Predictors of Prostate Cancer at Fusion Biopsy: The Role of Positive Family History, Hypertension, Diabetes, and Body Mass Index
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef] [PubMed]
- IARC; WHO. Data Visualization Tools for Exploring the Global Cancer Burden in 2020. Available online: https://gco.iarc.fr/today/home (accessed on 25 March 2023).
- Ann, W.H.; Hsing, A.W.; Chokkalingam, A.P. Prostate cancer epidemiology. Front. Biosci. 2006, 11, 1388–1413. [Google Scholar] [CrossRef]
- Hemminki, K. Familial risk and familial survival in prostate cancer. World J. Urol. 2012, 30, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Jansson, K.F.; Akre, O.; Garmo, H.; Bill-Axelson, A.; Adolfsson, J.; Stattin, P.; Bratt, O. Concordance of Tumor Differentiation Among Brothers with Prostate Cancer. Eur. Urol. 2012, 62, 656–661. [Google Scholar] [CrossRef]
- Haenszel, W.; Kurihara, M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J. Natl. Cancer Inst. 2012, 30, 143–148. [Google Scholar] [CrossRef]
- Shimizu, H.; Ross, R.K.; Bernstein, L.; Yatani, R.; E Henderson, B.; Mack, T.M. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br. J. Cancer 1991, 63, 963–966. [Google Scholar] [CrossRef]
- Haas, G.P.; Delongchamps, N.; Brawley, O.W.; Wang, C.Y.; De La Roza, G. The worldwide epidemiology of prostate cancer: Perspectives from autopsy studies. Can. J. Urol. 2008, 15, 3866–3871. [Google Scholar]
- Fleshner, K.; Carlsson, S.; Roobol, M.J. The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat. Rev. Urol. 2017, 14, 26–37. [Google Scholar] [CrossRef]
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The Epidemiology of Prostate Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a030361. [Google Scholar] [CrossRef]
- Vidal, A.C.; Howard, L.E.; Moreira, D.M.; Castro-Santamaria, R.; Andriole, G.L., Jr.; Freedland, S.J. Obesity Increases the Risk for High-Grade Prostate Cancer: Results from the REDUCE Study. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2936–2942. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Capuano, A.; Bellastella, G.; Maiorino, M.I.; Parretta, E.; Lenzi, A.; Giugliano, D. Effect of metabolic syndrome and its components on prostate cancer risk: Meta-analysis. J. Endocrinol. Investig. 2013, 36, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Eberli, D.; De Meerleer, G.; De Santis, M.; Gillessen, S.; Grummet, J.; Henry, A.M.; et al. EAU Guidelines. Edn. presented at the EAU Annual Congress Milan; EAU Guidelines Office: Arnhem, The Netherlands, 2023; ISBN 978-94-92671-19-6. Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed on 25 March 2023).
- Oderda, M.; Albisinni, S.; Benamran, D.; Calleris, G.; Ciccariello, M.; Dematteis, A.; Diamand, R.; Descotes, J.; Fiard, G.; Forte, V.; et al. Accuracy of elastic fusion biopsy: Comparing prostate cancer detection between targeted and systematic biopsy. Prostate 2023, 83, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Barentsz, J.O.; Weinreb, J.C.; Verma, S.; Thoeny, H.C.; Tempany, C.M.; Shtern, F.; Padhani, A.R.; Margolis, D.; Macura, K.J.; Haider, M.A.; et al. Synopsis of the PI-RADS v2 Guidelines for Multiparametric Prostate Magnetic Resonance Imaging and Recommendations for Use. Eur. Urol. 2016, 69, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, P.M.; Reitsma, J.B.; E Bruns, D.; A Gatsonis, C.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.W.; et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. Clin. Chem. 2015, 61, 1446–1452. [Google Scholar] [CrossRef]
- Pourmand, G.; Salem, S.; Mehrsai, A.; Lotfi, M.; Amirzargar, M.; Mazdak, H.; Roshani, A.; Kheirollahi, A.R.; Kalantar, E.; Baradaran, N.; et al. The risk factors of prostate cancer: A multicentric case-control study in Iran. Asian Pac. J. Cancer Prev. 2007, 8, 422–428. [Google Scholar]
- American Cancer Society: Facts & Figures 2015; American Cancer Society: Atlanta, GA, USA, 2015.
