Small Bowel Neuroendocrine Tumors—10-Year Experience of the Ottawa Hospital (TOH)
Abstract
- Brief Description
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient’s Characteristics
3.2. Sites
3.3. Clinical Presentations
3.4. Pathology
3.5. Secondary Malignancies
3.6. Management and Outcomes
3.7. Metastectomy and Local Therapy
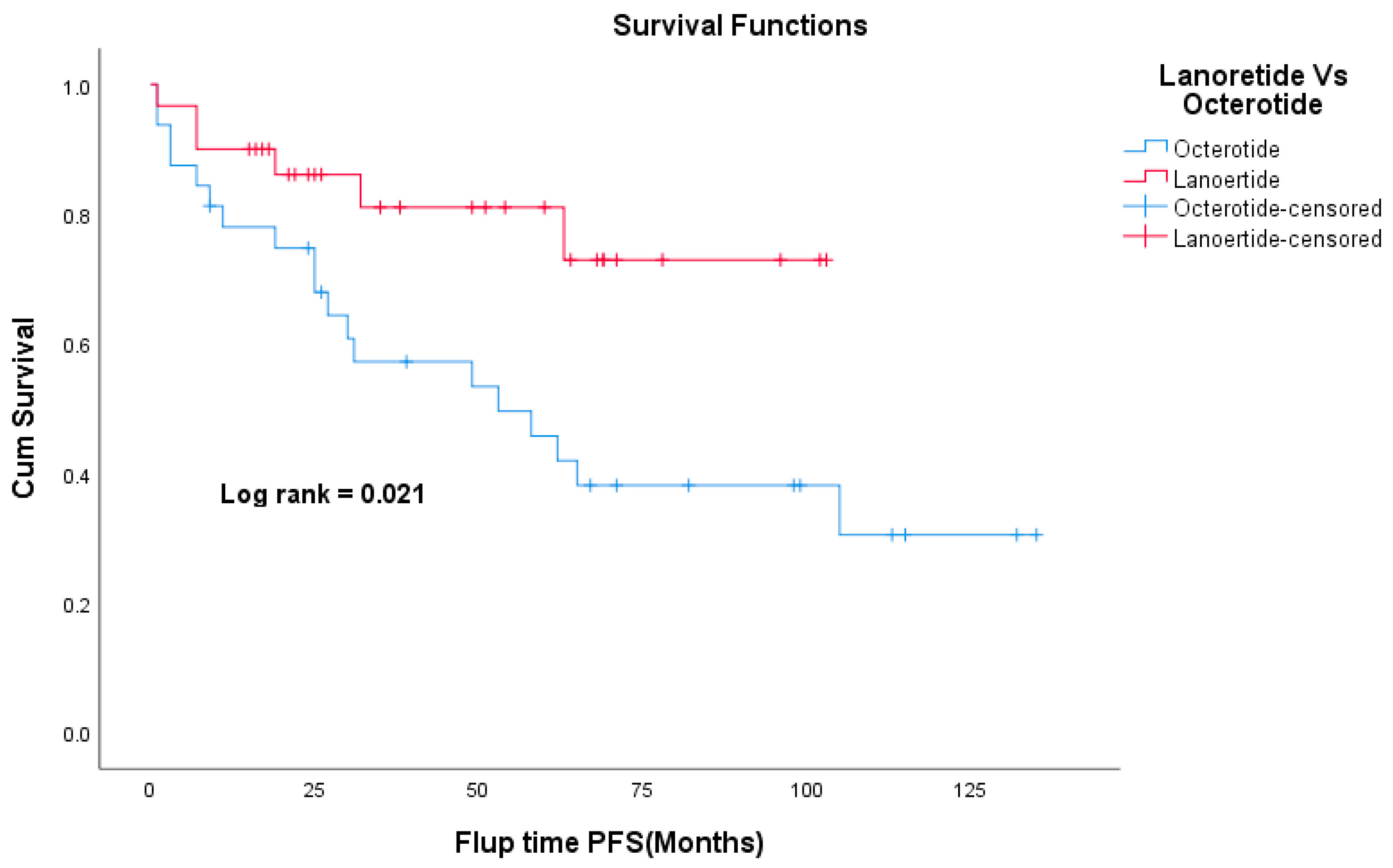
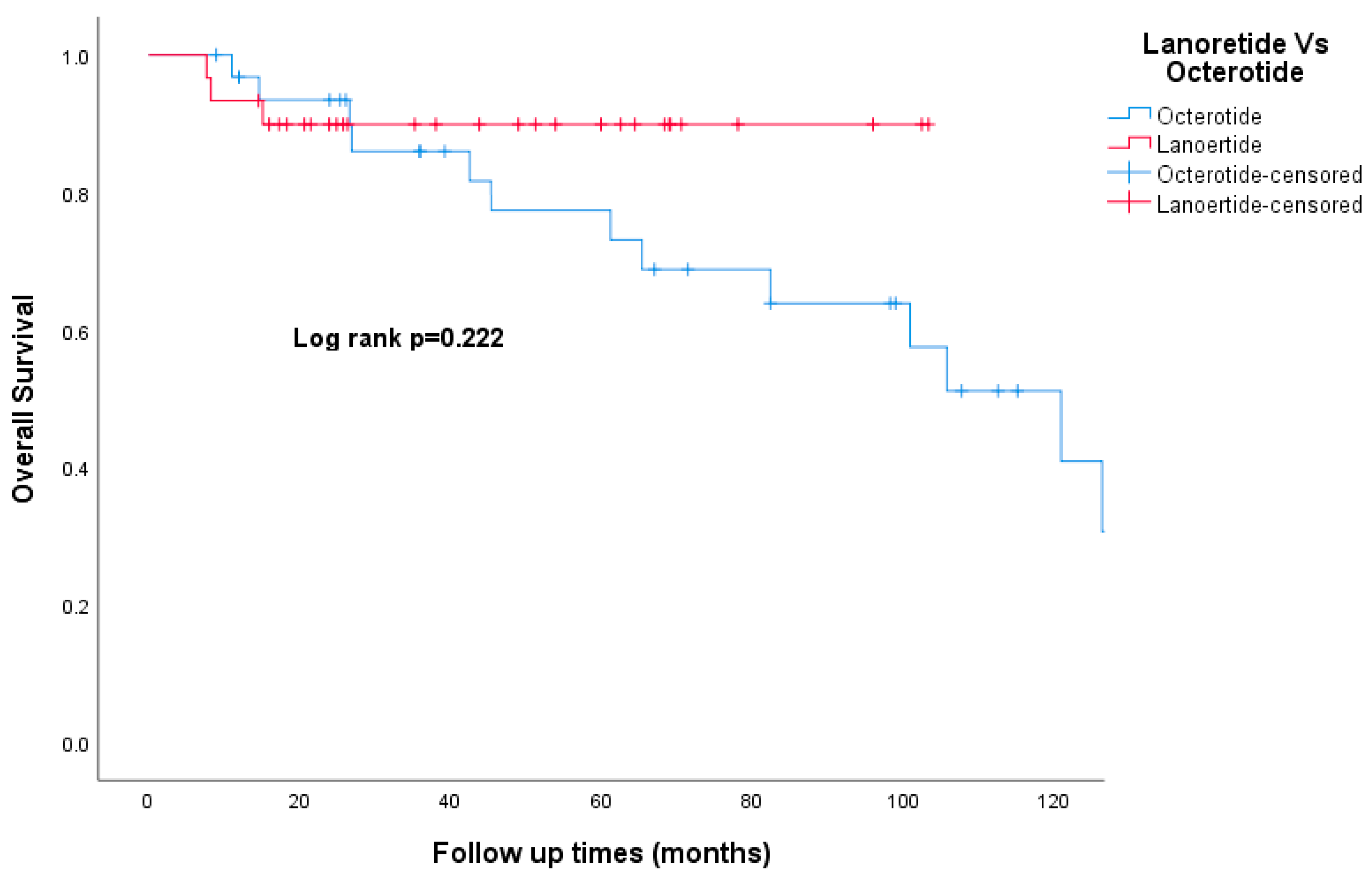
3.8. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bilimoria, K.Y.; Bentrem, D.J.; Wayne, J.D.; Ko, C.Y.M.; Bennett, C.L.; Talamonti, M.S. Small Bowel Cancer in the United States: Changes in epidemiology, treatment, and survival over the last 20 years. Ann. Surg. 2009, 249, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Öberg, K.E. Gastrointestinal neuroendocrine tumors. Ann. Oncol. 2010, 21 (Suppl. 7), vii72–vii80. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.G.; Sherman, S.K.; Howe, J.R. Small Bowel Neuroendocrine Tumors. Curr. Probl. Surg. 2020, 57, 100823. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; the WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Scarpa, A. The landscape of molecular alterations in pancreatic and small intestinal neuroendocrine tumours. Ann. d’Endocrinologie 2019, 80, 153–158. [Google Scholar] [CrossRef]
- Strosberg, J. Neuroendocrine tumours of the small intestine. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 755–773. [Google Scholar] [CrossRef]
- Fottner, C.; Ferrata, M.; Weber, M.M. Hormone secreting gastro-entero-pancreatic neuroendocrine neoplasias (GEP-NEN): When to consider, how to diagnose? Rev. Endocr. Metab. Disord. 2017, 18, 393–410. [Google Scholar] [CrossRef]
- Rossi, R.E.; Conte, D.; Elli, L.; Branchi, F.; Massironi, S. Endoscopic techniques to detect small-bowel neuroendocrine tumors: A literature review. United Eur. Gastroenterol. J. 2017, 5, 5–12. [Google Scholar] [CrossRef]
