In Vitro Assessment of the Synergistic Effect of Aspirin and 5-Fluorouracil in Colorectal Adenocarcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cellular-Viability Assessment
2.4. Cellular Morphology
2.5. Immunofluorescence
2.6. Wound-Healing Assay
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. Combination-Index Calculation
2.9. Statistical Analysis
3. Results
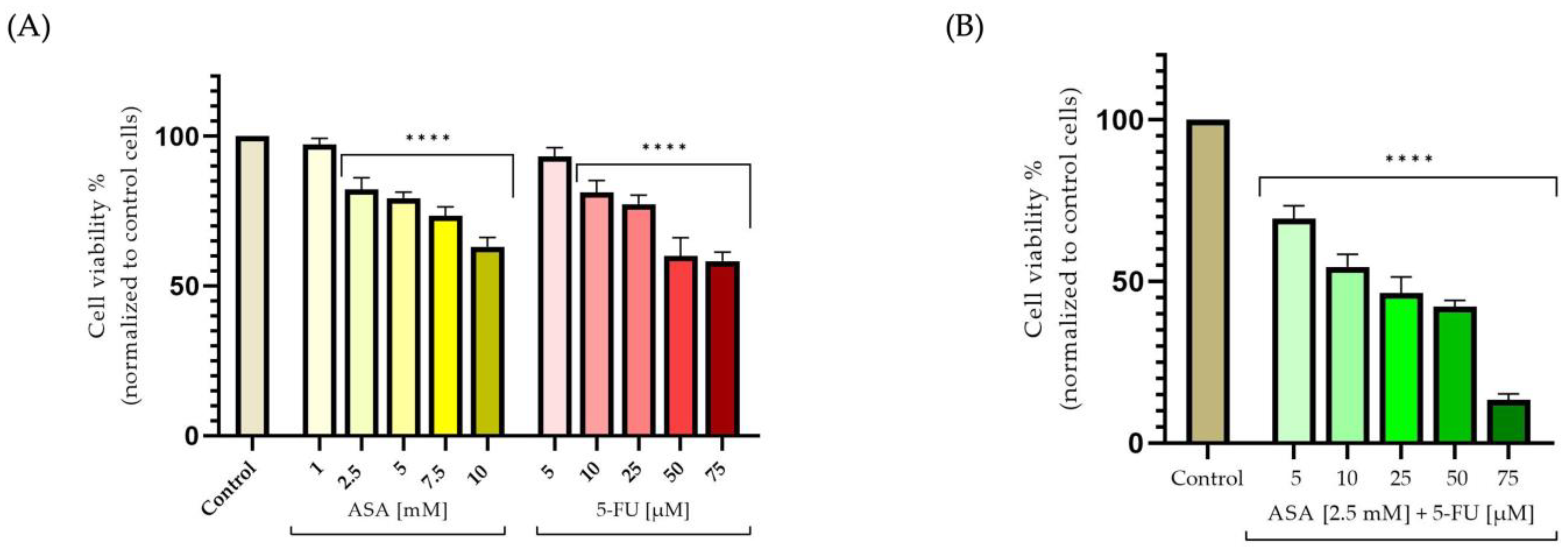
3.1. Cellular-Viability Assessment
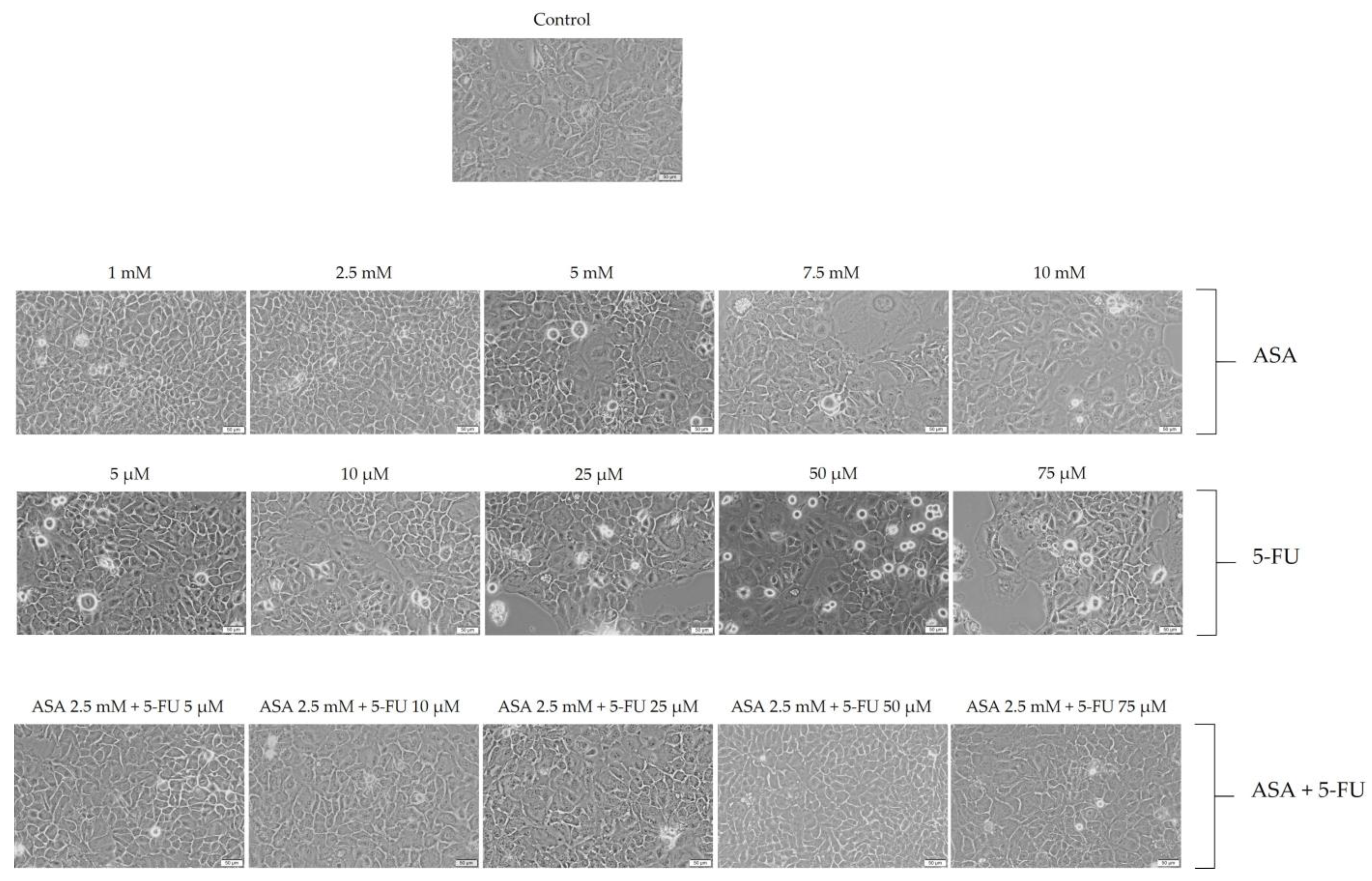
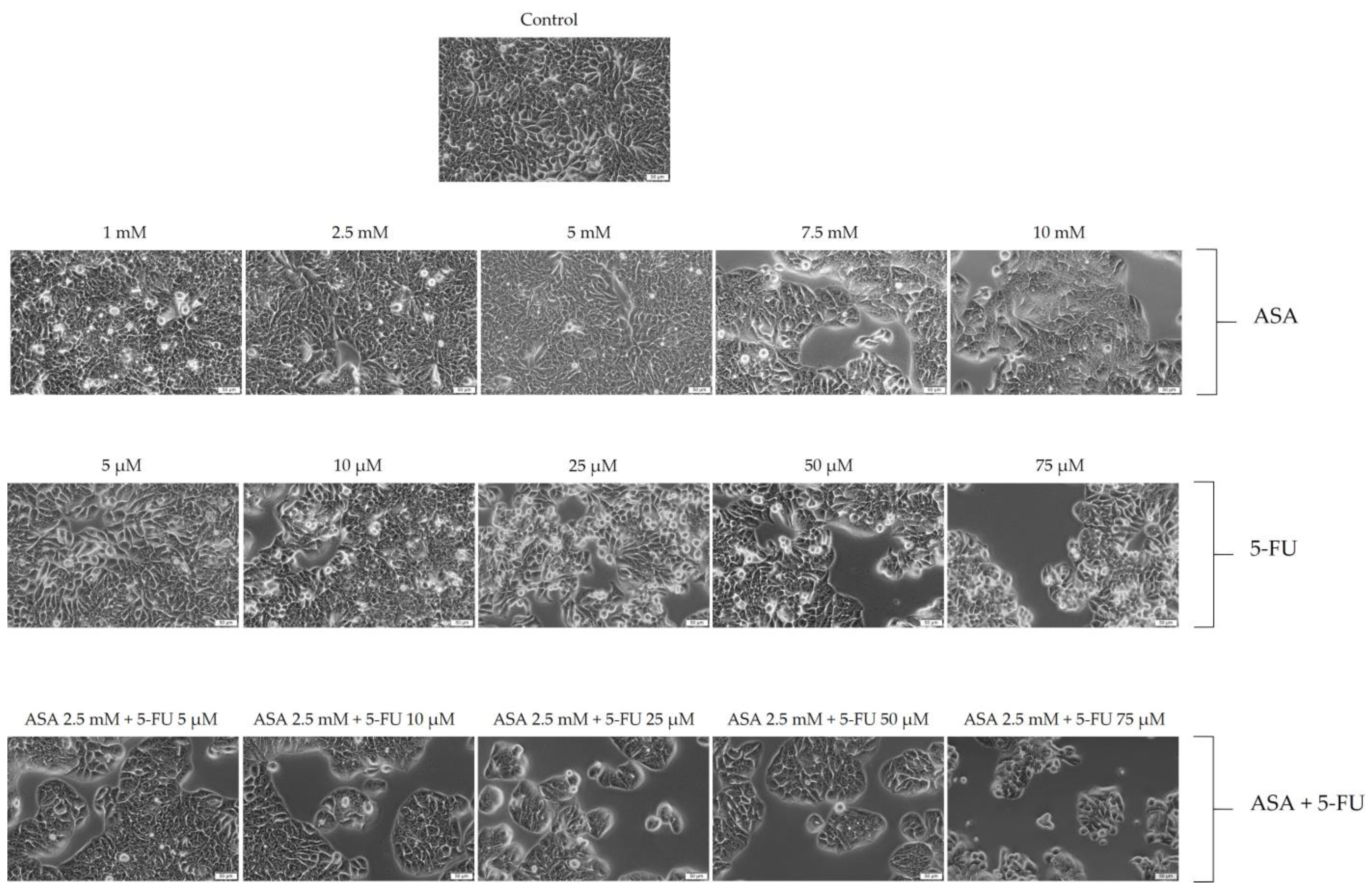
3.2. Cellular Morphology
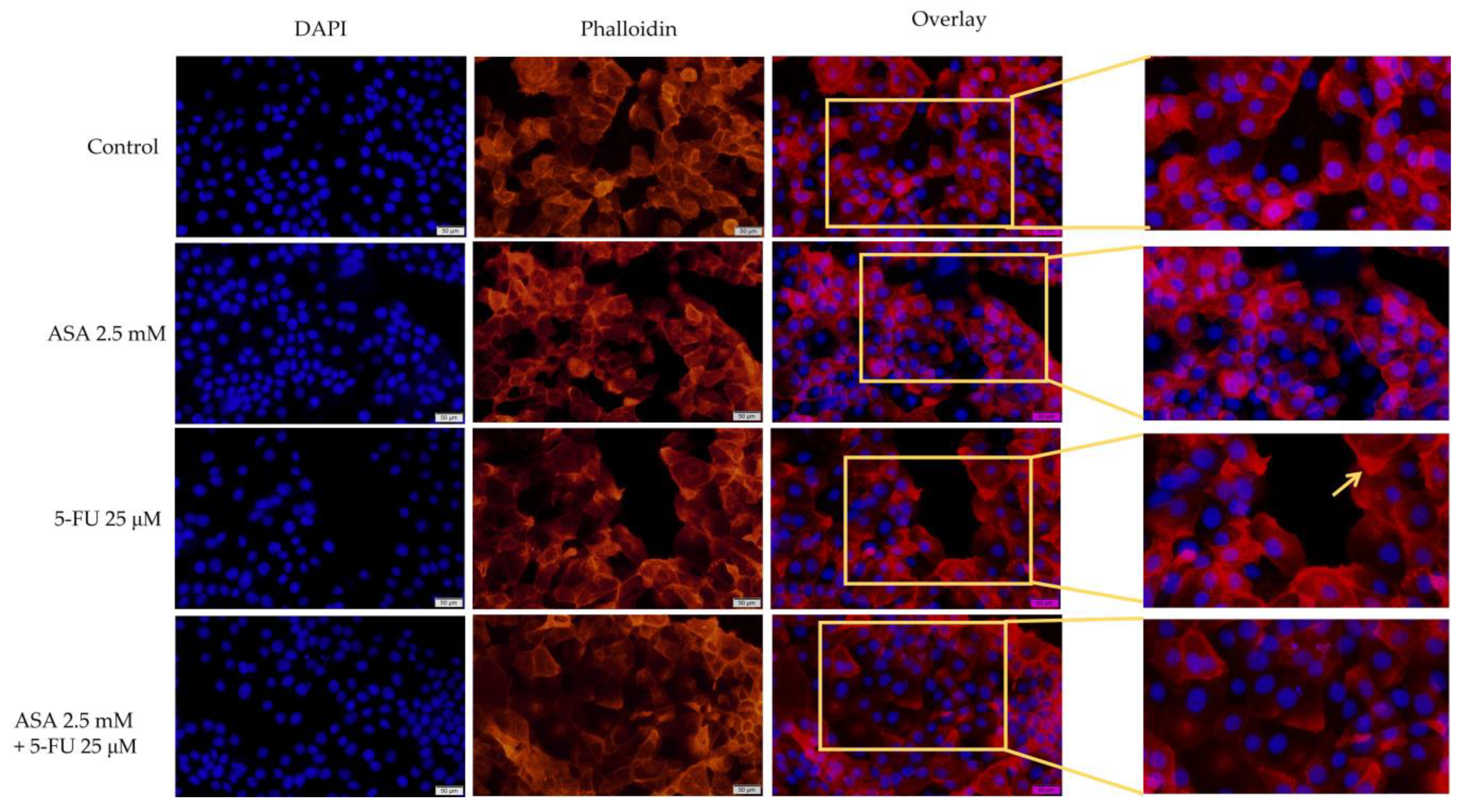
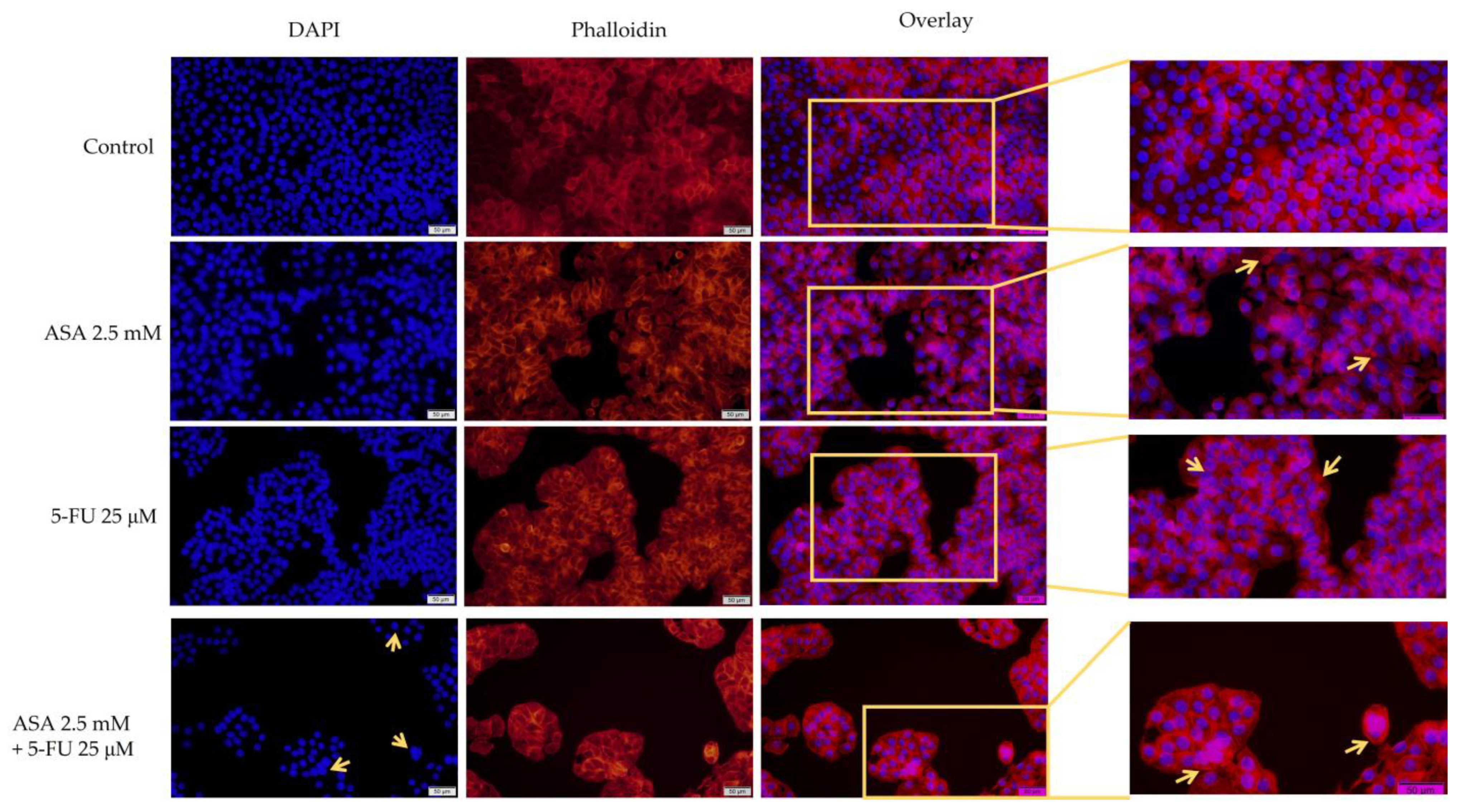
3.3. Immunofluorescence
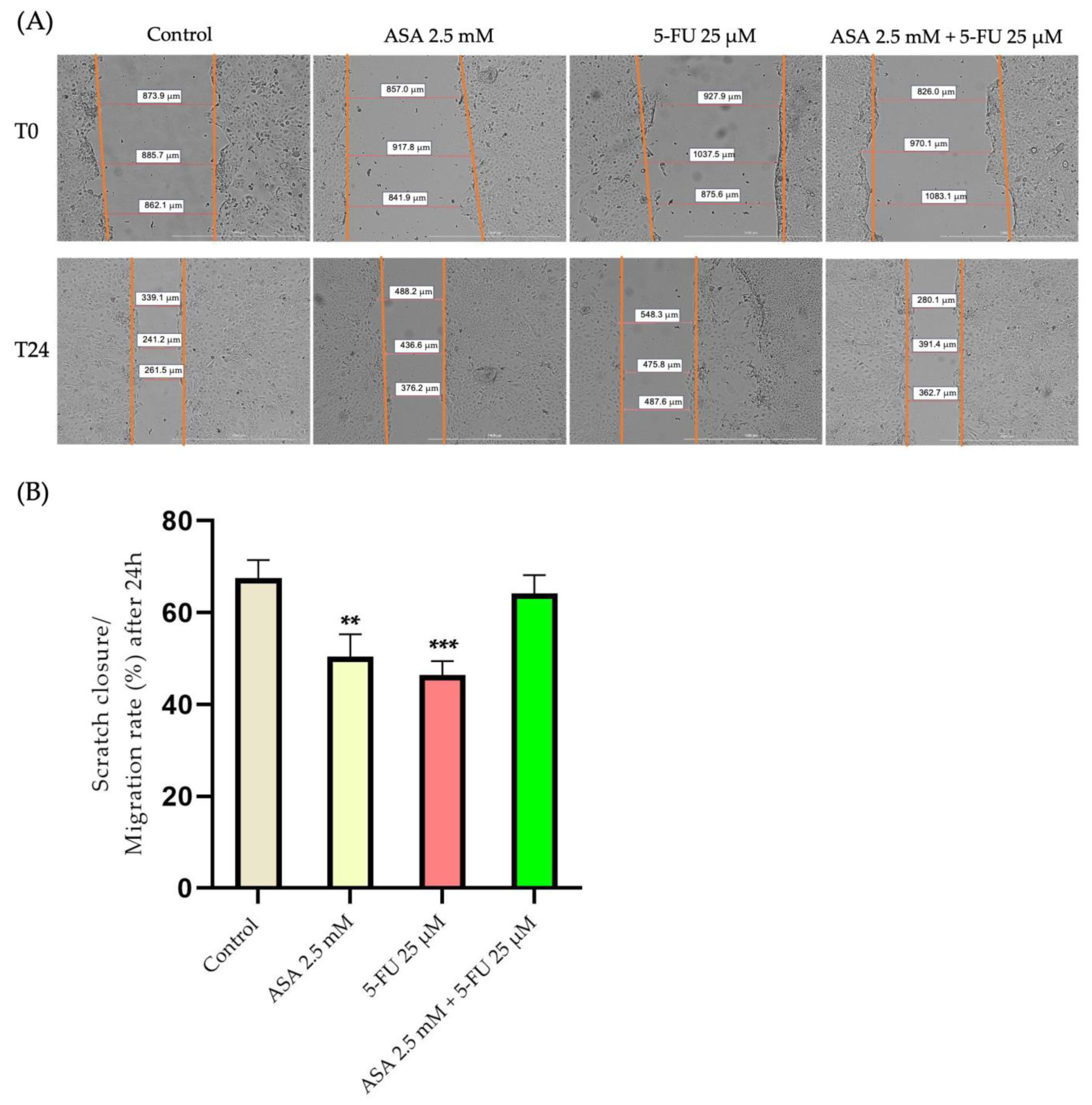
3.4. Wound-Healing Assay
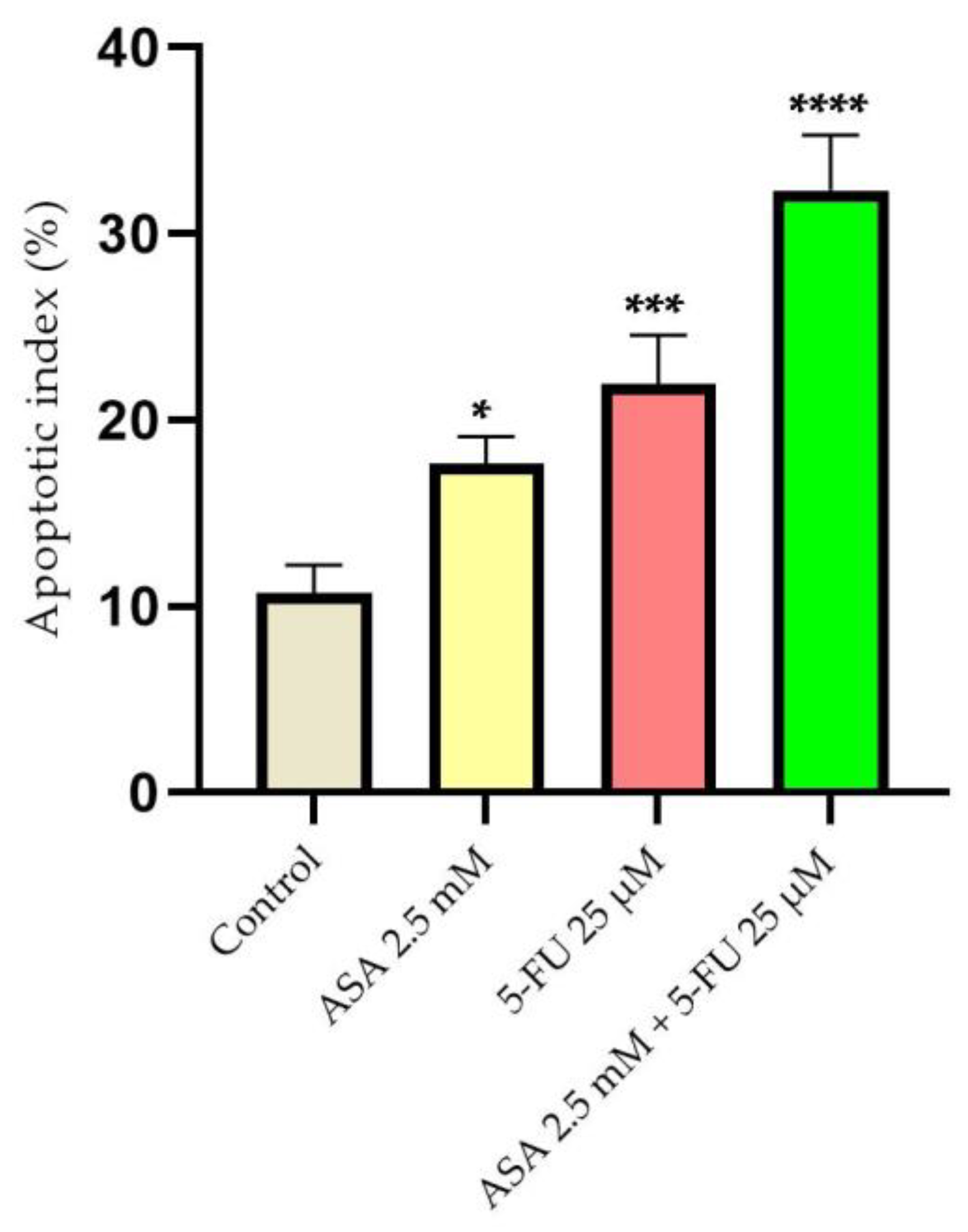
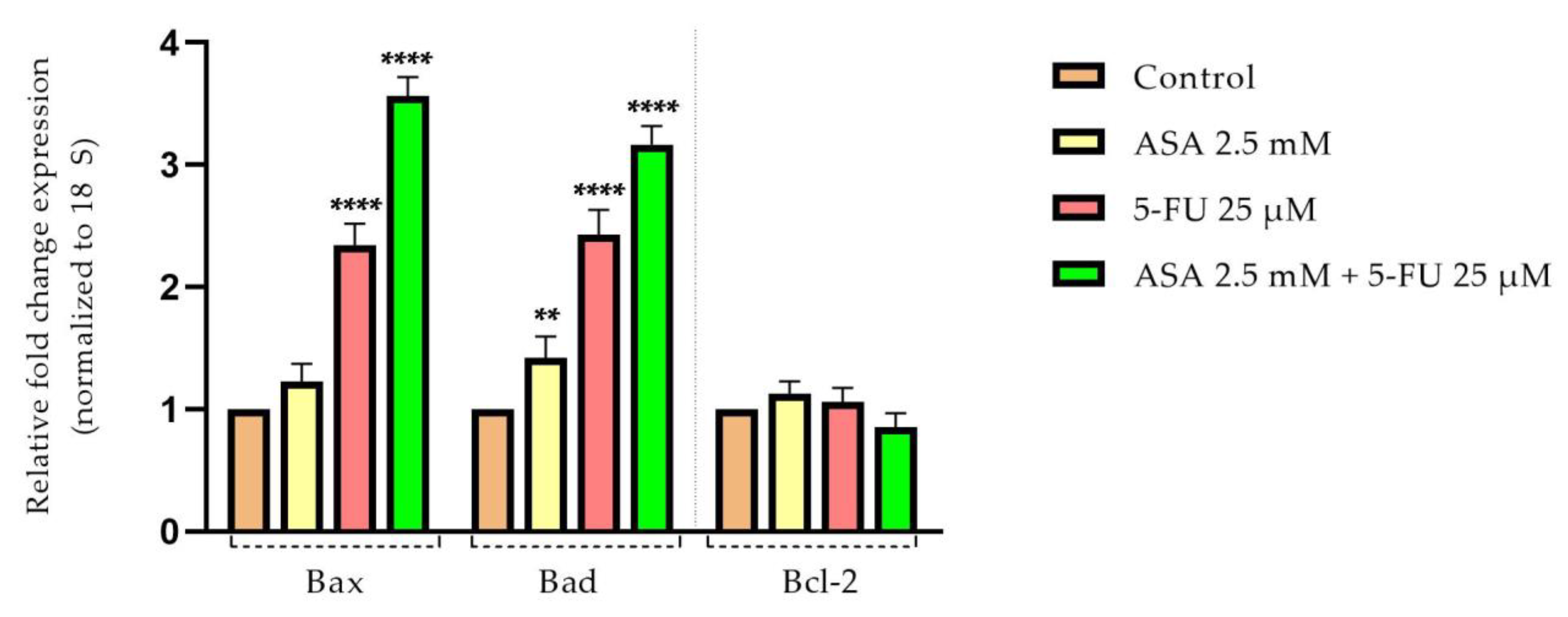
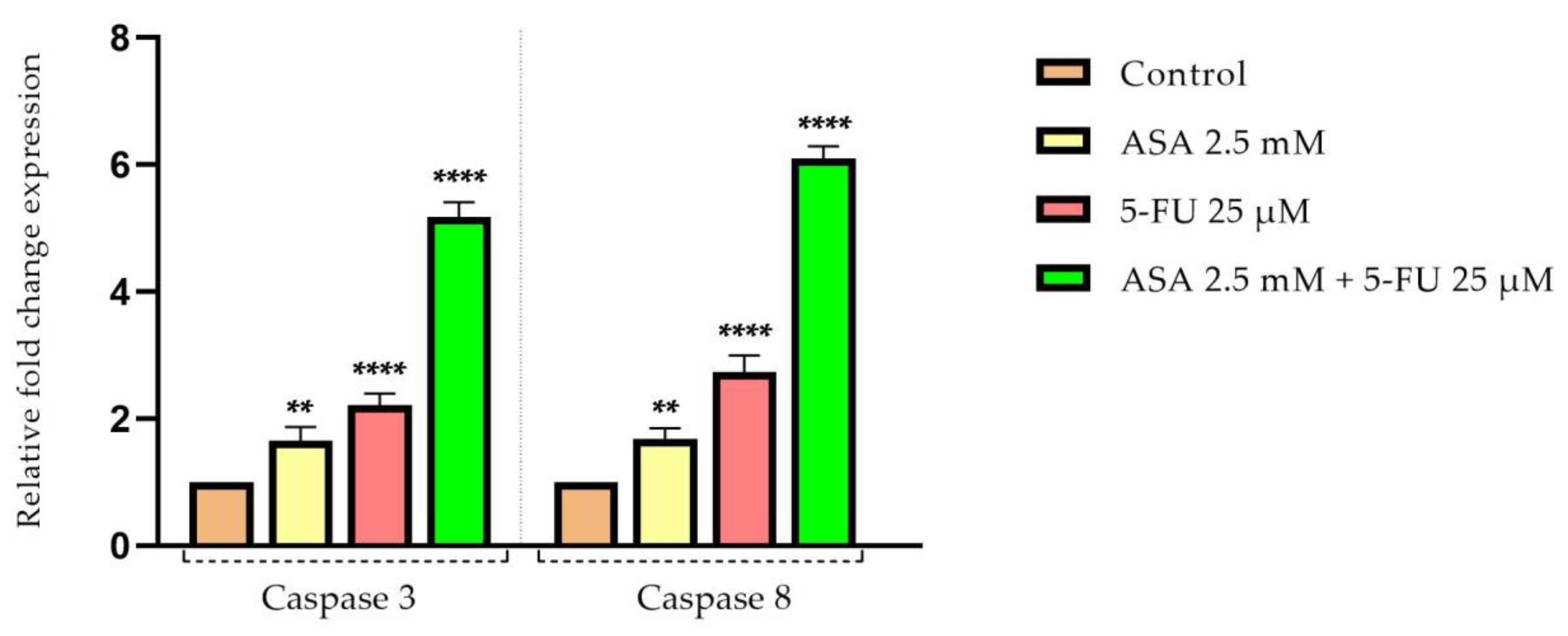
3.5. Quantification of Apoptotic Markers by Real-Time PCR
3.6. Combinatio-Index Calculation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; Van De Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Prim. 2015, 1, 15065. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Stryjkowska-Góra, A.; Rudzki, S. Risk Factors for the Diagnosis of Colorectal Cancer. Cancer Control 2022, 29, 10732748211056692. [Google Scholar] [CrossRef]
- Triantafillidis, J.K.; Nasioulas, G.; Kosmidis, P.A. Colorectal cancer and inflammatory bowel disease: Epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer. Res. 2009, 29, 2727–2737. [Google Scholar]
- Byrne, R.M.; Tsikitis, V.L. Colorectal polyposis and inherited colorectal cancer syndromes. Ann. Gastroenterol. 2018, 31, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Nash, G.F.; Hickish, T. Management of colorectal cancer and diabetes. J. R. Soc. Med. 2014, 107, 103–109. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Zhang, P.; Ai, L.; Liu, T. Cardiovascular Outcomes in the Patients with Colorectal Cancer: A Multi-Registry-Based Cohort Study of 197,699 Cases in the Real World. Front. Cardiovasc. Med. 2022, 9, 851833. [Google Scholar] [CrossRef]
- Chan, A.O.O.; Jim, M.H.; Lam, K.F.; Morris, J.S.; Siu, D.C.W.; Tong, T.; Ng, F.H.; Wong, S.Y.; Hui, W.M.; Chan, C.K.; et al. Prevalence of Colorectal Neoplasm among Patients with Newly Diagnosed Coronary Artery Disease. JAMA 2007, 298, 1412–1419. [Google Scholar] [CrossRef]
- Erichsen, R.; Sværke, C.; Sørensen, H.T.; Sandler, R.S.; Baron, J.A. Risk of Colorectal Cancer in Patients with Acute Myocardial Infarction and Stroke: A Nationwide Cohort Study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1994–1999. [Google Scholar] [CrossRef]
- Yang, S.Y.; Kim, Y.S.; Chung, S.J.; Song, J.H.; Choi, S.Y.; Park, M.J.; Yim, J.Y.; Lim, S.H.; Kim, D.; Kim, C.H.; et al. Association between colorectal adenoma and coronary atherosclerosis detected by CT coronary angiography in Korean men; a cross-sectional study. J. Gastroenterol. Hepatol. 2010, 25, 1795–1799. [Google Scholar] [CrossRef]
- Kenzik, K.M.; Balentine, C.; Richman, J.; Kilgore, M.; Bhatia, S.; Williams, G.R. New-Onset Cardiovascular Morbidity in Older Adults with Stage I to III Colorectal Cancer. J. Clin. Oncol. 2018, 36, 609–616. [Google Scholar] [CrossRef]
- Moghadamyeghaneh, Z.; Mills, S.D.; Carmichael, J.; Pigazzi, A.; Stamos, M.J. Risk Factors of Postoperative Myocardial Infarction after Colorectal Surgeries. Am. Surg. 2015, 81, 358–364. [Google Scholar] [CrossRef]
