Cancer Pain Management: A Narrative Review of Current Concepts, Strategies, and Techniques
Abstract
1. Introduction
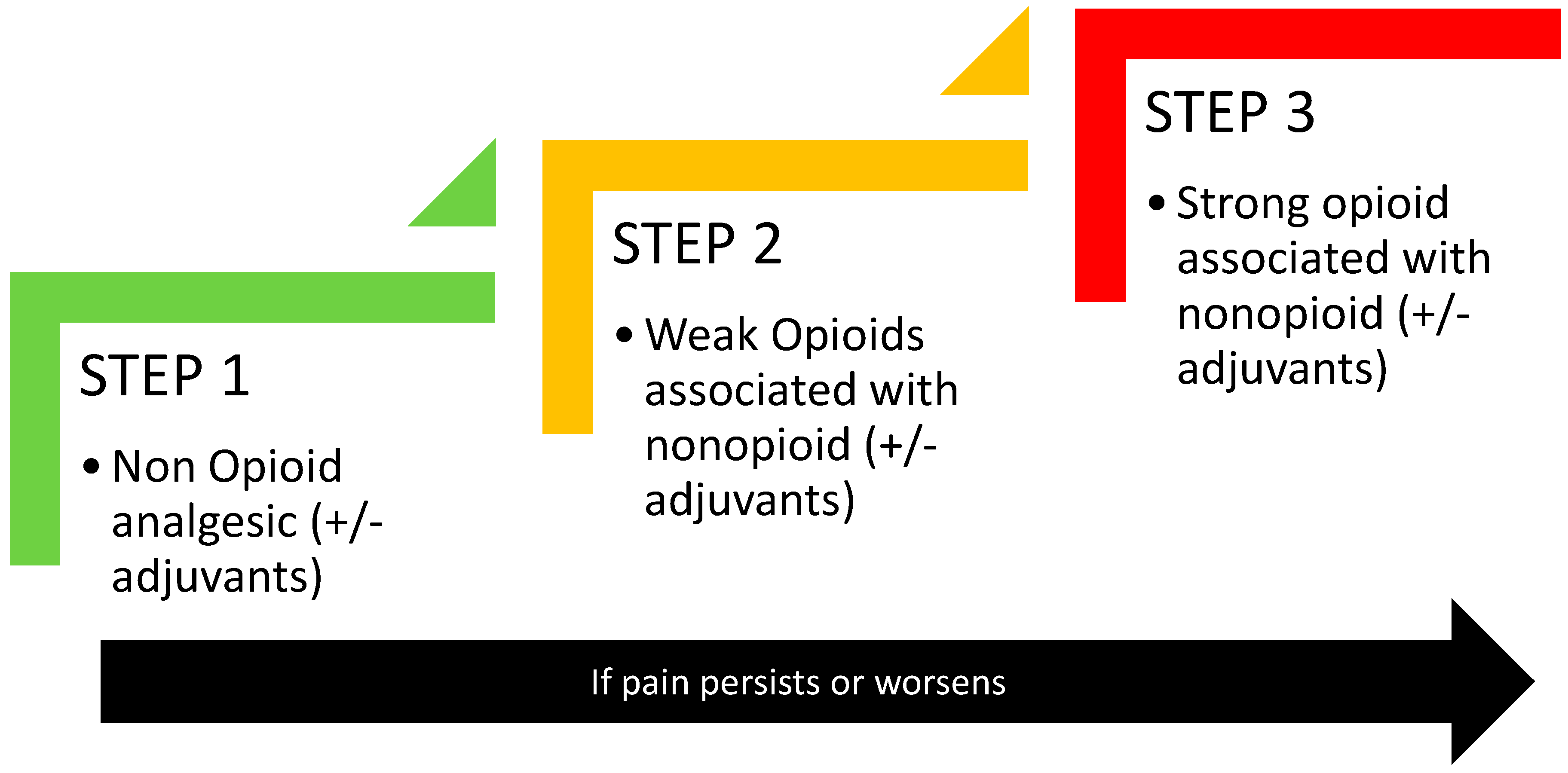
2. Opioids in Cancer Pain Management: An Update of the Mainstay Approach
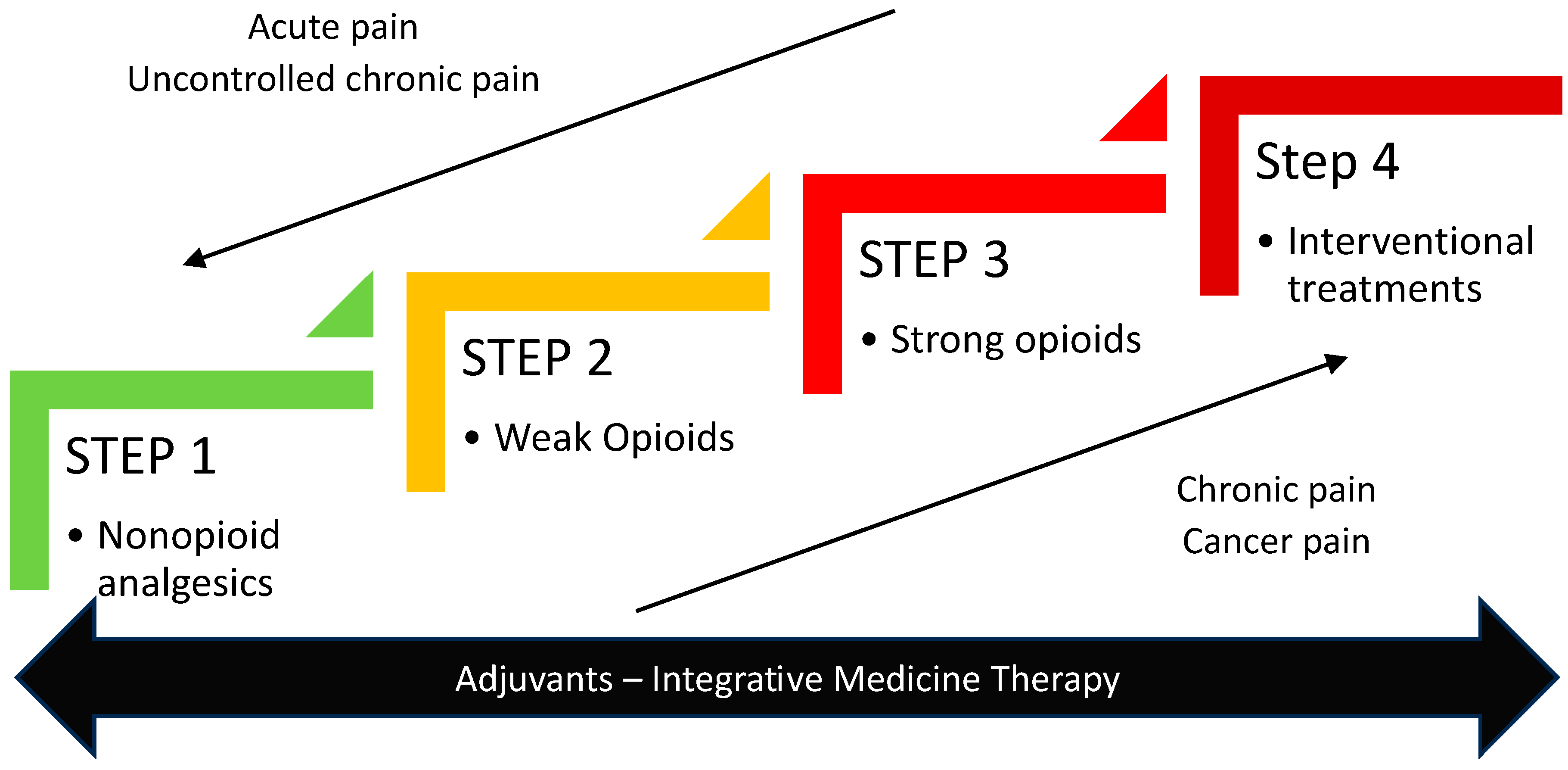
3. Optimizing Opioid Utilisation When Pain Remains Poorly Controlled
4. Adverse Effects and Harms Related to Long-Term Opioids Intake
5. The Problem of Neuropathic Pain
6. The Problem of Breakthrough Pain
7. More Personalized Treatment: Potential Benefits of Non-Invasive and Invasive Techniques in Pain Management
8. Psychological Support and Non-Pharmacological Therapies
9. Conclusions
| Treatment Options | Indications | References | |
|---|---|---|---|
| Analgesics | |||
| Nonopioid | Paracetamol, NSAIDs | Every patient if indicated | [15,16,17] |
| Weak opioids | Codeine, hydrocodone, tramadol | Mild-to-moderate pain in association with nonopioid Tramadol: second line for neuropathic pain | [16,17,18] |
| Strong opioids |
| Moderate to severe pain | [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] |
| Adjuvants | |||
| Ketamine | Refractory chronic pain | [24,38,39,40,41,42] | |
| Magnesium | Hypomagnesemia, refractory cancer pain | [43,44] | |
| Tricyclic antidepressants (TCA) | Amitriptyline, nortriptyline | Neuropathic pain | [61,62] |
| Serotonin and norepinephrine reuptake inhibitors (SNRI) | Duloxetine, venlafaxine | Neuropathic pain | [61,62,65] |
| Anticonvulsant | Gabapentin, pregabalin | Neuropathic pain | [61,62] |
| Cannabis-related medicines | Chronic pain | [48,49,50,51] | |
| Lidocaine infusion | Refractory neuropathic painRefractory visceral pain | [45,46,47] | |
| Topical treatment | Lidocaine capsaicin | Localized neuropathic pain | [68,69] |
| Interventional treatments | |||
| Photobiomodulation | Localized neuropathic pain | [83,84] | |
| Neuromodulation | Deep brain stimulation, transcranial magnetic stimulation, transcranial direct current stimulation | Under research | [82] |
| Spinal cord stimulation, dorsal root ganglion stimulation | Neuropathic pain | [82,86,87] | |
| Neuraxial drug delivery (morphine, ziconotide, local anaesthetic, baclofen, clonidine, ketamine) | Refractory pain, opioids intolerance, widespread bone metastases, specific location (i.e., pancreatic cancer) | [15,82,88,89,90,91] | |
Percutaneous neurolysis (cryoanalgesia, thermal neurotomy, pulsed radiofrequency)
| Neuropathic refractory pain
| [15,82,88,92] | |
| Cordotomy | Severe nociceptive or neuropathic pain in short term survival patients | [82] | |
| Percutaneous ablation of metastasis | Radiofrequency, cryotherapy | Metastatic bone lesions | [82] |
| Vertebroplasty and kyphoplasty | Vertebral instability and spinal cord compression consecutive to fracture | [82,93,94] | |
| Botulinum toxin | Neuropathic pain | [70,71,96,97,98,99] | |
| Integrative medicine | |||
| Hypnosis | Chronic pain | [100] | |
| Yoga Tai Chi and Qi Gong | Aromatase inhibitor-related joint pain Chronic pain | [92,100,101,102,103] | |
| Mindfulness and meditation | Cancer-related symptoms | [104,105] | |
| Cognitive behavioural strategies | Chronic pain | [105] | |
| Music therapy | Chronic pain | [105] | |
| Acupuncture | Chronic pain | [103,108,109,110,111,112] | |
| Massage therapy | Chronic pain | [100,102,114,115] | |
| Spiritual and religious interventions | Chronic pain | [92,116,117,118,119] | |
| Future therapeutic approach | |||
| Virtual reality | Under research | [120,121,122] | |
Author Contributions
Funding
Conflicts of Interest
References
- Bennett, M.I.; Kaasa, S.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D.; The IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: Chronic cancer-related pain. Pain 2019, 160, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Snijders, R.A.H.; Brom, L.; Theunissen, M.; van den Beuken-van Everdingen, M.H.J. Update on Prevalence of Pain in Patients with Cancer 2022: A Systematic Literature Review and Meta-Analysis. Cancers 2023, 15, 591. [Google Scholar] [CrossRef]