- Lardière-Deguelte, S.; de Mestier, L.; Appéré, F.; Vullierme, M.-P.; Zappa, M.; Hoeffel, C.; Noaves, M.; Brixi, H.; Hentic, O.; Ruszniewski, P.; et al. Toward a Preoperative Classification of Lymph Node Metastases in Patients with Small Intestinal Neuroendocrine Tumors in the Era of Intestinal-Sparing Surgery. Neuroendocrinology 2016, 103, 552–559. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Heede, K.V.D.; Chidambaram, S.; Van Slycke, S.; Brusselaers, N.; Warfvinge, C.F.; Ohlsson, H.; Nordenström, E.; Almquist, M. Effect of primary tumour resection without curative intent in patients with metastatic neuroendocrine tumours of the small intestine and right colon: Meta-analysis. Br. J. Surg. 2021, 109, 191–199. [Google Scholar] [CrossRef]
- Levy, S.; Arthur, J.D.; Banks, M.; Kok, N.F.M.; Fenwick, S.W.; Diaz-Nieto, R.; van Leerdam, M.E.; Cuthbertson, D.J.; Valk, G.D.; Kuhlmann, K.F.D.; et al. Primary Tumor Resection is Associated with Improved Disease-Specific Mortality in Patients with Stage IV Small Intestinal Neuroendocrine Tumors (NETs): A Comparison of Upfront Surgical Resection Versus a Watch and Wait Strategy in Two Specialist NET Centers. Ann. Surg. Oncol. 2022, 29, 7822–7832. [Google Scholar] [CrossRef] [PubMed]
- Frilling, A.; Modlin, I.M.; Kidd, M.; Russell, C.; Breitenstein, S.; Salem, R.; Kwekkeboom, D.; Lau, W.-Y.; Klersy, C.; Vilgrain, V.; et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014, 15, e8–e21. [Google Scholar] [CrossRef] [PubMed]
- Gedaly, R.; Daily, M.F.; Davenport, D.; McHugh, P.P.; Koch, A.; Angulo, P.; Hundley, J.C. Liver Transplantation for the Treatment of Liver Metastases From Neuroendocrine Tumors: An analysis of the UNOS database. Arch. Surg. 2011, 146, 953–958. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Pulvirenti, A.; Coppa, J. Neuroendocrine tumors metastatic to the liver: How to select patients for liver transplantation? J. Hepatol. 2007, 47, 460–466. [Google Scholar] [CrossRef]