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Haiman, C.A.; Chen, G.K.; Blot, W.J.; Strom, S.S.; Berndt, S.I.; Kittles, R.A.; Rybicki, B.A.; Isaacs, W.B.; Ingles, S.A.; Stanford, J.L.; et al. Characterizing Genetic Risk at Known Prostate Cancer Susceptibility Loci in African Americans. PLOS Genet. 2011, 7, e1001387. [Google Scholar] [CrossRef]
- Center, M.M.; Jemal, A.; Lortet-Tieulent, J.; Ward, E.; Ferlay, J.; Brawley, O.; Bray, F. International Variation in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2012, 61, 1079–1092. [Google Scholar] [CrossRef]
- Hemminki, K.; Czene, K. Attributable risks of familial cancer from the Family-Cancer Database. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1638–1644. [Google Scholar]
- Mucci, L.A.; Hjelmborg, J.B.; Harris, J.R.; Czene, K.; Havelick, D.J.; Scheike, T.; Graff, R.E.; Holst, K.; Möller, S.; Nordic Twin Study of Cancer (NorTwinCan) Collaboration. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA 2016, 315, 68–76. [Google Scholar] [CrossRef]
- Lange, E.M.; Salinas, C.A.; Zuhlke, K.A.; Ray, A.M.; Wang, Y.; Lu, Y.; Ho, L.A.; Luo, J.; Cooney, K. Early onset prostate cancer has a significant genetic component. Prostate 2012, 72, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Jahn, J.L.; Giovannucci, E.L.; Stampfer, M.J. The high prevalence of undiagnosed prostate cancer at autopsy: Implications for epidemiology and treatment of prostate cancer in the Prostate-specific Antigen-era. Int. J. Cancer 2015, 137, 2795–2802. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ma, J. Body Mass Index, Prostate Cancer–Specific Mortality, and Biochemical Recurrence: A Systematic Review and Meta-analysis. Cancer Prev. Res. 2011, 4, 486–501. [Google Scholar] [CrossRef]
- Bashir, M.N.; Ahmad, M.R.; Malik, A. Risk Factors of Prostate Cancer: A Case-control Study in Faisalabad, Pakistan. Asian Pac. J. Cancer Prev. 2014, 15, 10237–10240. [Google Scholar] [CrossRef]
- Rivera-Izquierdo, M.; de Rojas, J.P.; Martínez-Ruiz, V.; Pérez-Gómez, B.; Sánchez, M.-J.; Khan, K.S.; Jiménez-Moleón, J.J. Obesity as a Risk Factor for Prostate Cancer Mortality: A Systematic Review and Dose-Response Meta-Analysis of 280,199 Patients. Cancers 2021, 13, 4169. [Google Scholar] [CrossRef]