- Roberto, A.; Greco, M.T.; Uggeri, S.; Cavuto, S.; Deandrea, S.; Corli, O.; Apolone, G. Living systematic review to assess the analgesic undertreatment in cancer patients. Pain Pract. 2022, 22, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Roose, E.; Lahousse, A.; Mostaqim, K.; Reynebeau, I.; De Couck, M.; Beckwee, D.; Huysmans, E.; Bults, R.; van Wilgen, P.; et al. Pain and Opioid Use in Cancer Survivors: A Practical Guide to Account for Perceived Injustice. Pain Physician 2021, 24, 309–317. [Google Scholar] [PubMed]
- Boland, J.W.; Allgar, V.; Boland, E.G.; Bennett, M.I.; Kaasa, S.; Hjermstad, M.J.; Johnson, M. The relationship between pain, analgesics and survival in patients with advanced cancer; a secondary data analysis of the international European palliative care Cancer symptom study. Eur. J. Clin. Pharmacol. 2020, 76, 393–402. [Google Scholar] [CrossRef]
- De Groef, A.; Evenepoel, M.; Van Dijck, S.; Dams, L.; Haenen, V.; Wiles, L.; Catley, M.; Vogelzang, A.; Olver, I.; Hibbert, P.; et al. Feasibility and pilot testing of a personalized eHealth intervention for pain science education and self-management for breast cancer survivors with persistent pain: A mixed-method study. Support. Care Cancer 2023, 31, 119. [Google Scholar] [CrossRef]
- Kapoor, R.; Saxena, A.K.; Vasudev, P.; Sundriyal, D.; Kumar, A. Cancer induced bone pain: Current management and future perspectives. Med. Oncol. 2021, 38, 134. [Google Scholar] [CrossRef]
- Glare, P.; Aubrey, K.; Gulati, A.; Lee, Y.C.; Moryl, N.; Overton, S. Pharmacologic Management of Persistent Pain in Cancer Survivors. Drugs 2022, 82, 275–291. [Google Scholar] [CrossRef]
- Bouhassira, D.; Luporsi, E.; Krakowski, I. Prevalence and incidence of chronic pain with or without neuropathic characteristics in patients with cancer. Pain 2017, 158, 1118–1125. [Google Scholar]
- Preux, C.; Bertin, M.; Tarot, A.; Authier, N.; Pinol, N.; Brugnon, D.; Pereira, B.; Guastella, V. Prevalence of Opioid Use Disorder among Patients with Cancer-Related Pain: A Systematic Review. J. Clin. Med. 2022, 11, 1594. [Google Scholar] [CrossRef]
- Check, D.K.; Avecilla, R.A.V.; Mills, C.; Dinan, M.A.; Kamal, A.H.; Murphy, B.; Rezk, S.; Winn, A.; Oeffinger, K.C. Opioid Prescribing and Use Among Cancer Survivors: A Mapping Review of Observational and Intervention Studies. J. Pain Symptom Manage. 2022, 63, e397–e417. [Google Scholar] [CrossRef] [PubMed]
- van den Beuken-van Everdingen, M.H.J.; van Kuijk, S.M.J.; Janssen, D.J.A.; Joosten, E.A.J. Treatment of Pain in Cancer: Towards Personalised Medicine. Cancers 2018, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Drageset, J.; Sandvik, R.K.; Eide, L.S.P.; Austrheim, G.; Fox, M.; Beisland, E.G. Quality of life among cancer inpatients 80 years and older: A systematic review. Health Qual. Life Outcomes 2021, 19, 98. [Google Scholar] [CrossRef]
- Lemaire, A.; George, B.; Maindet, C.; Burnod, A.; Allano, G.; Minello, C. Opening up disruptive ways of management in cancer pain: The concept of multimorphic pain. Support. Care Cancer 2019, 27, 3159–3170. [Google Scholar] [CrossRef]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I.; Committee, E.G. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv166–iv191. [Google Scholar] [CrossRef] [PubMed]
- Aman, M.M.; Mahmoud, A.; Deer, T.; Sayed, D.; Hagedorn, J.M.; Brogan, S.E.; Singh, V.; Gulati, A.; Strand, N.; Weisbein, J.; et al. The American Society of Pain and Neuroscience (ASPN) Best Practices and Guidelines for the Interventional Management of Cancer-Associated Pain. J. Pain Res. 2021, 14, 2139–2164. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. In WHO Guidelines Approved by the Guidelines Review Committee; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Caraceni, A.; Hanks, G.; Kaasa, S.; Bennett, M.I.; Brunelli, C.; Cherny, N.; Dale, O.; De Conno, F.; Fallon, M.; Hanna, M.; et al. Use of opioid analgesics in the treatment of cancer pain: Evidence-based recommendations from the EAPC. Lancet Oncol. 2012, 13, e58–e68. [Google Scholar] [CrossRef] [PubMed]
- Wiffen, P.J.; Wee, B.; Derry, S.; Bell, R.F.; Moore, R.A. Opioids for cancer pain—An overview of Cochrane reviews. Cochrane Database Syst. Rev. 2017, 7, CD012592. [Google Scholar]