- Chang, H.-M.; Moudgil, R.; Scarabelli, T.; Okwuosa, T.M.; Yeh, E.T.H. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 1. J. Am. Coll Cardiol. 2017, 70, 2536–2551. [Google Scholar] [CrossRef] [PubMed]
- Cautela, J.; Lalevée, N.; Ammar, C.; Ederhy, S.; Peyrol, M.; Debourdeau, P.; Serin, D.; Le Dolley, Y.; Michel, N.; Orabona, M.; et al. Management and research in cancer treatment-related cardiovascular toxicity: Challenges and perspectives. Int. J. Cardiol. 2016, 224, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Tang, Y.; Jiang, Y.; Su, M.; Wang, X.; Xu, X.; Chen, Y. Cardiovascular Outcomes in the Patients with Primary Central Nervous System Lymphoma: A Multi-Registry Based Cohort Study of 4038 Cases. Front. Oncol. 2021, 11, 691038. [Google Scholar] [CrossRef]
- Ji, C.; Roy, M.D.; Golas, J.; Vitsky, A.; Ram, S.; Kumpf, S.W.; Martin, M.; Barletta, F.; Meier, W.A.; Hooper, A.T.; et al. Myocarditis in Cynomolgus Monkeys Following Treatment with Immune Checkpoint Inhibitors. Clin. Cancer Res. 2019, 25, 4735–4748. [Google Scholar] [CrossRef] [PubMed]
- Messersmith, W.A. NCCN Guidelines Updates: Management of Metastatic Colorectal Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 599–601. [Google Scholar]
- Cho, Y.-H.; Ro, E.J.; Yoon, J.-S.; Mizutani, T.; Kang, D.-W.; Park, J.-C.; Kim, T.I.; Clevers, H.; Choi, K.-Y. 5-FU promotes stemness of colorectal cancer via p53-mediated WNT/β-catenin pathway activation. Nat. Commun. 2020, 11, 5321. [Google Scholar] [CrossRef]
- Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020, 111, 3142–3154. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Cai, H.; Du, B.; Zhang, L.; Ma, W.; Hu, Y.; Feng, S.; Miao, G. MACC1 facilitates chemoresistance and cancer stem cell-like properties of colon cancer cells through the PI3K/AKT signaling pathway. Mol. Med. Rep. 2017, 16, 8747–8754. [Google Scholar] [CrossRef]
- Azwar, S.; Seow, H.F.; Abdullah, M.; Faisal Jabar, M.; Mohtarrudin, N. Recent Updates on Mechanisms of Resistance to 5-Fluorouracil and Reversal Strategies in Colon Cancer Treatment. Biology 2021, 10, 854. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Zhou, H.; Wang, Z.; You, Q.; Chen, J.; Lu, H.; Zhang, J. Aspirin increases chemosensitivity of colorectal cancer cells and inhibits the expression of toll-like receptor 4. Cancer Cell Int. 2023, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, M.; Pogorzelska, A.; Wiktorska, K. Synergistic Interaction between 5-FU and an Analog of Sulforaphane—2-Oxohexyl Isothiocyanate—In an In Vitro Colon Cancer Model. Molecules 2021, 26, 3019. [Google Scholar] [CrossRef]
- Elwood, P.; Protty, M.; Morgan, G.; Pickering, J.; Delon, C.; Watkins, J. Aspirin and cancer: Biological mechanisms and clinical outcomes. Open Biol. 2022, 12, 220124. [Google Scholar] [CrossRef]
- Rose, P.W.; Watson, E.K.; Jenkins, L.S. Aspirin for prevention of cancer and cardiovascular disease. Br. J. Gen. Pract. 2011, 61, 412–415. [Google Scholar] [CrossRef]
- Cuzick, J.; Thorat, M.A.; Bosetti, C.; Brown, P.H.; Burn, J.; Cook, N.R.; Ford, L.G.; Jacobs, E.J.; Jankowski, J.A.; La Vecchia, C.; et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann. Oncol. 2015, 26, 47–57. [Google Scholar] [CrossRef]
- Zheng, S.L.; Roddick, A.J. Association of Aspirin Use for Primary Prevention with Cardiovascular Events and Bleeding Events: A Systematic Review and Meta-Analysis. JAMA 2019, 321, 277–287. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Kim, S.Y.; Kim, J.H.; Lee, J.H.; Kim, Y.-W.; Ryu, K.W.; Park, J.-H.; Choi, I.J. Long-Term Low-Dose Aspirin Use Reduces Gastric Cancer Incidence: A Nationwide Cohort Study. Cancer Res. Treat. 2016, 48, 798–805. [Google Scholar] [CrossRef]
