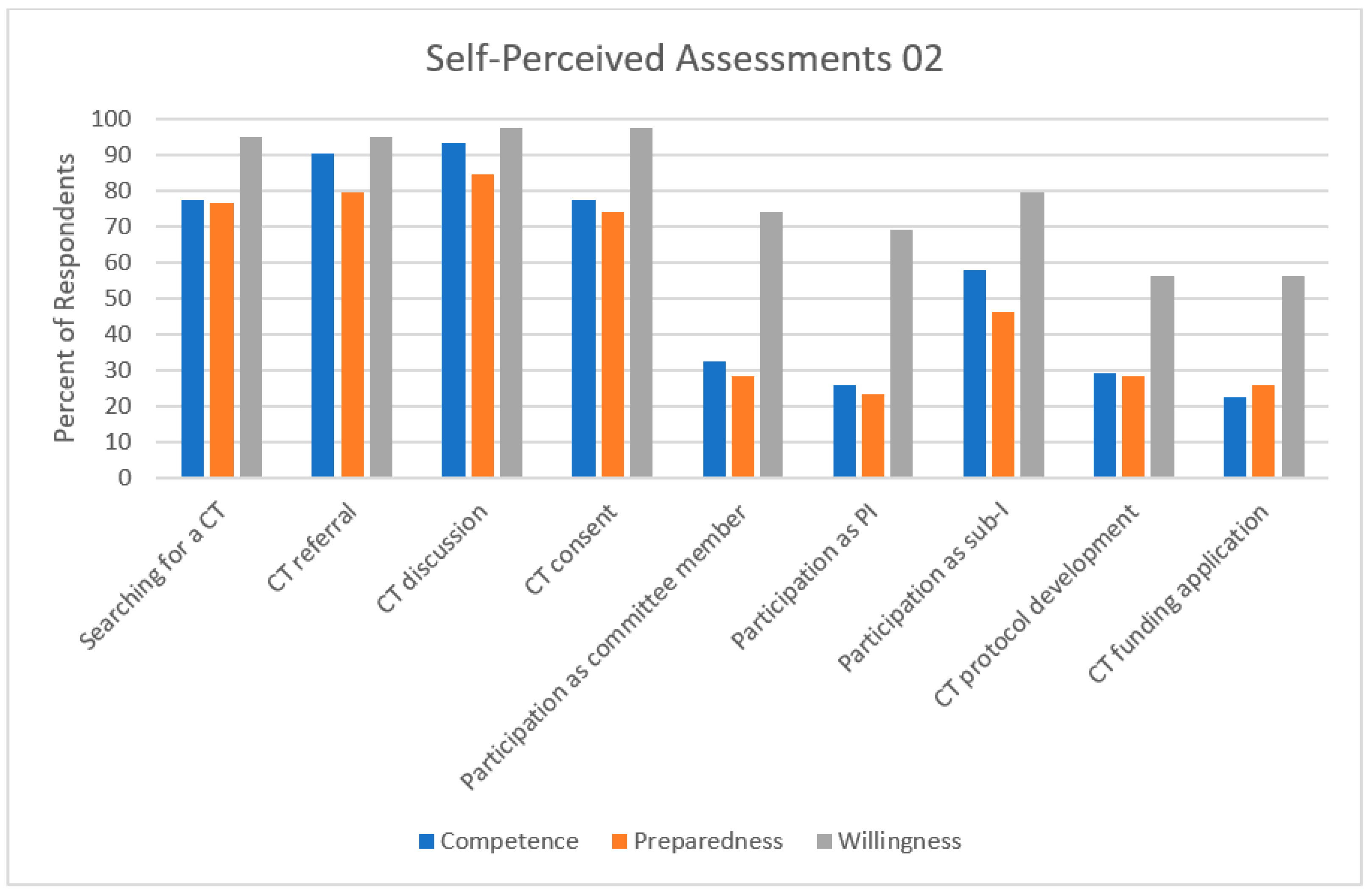
3.2. Self-Perceived Assessments
3.3. Perceived Role of the Trainee
4. Discussion
4.1. Limitations
4.2. Special Considerations and Next Steps
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, A.; Bergan, R.C. Clinical trial design: Past, present, and future in the context of big data and precision medicine. Cancer 2020, 126, 4838–4846. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.R.; Weiner, B.J.; Reeve, B.B.; Weinberger, M.; Minasian, L.M.; Good, M.J. Organizational and physician factors associated with patient enrollment in cancer clinical trials. Clin. Trials 2014, 11, 565–575. [Google Scholar] [CrossRef]
- Grunfeld, E.; Zitzelsberger, L.; Coristine, M.; Aspelund, F. Barriers and facilitators to enrollment in cancer clinical trials. Cancer 2002, 95, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- ASCO. American Society of Clinical Oncology Policy Statement: Oversight of Clinical Research. J. Clin. Oncol. 2003, 21, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.A.H.; Balneaves, L.G. Cancer patient decision making related to clinical trial participation: An integrative review with implications for patients’ relational autonomy. Support. Care Cancer 2015, 23, 1169–1196. [Google Scholar] [CrossRef]
- ACS. Barriers to Patient Enrollment in Therapeutic Clinical Trials for Cancer: A Landscape Report. Updated 2018. Available online: https://www.fightcancer.org/sites/default/files/National%20Documents/Clinical-Trials-Landscape-Report.pdf (accessed on 22 June 2023).