- Corli, O.; Floriani, I.; Roberto, A.; Montanari, M.; Galli, F.; Greco, M.T.; Caraceni, A.; Kaasa, S.; Dragani, T.A.; Azzarello, G.; et al. Are strong opioids equally effective and safe in the treatment of chronic cancer pain? A multicenter randomized phase IV ‘real life’ trial on the variability of response to opioids. Ann. Oncol. 2016, 27, 1107–1115. [Google Scholar] [CrossRef]
- Fallon, M.; Dierberger, K.; Leng, M.; Hall, P.S.; Allende, S.; Sabar, R.; Verastegui, E.; Gordon, D.; Grant, L.; Lee, R.; et al. An international, open-label, randomised trial comparing a two-step approach versus the standard three-step approach of the WHO analgesic ladder in patients with cancer. Ann. Oncol. 2022, 33, 1296–1303. [Google Scholar] [CrossRef]
- Crush, J.; Levy, N.; Knaggs, R.D.; Lobo, D.N. The pitfalls of labelling opioids as weak or strong. Response to Br J Anaesth 2022; 129, e150-e153. Br. J. Anaesth. 2023, 130, e16–e17. [Google Scholar] [CrossRef]
- Corli, O.; Roberto, A.; Bennett, M.I.; Galli, F.; Corsi, N.; Rulli, E.; Antonione, R. Nonresponsiveness and Susceptibility of Opioid Side Effects Related to Cancer Patients’ Clinical Characteristics: A Post-Hoc Analysis. Pain Pract. 2018, 18, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Lavand’homme, P.; Steyaert, A. Opioid-free anesthesia opioid side effects: Tolerance and hyperalgesia. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 487–498. [Google Scholar] [CrossRef]
- Treillet, E.; Laurent, S.; Hadjiat, Y. Practical management of opioid rotation and equianalgesia. J. Pain Res. 2018, 11, 2587–2601. [Google Scholar] [CrossRef]
- Schuster, M.; Bayer, O.; Heid, F.; Laufenberg-Feldmann, R. Opioid Rotation in Cancer Pain Treatment. Dtsch. Arztebl. Int. 2018, 115, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Heung, Y.; Reddy, A. How to Use Methadone in an Era of an Opioid Epidemic. Curr. Treat. Options Oncol. 2020, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Adile, C.; Ferrera, P.; Pallotti, M.C.; Ricci, M.; Bonanno, G.; Casuccio, A. Methadone as First-line Opioid for the Management of Cancer Pain. Oncologist 2022, 27, 323–327. [Google Scholar] [CrossRef]
- Lukin, B.; Greenslade, J.; Kearney, A.M.; Douglas, C.; Howell, T.; Barras, M.; Good, P. Conversion of other opioids to methadone: A retrospective comparison of two methods. BMJ Support. Palliat. Care 2020, 10, 201–204. [Google Scholar] [CrossRef]
- Nicholson, A.B.; Watson, G.R.; Derry, S.; Wiffen, P.J. Methadone for cancer pain. Cochrane Database Syst. Rev. 2017, 2, CD003971. [Google Scholar] [CrossRef]
- Courtemanche, F.; Dao, D.; Gagne, F.; Tremblay, L.; Neron, A. Methadone as a Coanalgesic for Palliative Care Cancer Patients. J. Palliat. Med. 2016, 19, 972–978. [Google Scholar] [CrossRef]
- Furst, P.; Lundstrom, S.; Klepstad, P.; Strang, P. The Use of Low-Dose Methadone as Add-On to Regular Opioid Therapy in Cancer-Related Pain at End of Life: A National Swedish Survey in Specialized Palliative Care. J. Palliat. Med. 2020, 23, 226–232. [Google Scholar] [CrossRef]
- Kress, H.G. Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine. Eur. J. Pain 2009, 13, 219–230. [Google Scholar] [CrossRef]
- Ahn, J.S.; Lin, J.; Ogawa, S.; Yuan, C.; O’Brien, T.; Le, B.H.; Bothwell, A.M.; Moon, H.; Hadjiat, Y.; Ganapathi, A. Transdermal buprenorphine and fentanyl patches in cancer pain: A network systematic review. J. Pain Res. 2017, 10, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Hansen, M.; Bromham, N.; Taubert, M.; Arnold, S.; Hilgart, J.S. Buprenorphine for treating cancer pain. Cochrane Database Syst. Rev. 2015, 2015, CD009596. [Google Scholar] [CrossRef] [PubMed]
- Kress, H.G.; Coluzzi, F. Tapentadol in the management of cancer pain: Current evidence and future perspectives. J. Pain Res. 2019, 12, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Takemura, M.; Niki, K.; Okamoto, Y.; Matsuda, Y.; Omae, T.; Takagi, T.; Ueda, M. Tapentadol in Cancer Patients with Neuropathic Pain: A Comparison of Methadone, Oxycodone, Fentanyl, and Hydromorphone. Biol. Pharm. Bull. 2021, 44, 1286–1293. [Google Scholar] [CrossRef]
- Culp, C.; Kim, H.K.; Abdi, S. Ketamine Use for Cancer and Chronic Pain Management. Front. Pharmacol. 2020, 11, 599721. [Google Scholar] [CrossRef] [PubMed]