- Bandini, M.; Gandaglia, G.; Briganti, A. Obesity and prostate cancer. Curr. Opin. Urol. 2017, 27, 415–421. [Google Scholar] [CrossRef]
- Wallner, L.P.; Morgenstern, H.; McGree, M.E.; Jacobson, D.J.; Sauver, J.L.S.; Jacobsen, S.J.; Sarma, A.V. The effects of metabolic conditions on prostate cancer incidence over 15 years of follow-up: Results from the Olmsted County Study. BJU Int. 2011, 107, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Lapierre, A.; Spence, A.R.; Karakiewicz, P.I.; Aprikian, A.G.; Saad, F.; Parent, M.É. Metabolic syndrome and prostate cancer risk in a population-based case–control study in Montreal, Canada. BMC Public Health 2015, 15, 913. [Google Scholar] [CrossRef]
- Bhindi, B.; Locke, J.; Alibhai, S.M.; Kulkarni, G.S.; Margel, D.S.; Hamilton, R.J.; Finelli, A.; Trachtenberg, J.; Zlotta, A.R.; Toi, A.; et al. Dissecting the Association Between Metabolic Syndrome and Prostate Cancer Risk: Analysis of a Large Clinical Cohort. Eur. Urol. 2015, 67, 64–70. [Google Scholar] [CrossRef]
- Montano, D. Association Between Socioeconomic Determinants and the Metabolic Syndrome in the German Health Interview and Examination Survey for Adults (DEGS1)—A Mediation Analysis. Rev. Diabet. Stud. 2017, 14, 279–294. [Google Scholar] [CrossRef]
- Kaneko, M.; Sugano, D.; Lebastchi, A.H.; Duddalwar, V.; Nabhani, J.; Haiman, C.; Gill, I.S.; Cacciamani, G.E.; Abreu, A.L. Techniques and Outcomes of MRI-TRUS Fusion Prostate Biopsy. Curr. Urol. Rep. 2021, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Oderda, M.; Marra, G.; Albisinni, S.; Altobelli, E.; Baco, E.; Beatrici, V.; Cantiani, A.; Carbone, A.; Ciccariello, M.; Descotes, J.-L.; et al. Accuracy of elastic fusion biopsy in daily practice: Results of a multicenter study of 2115 patients. Int. J. Urol. 2018, 25, 990–997. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. ECISION Study Group Collaborators. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Pagniez, M.A.; Kasivisvanathan, V.; Puech, P.; Drumez, E.; Villers, A.; Olivier, J. Predictive Factors of Missed Clinically Significant Prostate Cancers in Men with Negative Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis. J. Urol. 2020, 204, 24–32. [Google Scholar] [CrossRef] [PubMed]