- Perisetti, A.; Goyal, H.; Tharian, B.; Inamdar, S.; Mehta, J.L. Aspirin for prevention of colorectal cancer in the elderly: Friend or foe? Ann. Gastroenterol. 2021, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, L.; Ai, G.; Spitale, R.C.; Bhat, G.J. Molecular targets of aspirin and cancer prevention. Br. J. Cancer 2014, 111, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Kumar, D.R.; Altinoz, M.A.; Bhat, G.J. Mechanisms of Colorectal Cancer Prevention by Aspirin—A Literature Review and Perspective on the Role of COX-Dependent and -Independent Pathways. Int. J. Mol. Sci. 2020, 21, 9018. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Zhu, W.; Zhang, H.; Hu, S.; Cong, X. Growth inhibition of mesenchymal stem cells by aspirin: Involvement of the wnt/β-catenin signal pathway. Clin. Exp. Pharmacol. Physiol. 2006, 33, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Goessling, W.; North, T.E.; Loewer, S.; Lord, A.M.; Lee, S.; Stoick-Cooper, C.L.; Weidinger, G.; Puder, M.; Daley, G.Q.; Moon, R.T.; et al. Genetic Interaction of PGE2 and Wnt Signaling Regulates Developmental Specification of Stem Cells and Regeneration. Cell 2009, 136, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Understanding why aspirin prevents cancer and why consuming very hot beverages and foods increases esophageal cancer risk. Controlling the division rates of stem cells is an important strategy to prevent cancer. Oncoscience 2015, 2, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Kurtova, A.V.; Xiao, J.; Mo, Q.; Pazhanisamy, S.; Krasnow, R.; Lerner, S.P.; Chen, F.; Roh, T.T.; Lay, E.; Ho, P.L.; et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 2015, 517, 209–213. [Google Scholar] [CrossRef]
- Zhang, X.; Du, R.; Luo, N.; Xiang, R.; Shen, W. Aspirin mediates histone methylation that inhibits inflammation-related stemness gene expression to diminish cancer stemness via COX-independent manner. Stem Cell Res. Ther. 2020, 11, 370. [Google Scholar] [CrossRef]
- Giampieri, R.; Restivo, A.; Pusceddu, V.; Del Prete, M.; Maccaroni, E.; Bittoni, A.; Faloppi, L.; Andrikou, K.; Bianconi, M.; Cabras, F.; et al. The Role of Aspirin as Antitumoral Agent for Heavily Pretreated Patients with Metastatic Colorectal Cancer Receiving Capecitabine Monotherapy. Clin. Color. Cancer 2017, 16, 38–43. [Google Scholar] [CrossRef]
- Chioran, D.; Sitaru, A.; Macasoi, I.; Pinzaru, I.; Sarau, C.A.; Dehelean, C.; Dinu, S.; Szuhanek, C.; Zetu, I.N.; Serafin, A.C.; et al. Nicotine Exerts Cytotoxic Effects in a Panel of Healthy Cell Lines and Strong Irritating Potential on Blood Vessels. Int. J. Environ. Res. Public Health 2022, 19, 8881. [Google Scholar] [CrossRef]
- Kis, A.M.; Macasoi, I.; Paul, C.; Radulescu, M.; Buzatu, R.; Watz, C.G.; Cheveresan, A.; Berceanu, D.; Pinzaru, I.; Dinu, S.; et al. Methotrexate and Cetuximab—Biological Impact on Non-Tumorigenic Models: In Vitro and In Ovo Assessments. Medicina 2022, 58, 167. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Rednic, R.; Macasoi, I.; Pinzaru, I.; Dehelean, C.A.; Tomescu, M.-C.; Susan, M.; Feier, H. Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells. Life 2022, 12, 1855. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.M.; Al Doghaither, H.A.; Al-Ghafari, A.B. General insight into cancer: An overview of colorectal cancer (Review). Mol. Clin. Oncol. 2021, 15, 271. [Google Scholar] [CrossRef]