- Blonk, M.I.; Koder, B.G.; van den Bemt, P.M.; Huygen, F.J. Use of oral ketamine in chronic pain management: A review. Eur. J. Pain 2010, 14, 466–472. [Google Scholar] [CrossRef]
- Bell, R.F.; Eccleston, C.; Kalso, E.A. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst. Rev. 2017, 6, Cd003351. [Google Scholar] [CrossRef]
- Cheung, K.W.A.; Chan, P.C.; Lo, S.H. The use of ketamine in the management of refractory cancer pain in a palliative care unit. Ann. Palliat. Med. 2020, 9, 4478–4489. [Google Scholar] [CrossRef]
- Courade, M.; Bertrand, A.; Guerrini-Rousseau, L.; Pagnier, A.; Levy, D.; Lervat, C.; Cojean, N.; Ribrault, A.; Dugue, S.; Thouvenin, S.; et al. Low-dose ketamine adjuvant treatment for refractory pain in children, adolescents and young adults with cancer: A pilot study. BMJ Support. Palliat. Care 2022, 12, e656–e663. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Saca, J.M.; Lopez-Picazo, J.M.; Larumbe, A.; Urdiroz, J.; Centeno, C. Hypomagnesemia as a possible explanation behind episodes of severe pain in cancer patients receiving palliative care. Support. Care Cancer 2013, 21, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, V.; Pelissier, T.; Cazanga, V.; Hernandez, A.; Constandil, L. Magnesium Salt, a Simple Strategy to Improve Methadone Analgesia in Chronic Pain: An Isobolographic Preclinical Study in Neuropathic Mice. Front. Pharmacol. 2020, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Sanderson, C.R.; Xuan, W.; Agar, M. Lidocaine for Cancer Pain in Adults: A Systematic Review and Meta-Analysis. J. Palliat. Med. 2019, 22, 326–334. [Google Scholar] [CrossRef]
- Estebe, J.-P. Intravenous lidocaine. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 513–521. [Google Scholar] [CrossRef]
- Chong, P.H.; Yeo, Z.Z. Parenteral Lidocaine for Complex Cancer Pain in the Home or Inpatient Hospice Setting: A Review and Synthesis of the Evidence. J. Palliat. Med. 2021, 24, 1154–1160. [Google Scholar] [CrossRef]
- Boland, E.G.; Bennett, M.I.; Allgar, V.; Boland, J.W. Cannabinoids for adult cancer-related pain: Systematic review and meta-analysis. BMJ Support. Palliat. Care 2020, 10, 14–24. [Google Scholar] [CrossRef]
- Fisher, E.; Moore, R.A.; Fogarty, A.E.; Finn, D.P.; Finnerup, N.B.; Gilron, I.; Haroutounian, S.; Krane, E.; Rice, A.S.C.; Rowbotham, M.; et al. Cannabinoids, cannabis, and cannabis-based medicine for pain management: A systematic review of randomised controlled trials. Pain 2021, 162, S45–S66. [Google Scholar] [CrossRef]
- Häuser, W.; Fitzcharles, M.A.; Radbruch, L.; Petzke, F. Cannabinoids in Pain Management and Palliative Medicine. Dtsch. Arztebl. Int. 2017, 114, 627–634. [Google Scholar] [CrossRef]
- Wang, L.; Hong, P.J.; May, C.; Rehman, Y.; Oparin, Y.; Hong, C.J.; Hong, B.Y.; AminiLari, M.; Gallo, L.; Kaushal, A.; et al. Medical cannabis or cannabinoids for chronic non-cancer and cancer related pain: A systematic review and meta-analysis of randomised clinical trials. BMJ 2021, 374, n1034. [Google Scholar] [CrossRef]
- Bates, N.; Bello, J.K.; Osazuwa-Peters, N.; Sullivan, M.D.; Scherrer, J.F. Depression and Long-Term Prescription Opioid Use and Opioid Use Disorder: Implications for Pain Management in Cancer. Curr. Treat. Options Oncol. 2022, 23, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Kim, H.G.; Song, I.A. New, long-term opioid use after lung cancer surgery is associated with reduced 2-year survival: A retrospective population-based cohort study in South Korea. Reg. Anesth. Pain Med. 2022, 47, 678–683. [Google Scholar]
- Sandbrink, F.; Murphy, J.L.; Johansson, M.; Olson, J.L.; Edens, E.; Clinton-Lont, J.; Sall, J.; Spevak, C.; VA/DoD Guideline Development Group. The Use of Opioids in the Management of Chronic Pain: Synopsis of the 2022 Updated, U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guideline. Ann. Intern. Med. 2023, 176, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, C.; Bennett, M.I.; Kaasa, S.; Fainsinger, R.; Sjgren, P.; Mercadante, S.; Lohre, E.T.; Caraceni, A.; European Association for Palliative Care Research Network. International Association for the Study of Pain Cancer Pain Special Interest G. Classification of neuropathic pain in cancer patients: A Delphi expert survey report and EAPC/IASP proposal of an algorithm for diagnostic criteria. Pain 2014, 155, 2707–2713. [Google Scholar] [CrossRef]