- Unger, J.M.; Vaidya, R.; Hershman, D.L.; Minasian, L.M.; Fleury, M.E. Systematic Review and Meta-Analysis of the Magnitude of Structural, Clinical, and Physician and Patient Barriers to Cancer Clinical Trial Participation. JNCI J. Natl. Cancer Inst. 2019, 111, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.B.; Wotherspoon, A.C.; Morris, M.; Wilson, R.H.; Chen, J.J.; Cairnduff, V.; Morgan, E.; Devlin, A.; Gavin, A.T. A population-level investigation of cancer clinical trials participation in a UK region. Eur. J. Cancer Prev. 2017, 26, S229–S235. [Google Scholar] [CrossRef]
- Canadian Partnership Against Cancer. Cancer System Performance Report. Updated 2018. Available online: https://www.partnershipagainstcancer.ca/topics/2018-cancer-system-performance-report/ (accessed on 22 June 2023).
- 3CTN. Canadian Cancer Clinical Trials Network. Updated 2022. Available online: https://3ctn.ca/ (accessed on 22 June 2023).
- Mannel, R.S.; Walker, J.L.; Gould, N.; Scribner, D.R.; Kamelle, S.; Tillmanns, T.; McMeekin, S.D.; Gold, M.A. Impact of Individual Physicians on Enrollment of Patients into Clinical Trials. Am. J. Clin. Oncol. 2003, 26, 171–173. [Google Scholar] [CrossRef]
- Briel, M.; Elger, B.S.; McLennan, S.; Schandelmaier, S.; von Elm, E.; Satalkar, P. Exploring reasons for recruitment failure in clinical trials: A qualitative study with clinical trial stakeholders in Switzerland, Germany, and Canada. Trials 2021, 22, 844. [Google Scholar] [CrossRef]
- Chen, D.T. Clinical Research and the Physician–Patient Relationship. Ann. Intern. Med. 2003, 138, 669–672. [Google Scholar]
- Mann, S.; Truelove, A.H.; Beesley, T.; Howden, S.; Egan, R. Resident perceptions of Competency-Based Medical Education. Can. Med. Educ. J. 2020, 11, e31–e43. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Kazemi, G.; Hsu, T.; Levine, O.; Basi, S.K.; Henning, J.W.; Sussman, J.; Mukherjee, S.D. Implementing Changes to a Residency Program Curriculum before Competency-Based Medical Education: A Survey of Canadian Medical Oncology Program Directors. Curr. Oncol. 2020, 27, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.; Angelis, F.D.; Al-Asaaed, S.; Basi, S.K.; Tomiak, A.; Grenier, D.; Hammad, N.; Henning, J.W.; Berry, S.; Song, X.; et al. Ten ways to get a grip on designing and implementing a competency-based medical education training program. Can. Med. Educ. J. 2021, 12, e81–e87. [Google Scholar] [PubMed]
- Frank, J.R.; Snell, L.S.; Cate, O.T.; Holmboe, E.S.; Carraccio, C.; Swing, S.R.; Harris, P.; Glasgow, N.J.; Campbell, C.; Dath, D.; et al. Competency-based medical education: Theory to practice. Med. Teach. 2010, 32, 638–645. [Google Scholar] [CrossRef]
- Berman, A.T.; Shea, J.A.; Baffic, C.; Vapiwala, N. Is There a Need for Resident Training in Clinical Trial Design? Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 969–970. [Google Scholar] [CrossRef] [PubMed]
- Safa, M.; Jazieh, A.R. Lack of a Uniform Approach to Clinical Research Training for Hematology Oncology Fellows. J. Cancer Educ. 2006, 21, 166–168. [Google Scholar] [PubMed]
- Todd, R.F.; Gitlin, S.D.; Burns, L.J. Subspeciality training in hematology and oncology, 2003: Results of a survey of training program directors conducted by the American Society of Hematology. Blood 2004, 103, 4383–4388. [Google Scholar] [CrossRef]
- Royal College of Physicians and Surgeons of Canada. Medical Oncology Competencies. Updated 2018. Available online: https://www.royalcollege.ca/rcsite/home-e (accessed on 22 June 2023).
