Bilateral Renal Auto-Transplantation for Retroperitoneal Sarcomas: Is It Underutilized?
Abstract
1. Introduction
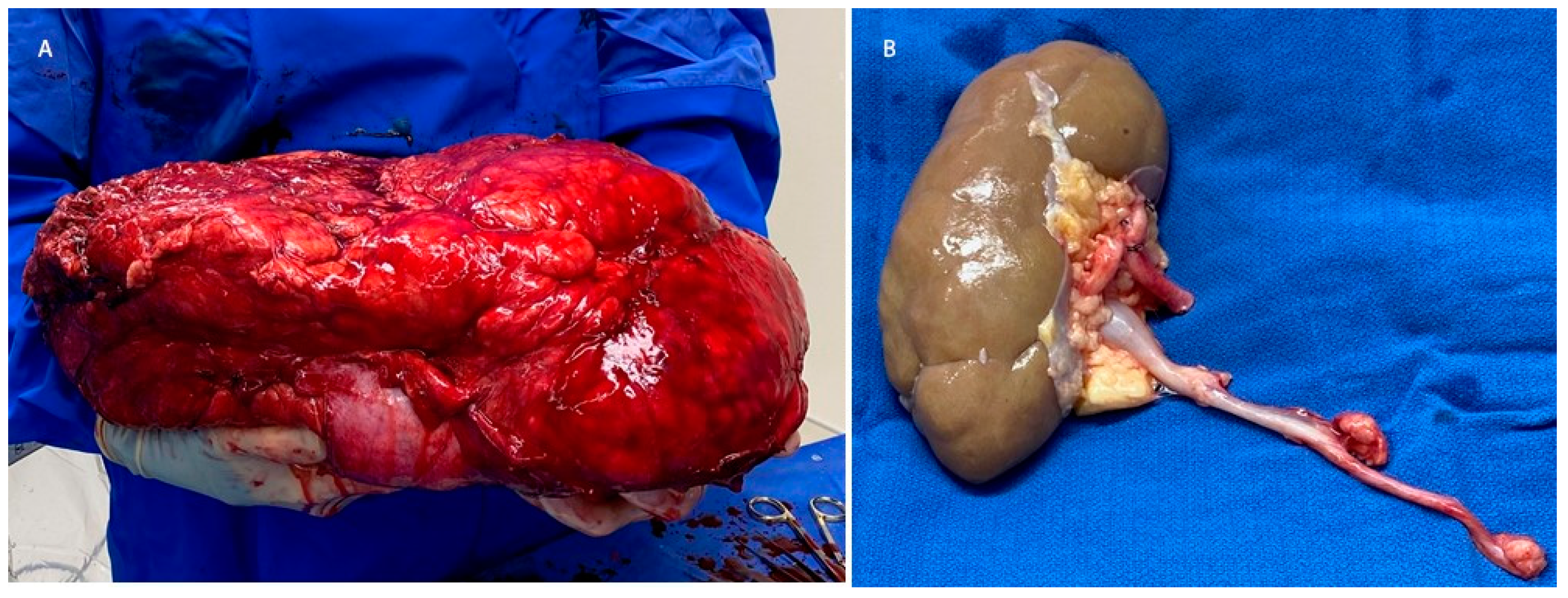
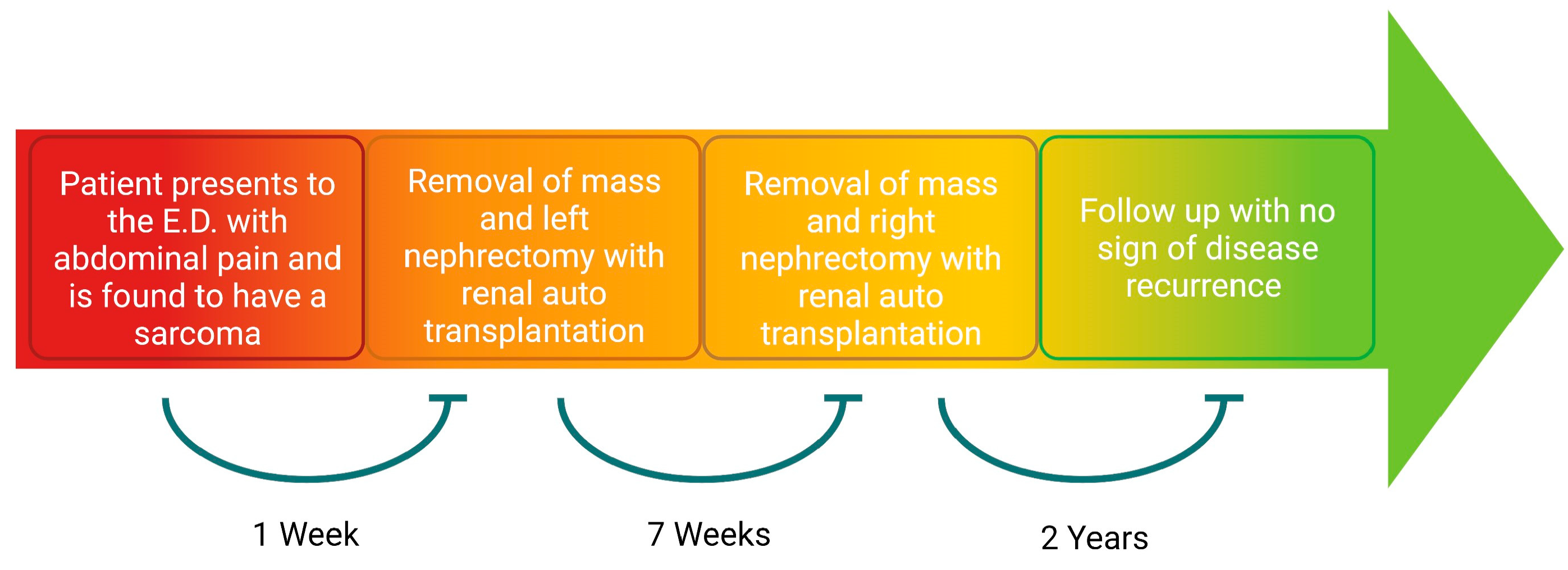
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, V.Y.; Scharschmidt, T.J.; Mayerson, J.L.; Fisher, J.L. Incidence and survival in sarcoma in the United States: A focus on musculoskeletal lesions. Anticancer Res. 2013, 33, 2597–2604. [Google Scholar]
- Choi, J.H.; Ro, J.Y. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv. Anat Pathol. 2021, 28, 44–58. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Koniaris, L.G.; Sola, J.E. Prognostication for trunk and retroperitoneal sarcomas. Ann. Surg. 2010, 252, 201–202. [Google Scholar] [CrossRef]
- Cho, C.W.; Lee, K.W.; Park, H.; Kim, H.J.; Park, J.B.; Choi, Y.-L.; Yu, J., II; Lee, S.J.; Choi, D., II; Lim, S.J. Clinical benefit and residual kidney function of en bloc nephrectomy for perirenal retroperitoneal sarcoma. Asia Pac. J. Clin. Oncol. 2018, 14, e465–e471. [Google Scholar] [CrossRef]
- Gronchi, A.; Strauss, D.C.; Miceli, R.; Bonvalot, S.; Swallow, C.; Hohenberger, P.; Van Coevorden, F.; Rutkowski, P.; Callegaro, D.; Hayes, A.J.; et al. Variability in Patterns of Recurrence After Resection of Primary Retroperitoneal Sarcoma (RPS): A Report on 1007 Patients From the Multi-institutional Collaborative RPS Working Group. Ann. Surg. 2016, 263, 1002–1009. [Google Scholar] [CrossRef]
- Swallow, C.J.; Strauss, D.C.; Bonvalot, S.; Rutkowski, P.; Desai, A.; Gladdy, R.A.; Gonzalez, R.; Gyorki, D.E.; Fairweather, M.; van Houdt, W.J.; et al. Management of Primary Retroperitoneal Sarcoma (RPS) in the Adult: An Updated Consensus Approach from the Transatlantic Australasian RPS Working Group. Ann. Surg. Oncol. 2021, 28, 7873–7888. [Google Scholar] [CrossRef]
- Milgrom, D.P.; Sehdev, A.; Kays, J.K.; Koniaris, L.G. Integrating therapies for surgical adult soft tissue sarcoma patients. Transl. Gastroenterol. Hepatol. 2018, 3, 88. [Google Scholar] [CrossRef]
- Gutierrez, J.C.; Perez, E.A.; Franceschi, D.; Moffat, F.L.; Livingstone, A.S.; Koniaris, L.G. Outcomes for soft-tissue sarcoma in 8249 cases from a large state cancer registry. J. Surg. Res. 2007, 141, 105–114. [Google Scholar] [CrossRef]