- Vernia, F.; Longo, S.; Stefanelli, G.; Viscido, A.; Latella, G. Dietary Factors Modulating Colorectal Carcinogenesis. Nutrients 2021, 13, 143. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, Y.; Luo, D.; Wang, J.; Liu, J.; Luo, Y.; Zhou, W.; Zhuo, Z.; Guo, K.; Zeng, R.; et al. Association between cardiovascular risk factors and colorectal cancer: A systematic review and meta-analysis of prospective cohort studies. Eclinicalmedicine 2021, 34, 100794. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Capodanno, D. Aspirin for Primary Prevention of Cardiovascular Disease in the 21st Century: A Review of the Evidence. Am. J. Cardiol. 2021, 144, S15–S22. [Google Scholar] [CrossRef] [PubMed]
- Tougeron, D.; Sha, D.; Manthravadi, S.; Sinicrope, F.A. Aspirin and Colorectal Cancer: Back to the Future. Clin. Cancer Res. 2014, 20, 1087–1094. [Google Scholar] [CrossRef]
- Patrignani, P.; Patrono, C. Aspirin and Cancer. J. Am. Coll. Cardiol. 2016, 68, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.T.; Arber, N.; Burn, J.; Chia, W.K.; Elwood, P.; Hull, M.A.; Logan, R.F.; Rothwell, P.M.; Schrör, K.; Baron, J.A. Aspirin in the Chemoprevention of Colorectal Neoplasia: An Overview. Cancer Prev. Res. 2012, 5, 164–178. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Kamran, S.; Sinniah, A.; Chik, Z.; Alshawsh, M.A. Diosmetin Exerts Synergistic Effects in Combination with 5-Fluorouracil in Colorectal Cancer Cells. Biomedicines 2022, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Moracci, L.; Sensi, F.; Biccari, A.; Crotti, S.; Gaio, E.; Benetti, F.; Traldi, P.; Pucciarelli, S.; Agostini, M. An investigation on [5 fluorouracil and epigallocatechin-3-gallate] complex activity on HT-29 cell death and its stability in gastrointestinal fluid. Oncotarget 2022, 13, 476–489. [Google Scholar] [CrossRef]
- Salerno, S.; Ståhlberg, A.; Holdfeldt, A.; Lindskog, E.B.; Landberg, G. 5-fluorouracil treatment of patient-derived scaffolds from colorectal cancer reveal clinically critical information. J. Transl. Med. 2022, 20, 209. [Google Scholar] [CrossRef]
- Srimuangwong, K.; Tocharus, C.; Yoysungnoen Chintana, P.; Suksamrarn, A.; Tocharus, J. Hexahydrocurcumin enhances inhibitory effect of 5-fluorouracil on HT-29 human colon cancer cells. World J. Gastroenterol. 2012, 18, 2383–2389. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Tang, X.-Q.; Sun, L.; Dong, L.; Qin, Y.; Liu, H.-Q.; Xia, H.; Cao, J.-G. Rosiglitazone enhances fluorouracil-induced apoptosis of HT-29 cells by activating peroxisome proliferator-activated receptor γ. World J. Gastroenterol. 2007, 13, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-J.; Rhee, J.-C.; Bae, Y.-M.; Chun, W.-J. Celecoxib attenuates 5-fluorouracil-induced apoptosis in HCT-15 and HT-29 human colon cancer cells. World J. Gastroenterol. 2007, 13, 1947–1952. [Google Scholar] [CrossRef]
- Colombo, I.; SanGiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT Cells as a Reliable In Vitro Differentiation Model to Dissect the Inflammatory/Repair Response of Human Keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef] [PubMed]
- Otto-Ślusarczyk, D.; Mielczarek-Puta, M.; Graboń, W. The Real Cytotoxic Effect of Artemisinins on Colon Cancer Cells in a Physiological Cell Culture Setting. How Composition of the Culture Medium Biases Experimental Findings. Pharmaceuticals 2021, 14, 976. [Google Scholar] [CrossRef]