- Zinboonyahgoon, N.; Luansritisakul, C. Neuropathic pain feature in cancer-induced bone pain: Does it matter? A prospective observational study. Korean J. Pain 2023, 36, 253–267. [Google Scholar] [CrossRef]
- Oh, S.Y.; Shin, S.W.; Koh, S.J.; Bae, S.B.; Chang, H.; Kim, J.H.; Kim, H.J.; Hong, Y.S.; Park, K.U.; Park, J.; et al. Multicenter, cross-sectional observational study of the impact of neuropathic pain on quality of life in cancer patients. Support. Care Cancer 2017, 25, 3759–3767. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.L.H.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T.; et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef]
- Mulvey, M.R.; Boland, E.G.; Bouhassira, D.; Freynhagen, R.; Hardy, J.; Hjermstad, M.J.; Mercadante, S.; Perez, C.; Bennett, M.I. Neuropathic pain in cancer: Systematic review, performance of screening tools and analysis of symptom profiles. Br. J. Anaesth. 2017, 119, 765–774. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Guan, J.; Tanaka, S.; Kawakami, K. Anticonvulsants or Antidepressants in Combination Pharmacotherapy for Treatment of Neuropathic Pain in Cancer Patients: A Systematic Review and Meta-analysis. Clin. J. Pain 2016, 32, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Iwase, S.; Miyaji, T.; Kawaguchi, T.; Ariyoshi, K.; Oyamada, S.; Satomi, E.; Ishiki, H.; Hasuo, H.; Sakuma, H.; et al. Additive Duloxetine for Cancer-Related Neuropathic Pain Nonresponsive or Intolerant to Opioid-Pregabalin Therapy: A Randomized Controlled Trial (JORTC-PAL08). J. Pain Symptom Manag. 2019, 58, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Iwase, S.; Miyaji, T.; Kawaguchi, T.; Ariyoshi, K.; Oyamada, S.; Satomi, E.; Ishiki, H.; Hasuo, H.; Sakuma, H.; et al. Predictors of duloxetine response in patients with neuropathic cancer pain: A secondary analysis of a randomized controlled trial-JORTC-PAL08 (DIRECT) study. Support. Care Cancer 2020, 28, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Curry, Z.A.; Dang, M.C.; Sima, A.P.; Abdullaziz, S.; Del Fabbro, E.G. Combination therapy with methadone and duloxetine for cancer-related pain: A retrospective study. Ann. Palliat. Med. 2021, 10, 2505–2511. [Google Scholar] [CrossRef]
- Driot, D.; Jouanjus, E.; Oustric, S.; Dupouy, J.; Lapeyre-Mestre, M. Patterns of gabapentin and pregabalin use and misuse: Results of a population-based cohort study in France. Br. J. Clin. Pharmacol. 2019, 85, 1260–1269. [Google Scholar] [CrossRef]
- Hahn, J.; Jo, Y.; Yoo, S.H.; Shin, J.; Yu, Y.M.; Ah, Y.M. Risk of major adverse events associated with gabapentinoid and opioid combination therapy: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 1009950. [Google Scholar] [CrossRef] [PubMed]
- Garzón-Rodríguez, C.; Casals Merchan, M.; Calsina-Berna, A.; López-Rómboli, E.; Porta-Sales, J. Lidocaine 5% patches as an effective short-term co-analgesic in cancer pain. Preliminary results. Support. Care Cancer 2013, 21, 3153–3158. [Google Scholar] [CrossRef]
- Cabezon-Gutierrez, L.; Custodio-Cabello, S.; Palka-Kotlowska, M.; Khosravi-Shahi, P. High-Dose 8% Capsaicin Patch in Treatment of Chemotherapy-Induced Peripheral Neuropathy. A Systematic Review. J. Pain Symptom Manag. 2020, 60, 1047–1054.e1041. [Google Scholar] [CrossRef]
- Attal, N.; de Andrade, D.C.; Adam, F.; Ranoux, D.; Teixeira, M.J.; Galhardoni, R.; Raicher, I.; Uceyler, N.; Sommer, C.; Bouhassira, D. Safety and efficacy of repeated injections of botulinum toxin A in peripheral neuropathic pain (BOTNEP): A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2016, 15, 555–565. [Google Scholar] [CrossRef]
- Lippi, L.; de Sire, A.; Folli, A.; D’Abrosca, F.; Grana, E.; Baricich, A.; Carda, S.; Invernizzi, M. Multidimensional Effectiveness of Botulinum Toxin in Neuropathic Pain: A Systematic Review of Randomized Clinical Trials. Toxins 2022, 14, 308. [Google Scholar] [CrossRef]