- Russo, P.; Kim, Y.; Ravindran, S.; Huang, W.; Brennan, M.F. Nephrectomy during operative management of retroperitoneal sarcoma. Ann. Surg. Oncol. 1997, 4, 421–424. [Google Scholar] [CrossRef]
- Kim, D.B.; Gray, R.; Li, Z.; Wasif, N.; Bagaria, S.P. Effect of nephrectomy for retroperitoneal sarcoma on post-operative renal function. J. Surg. Oncol. 2018, 117, 425–429. [Google Scholar] [CrossRef]
- Stahl, C.C.; Schwartz, P.B.; Ethun, C.G.; Marka, N.; Krasnick, B.A.; Tran, T.B.; Poultsides, G.A.; Roggin, K.K.; Fields, R.C.; Clarke, C.N.; et al. Renal Function After Retroperitoneal Sarcoma Resection with Nephrectomy: A Matched Analysis of the United States Sarcoma Collaborative Database. Ann. Surg. Oncol. 2021, 28, 1690–1696. [Google Scholar] [CrossRef]
- Rhu, J.; Cho, C.W.; Lee, K.W.; Park, H.; Park, J.B.; Choi, Y.-A.; Kim, S.J. Radical Nephrectomy for Primary Retroperitoneal Liposarcoma Near the Kidney has a Beneficial Effect on Disease-Free Survival. World J. Surg. 2018, 42, 254–262. [Google Scholar] [CrossRef]
- Scosyrev, E.; Messing, E.M.; Sylvester, R.; Campbell, S.; Van Poppel, H. Renal function after nephron-sparing surgery versus radical nephrectomy: Results from EORTC randomized trial 30904. Eur. Urol. 2014, 65, 372–377. [Google Scholar] [CrossRef]
- Kato, T.; Lobritto, S.J.; Tzakis, A.; Raveh, Y.; Sandoval, P.R.; Martinez, M.; Granowetter, L.; Armas, A.; Brown, R.; Emond, J. Multivisceral Ex Vivo Surgery for Tumors Involving Celiac and Superior Mesenteric Arteries. Am. J. Transplant. 2012, 12, 1323–1328. [Google Scholar] [CrossRef]
- MacNeill, A.J.; Gronchi, A.; Miceli, R.; Bonvalot, S.; Swallow, C.J.; Hohenberger, P.; Van Coevorden, F.; Rutkowski, P.; Callegaro, D.; Hayes, A.; et al. Postoperative Morbidity After Radical Resection of Primary Retroperitoneal Sarcoma: A Report From the Transatlantic RPS Working Group. Ann. Surg. 2018, 267, 959–964. [Google Scholar] [CrossRef]
- Paloyo, S.R.; Ramirez, A.D.; David-Paloyo, F.P.; Dofitas, R.B. Wide Excision of a Retroperitoneal Liposarcoma with En Bloc Ureterectomy and Renal Salvage by Autotransplantation. Case Rep. Transplant. 2019, 2019, 9725169. [Google Scholar] [CrossRef][Green Version]