- Paluszczak, J.; Kleszcz, R.; Studzińska-Sroka, E.; Krajka-Kuźniak, V. Lichen-derived caperatic acid and physodic acid inhibit Wnt signaling in colorectal cancer cells. Mol. Cell. Biochem. 2018, 441, 109–124. [Google Scholar] [CrossRef]
- Vo, N.T.K.; Shahid, M.; Seymour, C.B.; Mothersill, C.E. Effects of Dose Rate on the Reproductive Cell Death and Early Mitochondrial Membrane Potential in Different Human Epithelium-Derived Cells Exposed to Gamma Rays. Dose-Response 2019, 17, 1559325819852508. [Google Scholar] [CrossRef]
- Duarte, S.S.; Silva, D.K.F.; Lisboa, T.M.H.; Gouveia, R.G.; de Andrade, C.C.N.; de Sousa, V.M.; Ferreira, R.C.; de Moura, R.O.; Gomes, J.N.S.; da Silva, P.M.; et al. Apoptotic and antioxidant effects in HCT-116 colorectal carcinoma cells by a spiro-acridine compound, AMTAC-06. Pharmacol. Rep. 2022, 74, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Vo, N.T.K.; Seymour, C.B.; Mothersill, C.E. Parallel comparison of pre-conditioning and post-conditioning effects in human cancers and keratinocytes upon acute gamma irradiation. Int. J. Radiat. Biol. 2019, 95, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, R.; Jain, S.K.; Trivedi, P. Synergistic anti-cancer activity of combined 5-fuorouracil and gallic acid-stearylamine conjugate in A431 human squamous carcinoma cell line. Trop. J. Pharm. Res. 2019, 18, 471–477. [Google Scholar] [CrossRef]
- Szaryńska, M.; Olejniczak-Kęder, A.; Zubrzycki, A.; Wardowska, A.; Kmieć, Z. Aspirin Exerts Synergistic Effect with Anti-Fas Stimulation against Colorectal Cancer Stem Cells In Vitro. Appl. Sci. 2021, 11, 10009. [Google Scholar] [CrossRef]
- Ma, B.; Duan, X.; Zhou, Q.; Liu, J.; Yang, X.; Zhang, D.; Yang, S.; Du, Y.; Li, H.; Su, C. Use of aspirin in the prevention of colorectal cancer through TIGIT-CD155 pathway. J. Cell Mol. Med. 2019, 23, 4514–4522. [Google Scholar] [CrossRef]
- Pathi, S.; Jutooru, I.; Chadalapaka, G.; Nair, V.; Lee, S.-O.; Safe, S. Aspirin Inhibits Colon Cancer Cell and Tumor Growth and Downregulates Specificity Protein (Sp) Transcription Factors. PLoS ONE 2012, 7, e48208. [Google Scholar] [CrossRef]
- Levy, A. Correlation between In-Vitro and In-Vivo Studies Based on Pharmacokinetic Considerations. Am. J. Biomed. Sci. Res. 2020, 8, 48–50. [Google Scholar] [CrossRef]
- Soodi, D.; VanWormer, J.J.; Rezkalla, S.H. Aspirin in Primary Prevention of Cardiovascular Events. Clin. Med. Res. 2020, 18, 89–94. [Google Scholar] [CrossRef]
- Burn, J.; Sheth, H. The role of aspirin in preventing colorectal cancer. Br. Med. Bull. 2016, 119, 17–24. [Google Scholar] [CrossRef]
- Nan, H.; Hutter, C.M.; Lin, Y.; Jacobs, E.J.; Ulrich, C.M.; White, E.; Baron, J.A.; Berndt, S.I.; Brenner, H.; Butterbach, K.; et al. Association of Aspirin and NSAID Use with Risk of Colorectal Cancer According to Genetic Variants. JAMA 2015, 313, 1133–1142. [Google Scholar] [CrossRef]
- Bosetti, C.; Santucci, C.; Gallus, S.; Martinetti, M.; La Vecchia, C. Aspirin and the risk of colorectal and other digestive tract cancers: An updated meta-analysis through 2019. Ann. Oncol. 2020, 31, 558–568. [Google Scholar] [CrossRef]
- Ashktorab, H.; Dawkins, F.W.; Mohamed, R.; Larbi, D.; Smoot, D.T. Apoptosis Induced by Aspirin and 5-Fluorouracil in Human Colonic Adenocarcinoma Cells. Dig. Dis. Sci. 2005, 50, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Zhou, H.-Y.; Liu, P.; You, Q.; Kuang, F.; Shen, Y.-N.; Hu, Z.-Q. Aspirin inhibited the metastasis of colon cancer cells by inhibiting the expression of toll-like receptor 4. Cell Biosci. 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.; Patrikidou, A.; Tsapakidis, K.; Stathakis, N.E.; Papandreou, C.N. Combined treatment of colorectal cancer cell lines with aspirin and bortezomib. J. Clin. Oncol. 2007, 25, 14562. [Google Scholar] [CrossRef]