- Arbaiza, D.; Vidal, O. Tramadol in the treatment of neuropathic cancer pain: A double-blind, placebo-controlled study. Clin. Drug Investig. 2007, 27, 75–83. [Google Scholar] [CrossRef]
- Hartrick, C.T.; Rozek, R.J. Tapentadol in pain management: A mu-opioid receptor agonist and noradrenaline reuptake inhibitor. CNS Drugs 2011, 25, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Kress, H.G.; Koch, E.D.; Kosturski, H.; Steup, A.; Karcher, K.; Lange, B.; Dogan, C.; Etropolski, M.S.; Eerdekens, M. Tapentadol prolonged release for managing moderate to severe, chronic malignant tumor-related pain. Pain Physician 2014, 17, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Fawoubo, A.; Perceau-Chambard, E.; Ruer, M.; Filbet, M.; Tricou, C.; Economos, G. Methadone and neuropathic cancer pain subcomponents: A prospective cohort pilot study. BMJ Support. Palliat. Care 2021. [Google Scholar] [CrossRef]
- Mercadante, S.; Portenoy, R.K. Breakthrough cancer pain: Twenty-five years of study. Pain 2016, 157, 2657–2663. [Google Scholar] [CrossRef]
- Mercadante, S.; Portenoy, R.K. Understanding the Chameleonic Breakthrough Cancer Pain. Drugs 2021, 81, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Maltoni, M.; Russo, D.; Adile, C.; Ferrera, P.; Rossi, R.; Rosati, M.; Casuccio, A. The Prevalence and Characteristics of Breakthrough Cancer Pain in Patients Receiving Low Doses of Opioids for Background Pain. Cancers 2021, 13, 1058. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Schaffer, G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can. Fam. Physician 2010, 56, 514–517. [Google Scholar]
- Hagedorn, J.M.; Pittelkow, T.P.; Hunt, C.L.; D’Souza, R.S.; Lamer, T.J. Current Perspectives on Spinal Cord Stimulation for the Treatment of Cancer Pain. J. Pain Res. 2020, 13, 3295–3305. [Google Scholar] [CrossRef]
- Anekar, A.A.; Hendrix, J.M.; Cascella, M. WHO Analgesic Ladder. In StatPearls; StatPearls Publishing LLC.: Tampa, FL, USA, 2023. [Google Scholar]
- Allano, G.; George, B.; Minello, C.; Burnod, A.; Maindet, C.; Lemaire, A. Strategies for interventional therapies in cancer-related pain-a crossroad in cancer pain management. Support. Care Cancer 2019, 27, 3133–3145. [Google Scholar] [CrossRef]
- Robijns, J.; Nair, R.G.; Lodewijckx, J.; Arany, P.; Barasch, A.; Bjordal, J.M.; Bossi, P.; Chilles, A.; Corby, P.M.; Epstein, J.B.; et al. Photobiomodulation therapy in management of cancer therapy-induced side effects: WALT position paper 2022. Front. Oncol. 2022, 12, 927685. [Google Scholar] [CrossRef] [PubMed]
- Baxter, G.D.; Liu, L.; Petrich, S.; Gisselman, A.S.; Chapple, C.; Anders, J.J.; Tumilty, S. Low level laser therapy (Photobiomodulation therapy) for breast cancer-related lymphedema: A systematic review. BMC Cancer 2017, 17, 833. [Google Scholar] [CrossRef] [PubMed]
- Krames, E.S.; Rezai, A.R.; Peckham, P.H. Introduction. In Neuromodulation, 2nd ed.; Krames, E.S., Peckham, P.H., Rezai, A.R., Eds.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Peng, L.; Min, S.; Zejun, Z.; Wei, K.; Bennett, M.I. Spinal cord stimulation for cancer-related pain in adults. Cochrane Database Syst. Rev. 2015, 2015, Cd009389. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Her, Y.F.; Jin, M.Y.; Morsi, M.; Abd-Elsayed, A. Neuromodulation Therapy for Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review. Biomedicines 2022, 10, 1909. [Google Scholar] [CrossRef]
- Carvajal, G. Pancreatic Cancer Related Pain: Review of Pathophysiology and Intrathecal Drug Delivery Systems for Pain Management. Pain Physician 2021, 24, e583–e594. [Google Scholar]
- Deer, T.R.; Pope, J.E.; Hanes, M.C.; McDowell, G.C. Intrathecal Therapy for Chronic Pain: A Review of Morphine and Ziconotide as Firstline Options. Pain Med. 2019, 20, 784–798. [Google Scholar] [CrossRef]
- Perruchoud, C.; Dupoiron, D.; Papi, B.; Calabrese, A.; Brogan, S.E. Management of Cancer-Related Pain With Intrathecal Drug Delivery: A Systematic Review and Meta-Analysis of Clinical Studies. Neuromodulation 2022, 25, S1. [Google Scholar] [CrossRef]