- National Institute of Health. Definition of an Investigator. Updated 2022. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/investigator (accessed on 22 June 2023).
- Burns, K.E.A.; Duffett, M.; Kho, M.E.; Meade, M.O.; Adhikari, N.K.J.; Sinuff, T.; Cook, D.J. A guide for the design and conduct of self-administered surveys of clinicians. Can. Med. Assoc. J. 2008, 179, 245–252. [Google Scholar]
- Keeney, S.; Hasson, F.; McKenna, H. The Delphi Technique in Nursing and Health Research; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Yusoff, M.S.B. ABC of Content Validation and Content Validity Index Calculation. Educ. Med. J. 2019, 11, 49–54. [Google Scholar]
- CaRMS. Canadian Resident Matching Service. Updated 2023. Available online: https://www.carms.ca/ (accessed on 22 June 2023).
- Altahawi, F.; Sisk, B.; Poloskey, S.; Hicks, C.; Dannefer, E.F. Student perspectives on assessment: Experience in a competency-based portfolio system. Med. Teach. 2012, 34, 221–225. [Google Scholar] [CrossRef]
- Boet, S.; Pigford, A.A.E.; Naik, V.N. Program director and resident perspectives of a competency-based medical education anesthesia residency program in Canada: A needs assessment. Korean J. Med. Educ. 2016, 28, 157–168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ross, S.; Poth, C.A.; Donoff, M.G.; Papile, C.; Humphries, P.; Stasiuk, S.; Georgis, R. Involving users in the refinement of the competency-based achievement system: An innovative approach to competency-based assessment. Med. Teach. 2012, 34, e143–e147. [Google Scholar] [CrossRef] [PubMed]
- Canadian Cancer Trials Group. Who We Are. Updated 2021. Available online: https://www.ctg.queensu.ca/public/who-we-are (accessed on 22 June 2023).
- Statistics Canada. Census of Population. Updated 2016. Available online: https://www12.statcan.gc.ca/census-recensement/2016/ref/dict/geo049a-eng.cfm (accessed on 22 June 2023).
- Rahman, S.; Majumder, M.A.A.; Shaban, S.F.; Rahman, N.; Ahmed, M.; Abdulrahman, K.B.; D’souza, U.J. Physician participation in clinical research and trials: Issues and approaches. Adv. Med. Educ. Pract. 2011, 2, 85–93. [Google Scholar] [PubMed]
- Sumi, E.; Murayama, T.; Yokode, M. A survey of attitudes toward clinical research among physicians at Kyoto University Hospital. BMC Med. Educ. 2009, 9, 75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coomarasamy, A.; Khan, K.S. What is the evidence that postgraduate teaching in evidence-based medicine changes anything? A systematic review. BMJ 2004, 329, 1017. [Google Scholar] [CrossRef] [PubMed]
- Barron, D.; Khosa, D.; Jones-Bitton, A. Experiential Learning in Primary Care: Impact on Veterinary Students’ Communication Confidence. J. Exp. Educ. 2017, 40, 349–365. [Google Scholar]
- Burgess, A.; Diggele, C.; Mellis, C. Mentorship in the health professions: A review. Clin. Teach. 2018, 15, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Physicians and Surgeons of Canada. Competence by Design: Resource Directory. Updated 2023. Available online: https://royalcollege.ca/rcsite/cbd/cbd-tools-resources-e?N=10000023+10000026+4294967152&;No=6&Nrpp=6&searchstr=%27Workplace-based%20assessment%27 (accessed on 22 June 2023).
- Meijs, L.; Zusterzeel, R.; Wellens, H.J.; Gorgels, A.P. The Maastricht–Duke bridge: An era of mentoring in clinical research—A model for mentoring in clinical research—A tribute to Dr. Galen Wagner. J. Electrocardiol. 2017, 50, 16–20. [Google Scholar] [CrossRef]
- Spreafico, A.; Hansen, A.R.; Razak, A.R.A.; Bedard, P.L.; Siu, L.L. The Future of Clinical Trial Design in Oncology. Cancer Discov. 2021, 11, 822–837. [Google Scholar] [CrossRef]
- Murillo, H.; Reece, E.A.; Snyderman, R.; Sung, N.S. Meeting the Challenges Facing Clinical Research: Solutions Proposed by Leaders of Medical Specialty and Clinical Research Societies. Acad. Med. 2006, 81, 107–112. [Google Scholar] [CrossRef]
- Moya-Plana, A.; Tselikas, L.; Lambotte, O.; Temam, S.; Baere, T.D.; Deutsch, E.; Barlesi, F.; Blanchard, P.; Levy, A. Postgraduate oncology educational shifts during the COVID-19 pandemic: Results of faculty and medical student surveys. Esmo Open 2022, 7, 100451. [Google Scholar] [CrossRef]
- Unger, J.M.; Xiao, H.; LeBlanc, M.; Hershman, D.L.; Blanke, C.D. Cancer Clinical Trial Participation at the 1-Year Anniversary of the Outbreak of the COVID-19 Pandemic. JAMA Netw. Open 2021, 4, e2118433. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.S.; Holmboe, E.S.; Chandra, S. Competency-Based Medical Education: Considering Its Past, Present, and a Post–COVID-19 Era. Acad. Med. 2022, 97, S90–S97. [Google Scholar] [CrossRef]
- International Competency Based Medical Education. ICBME Collaboration. Updated 2018. Available online: https://gocbme.org/icbme-site/index.html (accessed on 22 June 2023).