- Zhuang, P.; Xie, L.; Zhang, Y.; Yuan, Y.; Liu, H.; Bi, C.; Zhao, H.; Li, Y.; Zhang, Y. Inhibition of desmoglein-1 by aspirin leads to synthetic lethality of keratinocytes in Shuanghuanglian-induced cutaneous eruption response. Toxicol. Lett. 2021, 349, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Tarnowski, B.I.; Spinale, F.G.; Nicholson, J.H. DAPI as a useful stain for nuclear quantitation. Biotech. Histochem. 1991, 66, 297–302. [Google Scholar] [CrossRef]
- Chazotte, B. Labeling Nuclear DNA Using DAPI. Cold Spring Harb. Protoc. 2011, 2011, pdb.prot5556. [Google Scholar] [CrossRef]
- Huang, Z.; Haugland, R.P.; You, W.; Haugland, R.P. Phallotoxin and actin binding assay by fluorescence enhancement. Anal. Biochem. 1992, 200, 199–204. [Google Scholar] [CrossRef]
- Bottone, M.G.; Santin, G.; Aredia, F.; Bernocchi, G.; Pellicciari, C.; Scovassi, A.I. Morphological Features of Organelles during Apoptosis: An Overview. Cells 2013, 2, 294–305. [Google Scholar] [CrossRef]
- Martelli, A.M.; Zweyer, M.; Ochs, R.L.; Tazzari, P.L.; Tabellini, G.; Narducci, P.; Bortul, R. Nuclear apoptotic changes: An overview. J. Cell. Biochem. 2001, 82, 634–646. [Google Scholar] [CrossRef]
- Yin, L.-M.; Campillo, C.; Gourlay, C.; Huang, J.; Ren, W.; Zhao, W.; Cao, L. Involvement of the Actin Machinery in Programmed Cell Death. Front. Cell Dev. Biol. 2021, 8, 634849. [Google Scholar] [CrossRef]
- Melak, M.; Plessner, M.; Grosse, R. Correction: Actin visualization at a glance. J. Cell Sci. 2017, 130, 1688. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-H.; Chakraborty, G.; Baek, K.; Yoon, H.S. Aspirin-induced Bcl-2 translocation and its phosphorylation in the nucleus trigger apoptosis in breast cancer cells. Exp. Mol. Med. 2013, 45, e47. [Google Scholar] [CrossRef]
- Massimi, I.; Guerriero, R.; Lotti, L.V.; Lulli, V.; Borgognone, A.; Romani, F.; Barillà, F.; Gaudio, C.; Gabbianelli, M.; Frati, L.; et al. Aspirin influences megakaryocytic gene expression leading to up-regulation of multidrug resistance protein-4 in human platelets. Br. J. Clin. Pharmacol. 2014, 78, 1343–1353. [Google Scholar] [CrossRef]
- Bashir, A.I.; Kankipati, C.S.; Jones, S.; Newman, R.M.; Safrany, S.; Perry, C.J.; Nicholl, I.D. A novel mechanism for the anticancer activity of aspirin and salicylates. Int. J. Oncol. 2019, 54, 1256–1270. [Google Scholar] [CrossRef] [PubMed]
- Colon, H. Prophylactic Effects of Dairy Kluyveromyces Marxianus YAS through Overexpression of BAX. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2016, 10, 142–152. [Google Scholar]
- Filgueiras, M.d.C.; Morrot, A.; Soares, P.M.G.; Costa, M.L.; Mermelstein, C. Effects of 5-Fluorouracil in Nuclear and Cellular Morphology, Proliferation, Cell Cycle, Apoptosis, Cytoskeletal and Caveolar Distribution in Primary Cultures of Smooth Muscle Cells. PLoS ONE 2013, 8, e63177. [Google Scholar] [CrossRef]
- Schaks, M.; Giannone, G.; Rottner, K. Actin dynamics in cell migration. Essays Biochem. 2019, 63, 483–495. [Google Scholar] [CrossRef]
- Stuelten, C.; Parent, C.A.; Montell, D.J. Cell motility in cancer invasion and metastasis: Insights from simple model organisms. Nat. Rev. Cancer 2018, 18, 296–312. [Google Scholar] [CrossRef]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In vitro Cell Migration and Invasion Assays. J. Vis. Exp. 2014, 88, e51046. [Google Scholar] [CrossRef]