- Duarte, R.; Copley, S.; Nevitt, S.; Maden, M.; Al-Ali, A.M.; Dupoiron, D.; Eldabe, S. Effectiveness and Safety of Intrathecal Drug Delivery Systems for the Management of Cancer Pain: A Systematic Review and Meta-Analysis. Neuromodulation 2022. [Google Scholar] [CrossRef]
- Lara-Solares, A.; Ahumada Olea, M.; Basantes Pinos, A.; Bistre Cohén, S.; Bonilla Sierra, P.; Duarte Juárez, E.R.; Símon Escudero, O.A.; Santacruz Escudero, J.G.; Flores Cantisani, J.A. Latin-American guidelines for cancer pain management. Pain Manag. 2017, 7, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, S.T.; Kirkegaard, A.O.; Carreon, L.; Rousing, R.; Andersen, M. Vertebroplasty or kyphoplasty as palliative treatment for cancer-related vertebral compression fractures: A systematic review. Spine J. 2019, 19, 1067–1075. [Google Scholar] [CrossRef]
- Mattie, R.; Brar, N.; Tram, J.T.; McCormick, Z.L.; Beall, D.P.; Fox, A.; Saltychev, M. Vertebral Augmentation of Cancer-Related Spinal Compression Fractures: A Systematic Review and Meta-Analysis. Spine 2021, 46, 1729–1737. [Google Scholar] [CrossRef]
- Samizadeh, S.; De Boulle, K. Botulinum neurotoxin formulations: Overcoming the confusion. Clin. Cosmet. Investig. Dermatol. 2018, 11, 273–287. [Google Scholar] [CrossRef]
- Mittal, S.O.; Jabbari, B. Botulinum Neurotoxins and Cancer-A Review of the Literature. Toxins 2020, 12, 32. [Google Scholar] [CrossRef]
- Kim, S.I.; Choi, Y. Botulinum Toxin Injection for Intractable Pain in Cancer Patients with Psoas Muscle Invasion. J. Pain Symptom Manag. 2022, 63, e441–e444. [Google Scholar] [CrossRef]
- Rostami, R.; Mittal, S.O.; Radmand, R.; Jabbari, B. Incobotulinum Toxin-A Improves Post-Surgical and Post-Radiation Pain in Cancer Patients. Toxins 2016, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Grenda, T.; Grenda, A.; Krawczyk, P.; Kwiatek, K. Botulinum toxin in cancer therapy—Current perspectives and limitations. Appl. Microbiol. Biotechnol. 2022, 106, 485–495. [Google Scholar] [CrossRef]
- Deng, G. Integrative Medicine Therapies for Pain Management in Cancer Patients. Cancer J. 2019, 25, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J.; Ismaila, N.; Bao, T.; Barton, D.; Ben-Arye, E.; Garland, E.L.; Greenlee, H.; Leblanc, T.; Lee, R.T.; Lopez, A.M.; et al. Integrative Medicine for Pain Management in Oncology: Society for Integrative Oncology–ASCO Guideline. J. Clin. Oncol. 2022, 40, 3998–4024. [Google Scholar] [CrossRef] [PubMed]
- De Groef, A.; Penen, F.; Dams, L.; Van der Gucht, E.; Nijs, J.; Meeus, M. Best-Evidence Rehabilitation for Chronic Pain Part 2: Pain during and after Cancer Treatment. J. Clin. Med. 2019, 8, 979. [Google Scholar] [CrossRef]
- Tao, W.W.; Jiang, H.; Tao, X.M.; Jiang, P.; Sha, L.Y.; Sun, X.C. Effects of Acupuncture, Tuina, Tai Chi, Qigong, and Traditional Chinese Medicine Five-Element Music Therapy on Symptom Management and Quality of Life for Cancer Patients: A Meta-Analysis. J. Pain Symptom Manag. 2016, 51, 728–747. [Google Scholar] [CrossRef]
- Ngamkham, S.; Holden, J.E.; Smith, E.L. A Systematic Review: Mindfulness Intervention for Cancer-Related Pain. Asia Pac. J. Oncol. Nurs. 2019, 6, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Ruano, A.; García-Torres, F.; Gálvez-Lara, M.; Moriana, J.A. Psychological and Non-Pharmacologic Treatments for Pain in Cancer Patients: A Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2022, 63, e505–e520. [Google Scholar] [CrossRef] [PubMed]
- Bradt, J.; Dileo, C.; Myers-Coffman, K.; Biondo, J. Music interventions for improving psychological and physical outcomes in people with cancer. Cochrane Database Syst. Rev. 2021, 10, Cd006911. [Google Scholar]
- Barke, A.; Koechlin, H.; Korwisi, B.; Locher, C. Emotional distress: Specifying a neglected part of chronic pain. Eur. J. Pain 2020, 24, 477–480. [Google Scholar] [CrossRef]
- Paley, C.A.; Johnson, M.I.; Tashani, O.A.; Bagnall, A.M. Acupuncture for cancer pain in adults. Cochrane Database Syst. Rev. 2015, 2015, Cd007753. [Google Scholar]
- Wen, J.; Chen, X.; Yang, Y.; Liu, J.; Li, E.; Liu, J.; Zhou, Z.; Wu, W.; He, K. Acupuncture Medical Therapy and its Underlying Mechanisms: A Systematic Review. Am. J. Chin. Med. 2021, 49, 1–23. [Google Scholar] [CrossRef]