- Ibrahim, H.; Abdel-Razig, S. Recalibrating our efforts: From globalisation to glocalisation of medical education. Postgrad. Med. J. 2021, 97, 545–546. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Responses—N (%) | |
|---|---|---|
| N | 41 | |
| Participant status | Resident | 10 (24.4) |
| Fellow | 14 (34.2) | |
| <5 years in practice | 17 (41.5) | |
| Age | Mean (standard deviation) | 33 (27, 43) |
| Gender | Male | 13 (33.3) |
| Female | 26 (66.7) | |
| Location of medical oncology subspecialty program | Atlantic Canada | 3 (7.3) |
| Quebec | 5 (12.2) | |
| Ontario | 25 (61.0) | |
| Central/Western Canada | 8 (19.5) | |
| Practice setting * | Academic | 11 (64.7) |
| Community | 4 (23.5) | |
| Mixed | 2 (11.8) | |
| Graduate Level Degree (master’s or PhD) | Yes | 16 (41.0) |
| No | 23 (59.0) | |
| Language of Questionnaire | English | 37 (90.2) |
| French | 4 (9.3) | |
| Question | Answer * | N | Good to Excellent Competence n (%) | p-Value |
|---|---|---|---|---|
| In your medical oncology subspecialty, how were you taught about participating in a clinical trial? | There was no teaching | 19 | 4 (21.1) | |
| Any teaching present | 12 | 7 (58.3) | 0.056 | |
| In clinic teaching * | 7 | 4 (57.1) | ||
| All other teaching ** | 24 | 7 (29.2) | 0.21 | |
| In your medical oncology subspecialty, how were you taught about critically appraising a clinical trial? | There was no teaching | 1 | 0 (0) | |
| Any teaching present | 30 | 11 (36.7) | 1.00 | |
| In clinic teaching * | 7 | 4 (57.1) | ||
| All other teaching ** | 24 | 7 (29.2) | 0.21 | |
| In your medical oncology subspecialty, how were you taught about clinical trials research methods? | There was no teaching | 3 | 1 (33.3) | |
| Any teaching present | 28 | 10 (35.7) | 1.00 | |
| In clinic teaching * | 16 | 7 (43.7) | ||
| All other teaching ** | 15 | 4 (26.7) | 0.46 | |
| Are you completing or have you completed a graduate level degree? | No | 16 | 6 (37.5) | |
| Yes | 13 | 4 (30.7) | 1.00 | |
| Upon completion of medical oncology subspecialty training, will you/did you feel the need to seek out additional clinical trials training? | No | 13 | 5 (38.5) | |
| Yes | 16 | 5 (31.3) | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Febbraro, M.; Kazemi, G.; Juergens, R.; Pond, G.R. Trainee Evaluations of Preparedness for Clinical Trials in Medical Oncology—A National Questionnaire. Curr. Oncol. 2023, 30, 7627-7637. https://doi.org/10.3390/curroncol30080553
Febbraro M, Kazemi G, Juergens R, Pond GR. Trainee Evaluations of Preparedness for Clinical Trials in Medical Oncology—A National Questionnaire. Current Oncology. 2023; 30(8):7627-7637. https://doi.org/10.3390/curroncol30080553
Chicago/Turabian StyleFebbraro, Michela, Ghazaleh Kazemi, Rosalyn Juergens, and Gregory R. Pond. 2023. "Trainee Evaluations of Preparedness for Clinical Trials in Medical Oncology—A National Questionnaire" Current Oncology 30, no. 8: 7627-7637. https://doi.org/10.3390/curroncol30080553
APA StyleFebbraro, M., Kazemi, G., Juergens, R., & Pond, G. R. (2023). Trainee Evaluations of Preparedness for Clinical Trials in Medical Oncology—A National Questionnaire. Current Oncology, 30(8), 7627-7637. https://doi.org/10.3390/curroncol30080553