- Yokomizo, T.; Liu, M.; Saeki, K. Aspirin delays skin wound healing by reducing the production of 12-hydroxyheptadecatrienoic acid, a ligand for BLT2 receptor. FASEB J. 2013, 27, 813.4. [Google Scholar] [CrossRef]
- Menter, D.G.; DuBois, R.N. Prostaglandins in Cancer Cell Adhesion, Migration, and Invasion. Int. J. Cell Biol. 2012, 2012, 723419. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; Magliocca, G.; Pepe, G.; Amodio, G.; Autore, G.; Campiglia, P.; Marzocco, S. Protective Effect of Pomegranate on Oxidative Stress and Inflammatory Response Induced by 5-Fluorouracil in Human Keratinocytes. Antioxidants 2021, 10, 203. [Google Scholar] [CrossRef]
- Yu, Z.; Chan, S.; Wang, X.; Sun, R.; Wang, M.; Wang, Z.; Zuo, X.; Chen, J.; Zhang, H.; Chen, W. 5-Fluorouracil Combined with Rutaecarpine Synergistically Suppresses the Growth of Colon Cancer Cells by Inhibiting STAT3. Drug Des. Dev. Ther. 2023, 17, 993–1006. [Google Scholar] [CrossRef]
- Wang, N.; Yang, L.; Dai, J.; Wu, Y.; Zhang, R.; Jia, X.; Liu, C. 5-FU inhibits migration and invasion of CRC cells through PI3K/AKT pathway regulated by MARCH1. Cell Biol. Int. 2021, 45, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Ki, S.H.; Park, E.Y.; Shin, S.M. 5-Fluorouracil inhibits cell migration by induction of Sestrin2 in colon cancer cells. Arch. Pharmacal Res. 2017, 40, 231–239. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Zhou, X.M.; Wong, B.C.Y.; Fan, X.M.; Zhang, H.B.; Lin, M.C.M.; Kung, H.F.; Fan, D.M.; Lam, S.K. Non-steroidal anti-inflammatory drugs induce apoptosis in gastric cancer cells through up-regulation of bax and bak. Carcinogenesis 2001, 22, 1393–1397. [Google Scholar] [CrossRef]
- Wu, D.-W.; Huang, C.-C.; Chang, S.-W.; Chen, T.-H.; Lee, H. Bcl-2 stabilization by paxillin confers 5-fluorouracil resistance in colorectal cancer. Cell Death Differ. 2015, 22, 779–789. [Google Scholar] [CrossRef]
- Violette, S.; Poulain, L.; Dussaulx, E.; Pepin, D.; Faussat, A.-M.; Chambaz, J.; Lacorte, J.-M.; Staedel, C.; Lesuffleur, T. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-XL in addition toBax andp53 status. Int. J. Cancer 2002, 98, 498–504. [Google Scholar] [CrossRef]
- Wang, W.; Guo, W.; Li, L.; Fu, Z.; Liu, W.; Gao, J.; Shu, Y.; Xu, Q.; Sun, Y.; Gu, Y. Andrographolide reversed 5-FU resistance in human colorectal cancer by elevating BAX expression. Biochem. Pharmacol. 2016, 121, 8–17. [Google Scholar] [CrossRef]
- Nita, M.; Nagawa, H.; Tominaga, O.; Tsuno, N.; Fujii, S.; Sasaki, S.; Fu, C.-G.; Takenoue, T.; Tsuruo, T.; Muto, T. 5-Fluorouracil induces apoptosis in human colon cancer cell lines with modulation of Bcl-2 family proteins. Br. J. Cancer 1998, 78, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Caspase activation, inhibition, and reactivation: A mechanistic view. Protein Sci. 2004, 13, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, M.; Günther, S.D.; Schwarzer, R.; Albert, M.-C.; Schorn, F.; Werthenbach, J.P.; Schiffmann, L.M.; Stair, N.; Stocks, H.; Seeger, J.M.; et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 2019, 575, 683–687. [Google Scholar] [CrossRef]
- Zimmermann, K.C.; Waterhouse, N.J.; Goldstein, J.C.; Schuler, M.; Green, D.R. Aspirin Induces Apoptosis through Release of Cytochrome c from Mitochondria. Neoplasia 2000, 2, 505–513. [Google Scholar] [CrossRef]