- Vogl, T.J.; Naguib, N.N.; Zangos, S.; Eichler, K.; Hedayati, A.; Nour-Eldin, N.-E.A. Liver metastases of neuroendocrine carcinomas: Interventional treatment via transarterial embolization, chemoembolization and thermal ablation. Eur. J. Radiol. 2009, 72, 517–528. [Google Scholar] [CrossRef]
- Kennedy, A.S.; Dezarn, W.A.; McNeillie, P.; Coldwell, D.; Nutting, C.; Carter, D.; Murthy, R.; Rose, S.; Warner, R.R.P.; Liu, D.; et al. Radioembolization for Unresectable Neuroendocrine Hepatic Metastases Using Resin 90Y-Microspheres: Early Results in 148 Patients. Am. J. Clin. Oncol. 2008, 31, 271–279. [Google Scholar] [CrossRef]
- McStay, M.K.G.; Maudgil, D.; Williams, M.; Tibballs, J.M.; Watkinson, A.F.; Caplin, M.E.; Buscombe, J.R. Large-Volume Liver Metastases from Neuroendocrine Tumors: Hepatic Intraarterial90Y-DOTA-Lanreotide as Effective Palliative Therapy. Radiology 2005, 237, 718–726. [Google Scholar] [CrossRef]
- Puccini, A.; Battaglin, F.; Lenz, H.-J. Management of Advanced Small Bowel Cancer. Curr. Treat. Options Oncol. 2018, 19, 69. [Google Scholar] [CrossRef]
- Rinke, A.; Müller, H.-H.; Schade-Brittinger, C.; Klose, K.-J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.-F.; Bläker, M.; et al. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors: A Report from the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Pavel, M.E.; Hainsworth, J.D.; Baudin, E.; Peeters, M.; Höersch, D.; Winkler, R.E.; Klimovsky, J.; Lebwohl, D.; Jehl, V.; Wolin, E.M.; et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): A randomised, placebo-controlled, phase 3 study. Lancet 2011, 378, 2005–2012. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- IBM Corp. Released IBM SPSS Statistics for Windows, Version 25.0; IBM Corp: Armonk, NY, USA, 2017.