- Fernandez, H.T.; Kim, P.T.; Anthony, T.L.; Hamman, B.L.; Goldstein, R.M.; Testa, G. Inferior vena cava reconstruction for leiomyosarcoma of Zone I-III requiring complete hepatectomy and bilateral nephrectomy with autotransplantation. J. Surg. Oncol. 2015, 112, 481–485. [Google Scholar] [CrossRef]
- Bansal, V.K.; Misra, M.C.; Sharma, A.; Chabbra, A.; Murmu, L.R. Giant retroperitoneal liposarcoma- renal salvage by autotransplantation. Indian J. Surg. 2013, 75, 159–161. [Google Scholar] [CrossRef]
- Kraybill, W.G.; Callery, M.P.; Heiken, J.P.; Flye, M.W. Radical resection of tumors of the inferior vena cava with vascular reconstruction and kidney autotransplantation. Surgery 1997, 121, 31–36. [Google Scholar] [CrossRef]
- Eriksson, M. Histology-driven chemotherapy of soft-tissue sarcoma. Ann. Oncol. 2010, 21, 270–276. [Google Scholar] [CrossRef]
- Bonvalot, S.; Gronchi, A.; Le Péchoux, C.; Swallow, C.J.; Strauss, D.; Meeus, P.; van Coevorden, F.; Stoldt, S.; Stoeckle, E.; Rutkowski, P.; et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1366–1377. [Google Scholar] [CrossRef]
- Crago, A.M.; Brennan, M.F. Principles in Management of Soft Tissue Sarcoma. Adv. Surg. 2015, 49, 107–122. [Google Scholar] [CrossRef]

| Author | Year | Case Description | Follow Up | Disease Status |
|---|---|---|---|---|
| Paloyo et al. | 2019 | A 47 × 34 × 17 cm 11 kg tumor encased the right kidney and ureter, requiring a right nephrectomy and auto-transplantation. The pathology demonstrated a low-grade, well-differentiated liposarcoma. Nuclear imaging demonstrated good renal function post-operatively [17]. | 6 months | No recurrence |
| Fernandez et al. | 2015 | A 13.5 cm tumor, encasing zone I–III of the IVC, requiring the resection of the IVC, a complete hepatectomy, and a bilateral nephrectomy with IVC reconstruction via a vascular graft, and the auto-transplantation of the liver and the left kidney. The pathology demonstrated a high-grade spindle cell sarcoma of vena cava origin. The liver function was found to be within normal limits, and the baseline creatinine increased to 1.8 [18]. | 1 year | No recurrence |