- Park, I.-S.; Jo, J.-R.; Hong, H.; Nam, K.-Y.; Kim, J.-B.; Hwang, S.-H.; Choi, M.-S.; Ryu, N.-H.; Jang, H.-J.; Lee, S.-H.; et al. Aspirin induces apoptosis in YD-8 human oral squamous carcinoma cells through activation of caspases, down-regulation of Mcl-1, and inactivation of ERK-1/2 and AKT. Toxicol. Vitr. 2010, 24, 713–720. [Google Scholar] [CrossRef]
- Mhaidat, N.M.; Bouklihacene, M.; Thorne, R.F. 5-Fluorouracil-induced apoptosis in colorectal cancer cells is caspase-9-dependent and mediated by activation of protein kinase C-δ. Oncol. Lett. 2014, 8, 699–704. [Google Scholar] [CrossRef]
- Drew, D.A.; Chan, A.T. Aspirin in the Prevention of Colorectal Neoplasia. Annu. Rev. Med. 2021, 72, 415–430. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Dong, H.; Wang, X.; Gao, F.; Zhang, S.; Zhang, X. Aspirin has a better effect on PIK3CA mutant colorectal cancer cells by PI3K/Akt/Raptor pathway. Mol. Med. 2020, 26, 14. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, T.; Mori, R.; Futamura, M.; Fukada, M.; Tanaka, H.; Yasufuku, I.; Sato, Y.; Iwata, Y.; Imai, T.; Imai, H.; et al. Mechanism of acquired 5FU resistance and strategy for overcoming 5FU resistance focusing on 5FU metabolism in colon cancer cell lines. Oncol. Rep. 2021, 45, 27. [Google Scholar] [CrossRef] [PubMed]
- Pouya, F.D.; Rasmi, Y.; Nemati, M. Signaling Pathways Involved in 5-FU Drug Resistance in Cancer. Cancer Investig. 2022, 40, 516–543. [Google Scholar] [CrossRef] [PubMed]



| Inhibitory Effect (Fa) | Combination Index (CI) | Dose ASA | Dose 5-FU | Dose-Reduction Index (DRI) ASA | Dose-Reduction Index (DRI) 5-FU |
|---|---|---|---|---|---|
| 0.92 | 199.548 | 2.5 mM | 5 μM | 0.01 | 1.90 |
| 0.91 | 110.082 | 2.5 mM | 10 μM | 0.009 | 2.07 |
| 0.91 | 274.483 | 2.5 mM | 25 μM | 0.003 | 2.07 |
| 0.87 | 79.970 | 2.5 mM | 50 μM | 0.012 | 2.77 |
| 0.85 | 55.189 | 2.5 mM | 75 μM | 0.018 | 3.11 |
| Inhibitory Effect (Fa) | Combination Index (CI) | Dose ASA | Dose 5-FU | Dose-Reduction Index (DRI) ASA | Dose-Reduction Index (DRI) 5-FU |
|---|---|---|---|---|---|
| 0.69 | 0.46 | 2.5 mM | 5 μM | 3.044 | 7.094 |
| 0.54 | 0.31 | 2.5 mM | 10 μM | 5.276 | 7.937 |
| 0.46 | 0.35 | 2.5 mM | 25 μM | 6.951 | 4.754 |
| 0.42 | 0.46 | 2.5 mM | 50 μM | 7.993 | 2.916 |
| 0.13 | 0.10 | 2.5 mM | 75 μM | 31.045 | 14.178 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susan, M.; Macasoi, I.; Pinzaru, I.; Dehelean, C.; Ilia, I.; Susan, R.; Ionita, I. In Vitro Assessment of the Synergistic Effect of Aspirin and 5-Fluorouracil in Colorectal Adenocarcinoma Cells. Curr. Oncol. 2023, 30, 6197-6219. https://doi.org/10.3390/curroncol30070460
Susan M, Macasoi I, Pinzaru I, Dehelean C, Ilia I, Susan R, Ionita I. In Vitro Assessment of the Synergistic Effect of Aspirin and 5-Fluorouracil in Colorectal Adenocarcinoma Cells. Current Oncology. 2023; 30(7):6197-6219. https://doi.org/10.3390/curroncol30070460
Chicago/Turabian StyleSusan, Monica, Ioana Macasoi, Iulia Pinzaru, Cristina Dehelean, Iosif Ilia, Razvan Susan, and Ioana Ionita. 2023. "In Vitro Assessment of the Synergistic Effect of Aspirin and 5-Fluorouracil in Colorectal Adenocarcinoma Cells" Current Oncology 30, no. 7: 6197-6219. https://doi.org/10.3390/curroncol30070460
APA StyleSusan, M., Macasoi, I., Pinzaru, I., Dehelean, C., Ilia, I., Susan, R., & Ionita, I. (2023). In Vitro Assessment of the Synergistic Effect of Aspirin and 5-Fluorouracil in Colorectal Adenocarcinoma Cells. Current Oncology, 30(7), 6197-6219. https://doi.org/10.3390/curroncol30070460