- Modlin, I.M.; Champaneria, M.C.; Chan, A.K.; Kidd, M. A Three-Decade Analysis of 3911 Small Intestinal Neuroendocrine Tumors: The Rapid Pace of No Progress. Off. J. Am. Coll. Gastroenterol. ACG 2007, 102, 1464–1473. Available online: https://journals.lww.com/ajg/Fulltext/2007/07000/A_Three_Decade_Analysis_of_3,911_Small_Intestinal.20.aspx (accessed on 28 April 2023). [CrossRef]
- Yao, J.C.; Hassan, M.M.; Phan, A.T.; Dagohoy, C.G.; Leary, C.C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Hakim, F.A.; Alexander, J.A.; Huprich, J.E.; Grover, M.; Enders, F.T. CT-Enterography May Identify Small Bowel Tumors Not Detected by Capsule Endoscopy: Eight Years Experience at Mayo Clinic Rochester. Dig. Dis. Sci. 2011, 56, 2914–2919. [Google Scholar] [CrossRef]
- Deppen, S.A.; Blume, J.; Bobbey, A.J.; Shah, C.; Graham, M.M.; Lee, P.; Delbeke, D.; Walker, R.C. 68Ga-DOTATATE Compared with 111In-DTPA-Octreotide and Conventional Imaging for Pulmonary and Gastroenteropancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. J. Nucl. Med. 2016, 57, 872–878. [Google Scholar] [CrossRef]
- Rossi, R.E.; Massironi, S. The Increasing Incidence of Neuroendocrine Neoplasms Worldwide: Current Knowledge and Open Issues. J. Clin. Med. 2022, 11, 3794. [Google Scholar] [CrossRef]
- Khan, M.S.; Kirkwood, A.; Tsigani, T.; Garcia-Hernandez, J.; Hartley, J.A.; Caplin, M.E.; Meyer, T. Circulating Tumor Cells as Prognostic Markers in Neuroendocrine Tumors. J. Clin. Oncol. 2013, 31, 365–372. [Google Scholar] [CrossRef]
- Modlin, I.M.; Drozdov, I.; Kidd, M. The Identification of Gut Neuroendocrine Tumor Disease by Multiple Synchronous Transcript Analysis in Blood. PLoS ONE 2013, 8, e63364. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.R.; Rendell, V.; Pokrzywa, C.; Lopez-Aguiar, A.G.; Cannon, J.; Poultsides, G.A.; Rocha, F.; Crown, A.; Beal, E.; Pawlik, T.M.; et al. Adjuvant therapy following resection of gastroenteropancreatic neuroendocrine tumors provides no recurrence or survival benefit. J. Surg. Oncol. 2020, 121, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Partelli, S.; Cirocchi, R.; Crippa, S.; Cardinali, L.; Fendrich, V.; Bartsch, D.K.; Falconi, M. Systematic review of active surveillance versus surgical management of asymptomatic small non-functioning pancreatic neuroendocrine neoplasms. J. Br. Surg. 2017, 104, 34–41. [Google Scholar] [CrossRef]
- Allaw, M.B.; Switchenko, J.M.; Khalil, L.; Wu, C.; Alese, O.B.; Akce, M.; Draper, A.; Jones, A.T.; El-Rayes, B.; Shaib, W. Comparing Somatostatin Analogs in the Treatment of Advanced Gastroenteropancreatic Neuroendocrine Tumors. Oncology 2022, 100, 131–139. [Google Scholar] [CrossRef]
- Paulson, A.S.; Bergsland, E.K. Systemic Therapy for Advanced Carcinoid Tumors: Where Do We Go from Here? J. Natl. Compr. Cancer Netw. 2012, 10, 785–793. [Google Scholar] [CrossRef][Green Version]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls (accessed on 17 April 2022).