- Birch, S.; Lee, M.S.; Alraek, T.; Kim, T.H. Evidence, safety and recommendations for when to use acupuncture for treating cancer related symptoms: A narrative review. Integr. Med. Res. 2019, 8, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wahner-Roedler, D.L.; Zhou, X.; Johnson, L.A.; Do, A.; Pachman, D.R.; Chon, T.Y.; Salinas, M.; Millstine, D.; Bauer, B.A. Acupuncture for palliative cancer pain management: Systematic review. BMJ Support. Palliat. Care 2021, 11, 264–270. [Google Scholar] [CrossRef]
- He, Y.; Guo, X.; May, B.H.; Zhang, A.L.; Liu, Y.; Lu, C.; Mao, J.J.; Xue, C.C.; Zhang, H. Clinical Evidence for Association of Acupuncture and Acupressure With Improved Cancer Pain: A Systematic Review and Meta-Analysis. JAMA Oncol. 2020, 6, 271–278. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Hsieh, Y.J.; Tsai, P.S. Systematic review and meta-analysis of acupuncture to reduce cancer-related pain. Eur. J. Cancer Care 2017, 26, e12457. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.Y.; Yeo, S.; Kim, S.H.; Lim, S. Meta-Analysis of Massage Therapy on Cancer Pain. Integr. Cancer Ther. 2015, 14, 297–304. [Google Scholar] [CrossRef]
- Boyd, C.; Crawford, C.; Paat, C.F.; Price, A.; Xenakis, L.; Zhang, W. The Impact of Massage Therapy on Function in Pain Populations-A Systematic Review and Meta-Analysis of Randomized Controlled Trials: Part II, Cancer Pain Populations. Pain Med. 2016, 17, 1553–1568. [Google Scholar] [CrossRef]
- Kamal, A.H.; Gradison, M.; Maguire, J.M.; Taylor, D.; Abernethy, A.P. Quality measures for palliative care in patients with cancer: A systematic review. J. Oncol. Pract. 2014, 10, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.P.B.; Lucchetti, G.; Menezes, P.R.; Vallada, H. Complementary religious and spiritual interventions in physical health and quality of life: A systematic review of randomized controlled clinical trials. PLoS ONE 2017, 12, e0186539. [Google Scholar] [CrossRef]
- Kruizinga, R.; Hartog, I.D.; Jacobs, M.; Daams, J.G.; Scherer-Rath, M.; Schilderman, J.B.; Sprangers, M.A.; Van Laarhoven, H.W. The effect of spiritual interventions addressing existential themes using a narrative approach on quality of life of cancer patients: A systematic review and meta-analysis. Psychooncology 2016, 25, 253–265. [Google Scholar] [CrossRef]
- Hindmarch, T.; Dalrymple, J.; Smith, M.; Barclay, S. Spiritual interventions for cancer pain: A systematic review and narrative synthesis. BMJ Support. Palliat. Care 2022, 12, 1–9. [Google Scholar] [CrossRef]
- Chow, H.; Hon, J.; Chua, W.; Chuan, A. Effect of Virtual Reality Therapy in Reducing Pain and Anxiety for Cancer-Related Medical Procedures: A Systematic Narrative Review. J. Pain Symptom Manag. 2021, 61, 384–394. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, J.; Han, R.; Peng, C.; Huang, A. Using Virtual Reality Exposure Therapy in Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Value Health 2022, 25, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, J.E.; Cheng, A.S.K.; Cheng, H.; Wefel, J.S. Meta-Analysis of the Efficacy of Virtual Reality-Based Interventions in Cancer-Related Symptom Management. Integr. Cancer Ther. 2019, 18, 1534735419871108. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mestdagh, F.; Steyaert, A.; Lavand’homme, P. Cancer Pain Management: A Narrative Review of Current Concepts, Strategies, and Techniques. Curr. Oncol. 2023, 30, 6838-6858. https://doi.org/10.3390/curroncol30070500
Mestdagh F, Steyaert A, Lavand’homme P. Cancer Pain Management: A Narrative Review of Current Concepts, Strategies, and Techniques. Current Oncology. 2023; 30(7):6838-6858. https://doi.org/10.3390/curroncol30070500
Chicago/Turabian StyleMestdagh, François, Arnaud Steyaert, and Patricia Lavand’homme. 2023. "Cancer Pain Management: A Narrative Review of Current Concepts, Strategies, and Techniques" Current Oncology 30, no. 7: 6838-6858. https://doi.org/10.3390/curroncol30070500
APA StyleMestdagh, F., Steyaert, A., & Lavand’homme, P. (2023). Cancer Pain Management: A Narrative Review of Current Concepts, Strategies, and Techniques. Current Oncology, 30(7), 6838-6858. https://doi.org/10.3390/curroncol30070500