| Bansal et al. | 2013 | A 40 × 35 × 35 cm 24 kg tumor, involving the right ureter and ileum, ultimately requiring a small bowel resection and a right nephrectomy with auto-transplantation. The pathology demonstrated a mixed-type liposarcoma. Post-operatively, nuclear imaging demonstrated good renal function [19]. | 63 months | Recurrence at 40 months, requiring reoperation. No further recurrence as of the 63 month follow-up |
| Kraybill et al. | 1997 | Two patients were described as having a sarcoma of the IVC, requiring the resection of the IVC and aorta, and a bilateral nephrectomy, via the vascular graft reconstruction of the IVC and aorta, and left renal auto-transplantation. The first patient had a 15 × 10 × 7 cm poorly differentiated spindle cell rhabdomyosarcoma. Post-operatively, she showed recovery in her creatinine to near the baseline. The second patient had a 4.5 cm moderately differentiated leiomyosarcoma. Post-operatively, her creatinine increased to 1.5 [20]. | Patient 1—8 months Patient 2—about 2.5 years | Patient 1—Recurrence in the right psoas muscle; patient declined further treatment Patient 2—Recurrence in the lung and liver |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, T.P.; Milgrom, D.P.; Nagaraju, S.; Goggins, W.C.; Samy, K.P.; Koniaris, L.G. Bilateral Renal Auto-Transplantation for Retroperitoneal Sarcomas: Is It Underutilized? Curr. Oncol. 2023, 30, 7620-7626. https://doi.org/10.3390/curroncol30080552
Robinson TP, Milgrom DP, Nagaraju S, Goggins WC, Samy KP, Koniaris LG. Bilateral Renal Auto-Transplantation for Retroperitoneal Sarcomas: Is It Underutilized? Current Oncology. 2023; 30(8):7620-7626. https://doi.org/10.3390/curroncol30080552
Chicago/Turabian StyleRobinson, Tyler P., Daniel P. Milgrom, Santosh Nagaraju, William C. Goggins, Kannan P. Samy, and Leonidas G. Koniaris. 2023. "Bilateral Renal Auto-Transplantation for Retroperitoneal Sarcomas: Is It Underutilized?" Current Oncology 30, no. 8: 7620-7626. https://doi.org/10.3390/curroncol30080552
APA StyleRobinson, T. P., Milgrom, D. P., Nagaraju, S., Goggins, W. C., Samy, K. P., & Koniaris, L. G. (2023). Bilateral Renal Auto-Transplantation for Retroperitoneal Sarcomas: Is It Underutilized? Current Oncology, 30(8), 7620-7626. https://doi.org/10.3390/curroncol30080552