- Garcia-Carbonero, R.; Capdevila, J.; Crespo-Herrero, G.; Díaz-Pérez, J.A.; del Prado, M.P.M.; Orduña, V.A.; Sevilla-García, I.; Villabona-Artero, C.; Beguiristain-Gómez, A.; Llanos-Muñoz, M.; et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): Results from the National Cancer Registry of Spain (RGETNE). Ann. Oncol. 2010, 21, 1794–1803. [Google Scholar] [CrossRef]
- Hallet, J.; Law, C.H.L.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Albertelli, M.; Dotto, A.; Nista, F.; Veresani, A.; Patti, L.; Gay, S.; Sciallero, S.; Boschetti, M.; Ferone, D. Present and future of immunotherapy in Neuroendocrine Tumors. Rev. Endocr. Metab. Disord. 2021, 22, 615–636. [Google Scholar] [CrossRef] [PubMed]



| n = 177 | (%) | ||
|---|---|---|---|
| Age | Mean | 66 | |
| Gender | Male | 91 | 51% |
| Female | 86 | 49% | |
| Comorbidities | HTN | 74 | 42% |
| DM | 22 | 12% | |
| IHD | 34 | 19% | |
| Clinical presentation | Diarrhea | 42 | 24% |
| Abdominal pain | 81 | 46% | |
| GI bleeding | 10 | 6% | |
| Anemia | 13 | 7% | |
| Bowel obstruction | 23 | 13% | |
| Carcinoid symptoms | 23 | 13% | |
| Duration of symptoms (pre-diagnosis) | <14 days | 21 | 12% |
| >14 day | 16 | 9% | |
| >2 months | 21 | 12% | |
| >6 months | 42 | 24% | |
| NA | 43 | 24% | |
| Mode of initial diagnosis | CT | 117 | 66% |
| Endoscopy | 26 | 15% | |
| Surgical exploration | 4 | 2% | |
| Ultrasound | 3 | 2% | |
| NA | 24 | 14% | |
| Curative surgery | 120 | 68% | |
| n = 177 | (%) | ||
|---|---|---|---|
| Site | Duodenum | 16 | 9% |
| Jejunum | 12 | 7% | |
| Ileum | 94 | 53% | |
| Small bowel, Unspecified | 55 | 31% | |
| Differentiation | Well | 148 | 84% |
| Moderate | 4 | 2% | |
| Poor | 2 | 1% | |
| NA | 23 | 13% | |
| Grade | 1 | 98 | 54% |
| 2 | 48 | 27% | |
| 3 | 1 | 6% | |
| NA | 30 | 17% | |
| Ki67 | <20% | 167 | 94% |
| ≥20% | 6 | 3% | |
| NA | 4 | 3% | |
| Functioning | Functioning | 32 | 18% |
| Non-functioning | 113 | 64% | |
| NA | 32 | 18% | |
| TNM stage | I | 7 | 4% |
| II | 12 | 7% | |
| III | 75 | 42% | |
| IV | 73 | 41% | |
| NA | 10 | 6% | |
| Sites of metasteses (stage IV) | Liver | 55 | |
| Peritoneum | 36 | ||
| Lymph nodes | 20 | ||
| Lung | 6 | ||
| Bone | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfagih, A.; AlJassim, A.; Alqahtani, N.; Vickers, M.; Goodwin, R.; Asmis, T. Small Bowel Neuroendocrine Tumors—10-Year Experience of the Ottawa Hospital (TOH). Curr. Oncol. 2023, 30, 7508-7519. https://doi.org/10.3390/curroncol30080544
Alfagih A, AlJassim A, Alqahtani N, Vickers M, Goodwin R, Asmis T. Small Bowel Neuroendocrine Tumors—10-Year Experience of the Ottawa Hospital (TOH). Current Oncology. 2023; 30(8):7508-7519. https://doi.org/10.3390/curroncol30080544
Chicago/Turabian StyleAlfagih, Abdulhameed, Abdulaziz AlJassim, Nasser Alqahtani, Michael Vickers, Rachel Goodwin, and Timothy Asmis. 2023. "Small Bowel Neuroendocrine Tumors—10-Year Experience of the Ottawa Hospital (TOH)" Current Oncology 30, no. 8: 7508-7519. https://doi.org/10.3390/curroncol30080544
APA StyleAlfagih, A., AlJassim, A., Alqahtani, N., Vickers, M., Goodwin, R., & Asmis, T. (2023). Small Bowel Neuroendocrine Tumors—10-Year Experience of the Ottawa Hospital (TOH). Current Oncology, 30(8), 7508-7519. https://doi.org/10.3390/curroncol30080544