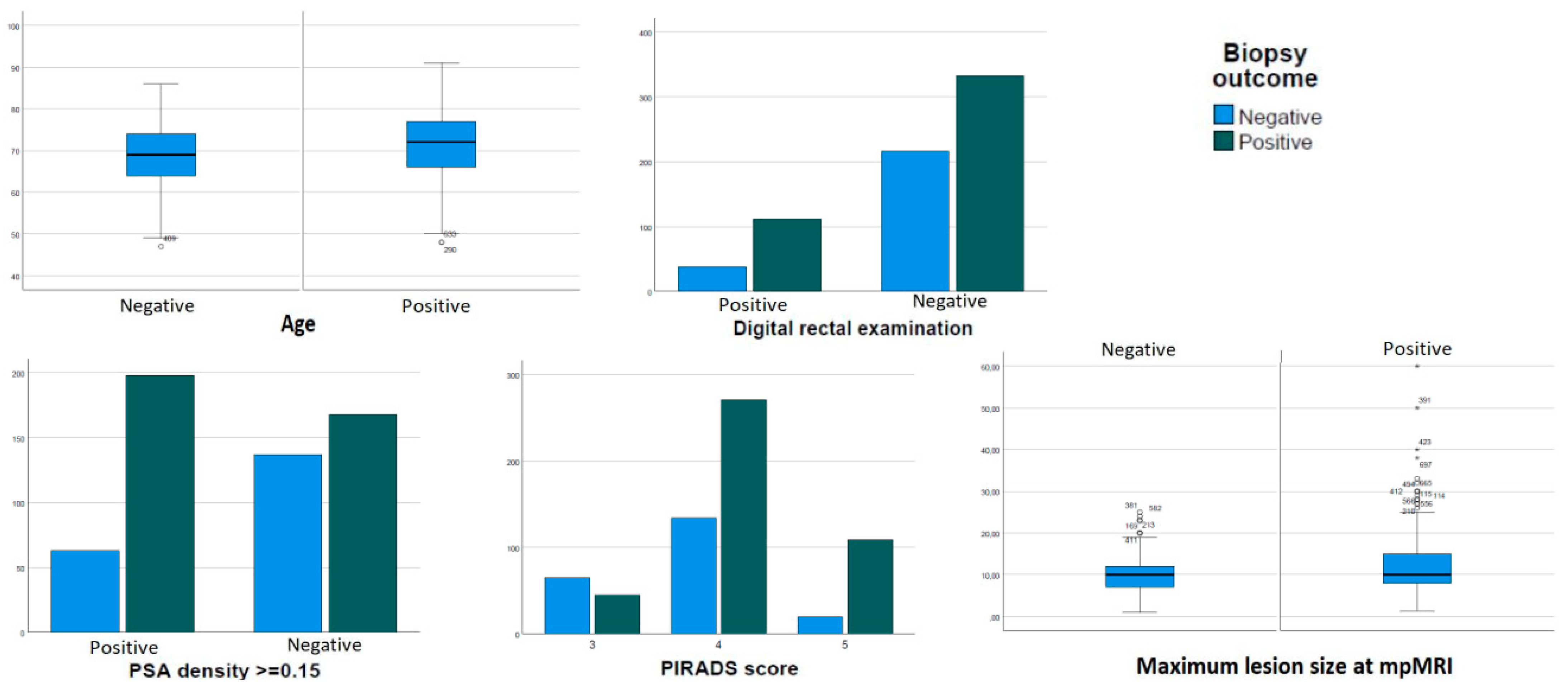
| Variables | All Patients | Missing Data | Patients with Positive Biopsy | Patients with Negative Biopsy | p |
|---|---|---|---|---|---|
| Patients | 736 | - | 465 (63.2%) | 271 (36.8%) | |
| Age; years; median (IQR) | 71 (11) | 1 (0.1%) | 72 (11) | 69 (10) | <0.001 |
| BMI; mean (SD) | 25.8 (3.4) | 370 (50.2%) | 25.8 (3.4) | 25.9 (3.5) | 0.78 |
| Hypertension; n (%) | 399 (54.2%) | 13 (1.8%) | 253 (63.4%) | 146 (36.6%) | 1.00 |
| Diabetes; n (%) | 66 (9%) | 27 (3.7%) | 46 (69.7%) | 20 (30.3%) | 0.28 |
| Positive family history for PCa; n (%) | 55 (7.5%) | 97 (13.2%) | 36 (65.5%) | 19 (34.5%) | 0.77 |
| PSA; ng/mL; median (IQR) | 6.5 (4.3) | 7 (0.9%) | 6.8 (4.5) | 6.1 (3.8) | 0.13 |
| PSA density; ng/mL/mL; median (IQR) | 0.14 (0.12) | 170 (23.1%) | 0.16 (0.12) | 0.11 (0.10) | <0.001 |
| PSA density ≥0.15; n (%) | 261 (35.5) | 170 (23.1%) | 198 (75.9%) | 63 (24.1%) | <0.001 |
| Positive DRE; n (%) | 150 (20.4) | 37 (5.0%) | 112 (74.7%) | 38 (25.3%) | 0.002 |
| Prostate volume; cc; median (IQR) | 48 (35) | 168 (22.8%) | 42 (26) | 60 (40) | <0.001 |
| Previous negative biopsies; n (%) | 168 (22.8%) | 5 (0.7%) | 105 (62.5%) | 63 (37.5%) | 0.85 |
| Single mpMRI target; n (%) | 544 (73.9%) | 55 (7.5%) | 340 (62.5%) | 204 (37.5%) | 0.009 |
| Size of targets; mm; mean (SD) | 11.5 (6.2) | 106 (14.4%) | 12.3 (6.9) | 10.1 (4.4) | <0.001 |
| PIRADS of targets (maximum score if multiple); n (%) | |||||
| 110 (14.9%) | 92 (12.5%) | 45 (40.9%) | 65 (59.1%) | <0.001 |
| 405 (55.0%) | 271 (66.9%) | 134 (33.1%) | ||
| 129 (17.5%) | 109 (84.5%) | 20 (15.5%) | ||
| Cancer detection rate; n (%) | 465 (63.2%) | 0 (0%) | 465 (63.2%) | - | - |
| Clinically significant cancer detection rate; n (%) | 432 (58.7%) | 0 (0%) | 432 (58.7%) | - | - |
| PCa ISUP score; n (%) | |||||
| 33 (7.0%) | 33 (7.0%) | |||
| 199 (42.8%) | 199 (42.8%) | |||
| 144 (31.0%) | 144 (31.0%) | |||
| 61 (13.2%) | 61 (13.2%) | |||
| 28 (6.0%) | 28 (6.0%) |
| All PCa | CsPCa | |||
|---|---|---|---|---|
| Variable | Uni-Variable | Multi-Variable | Uni-Variable | Multi-Variable |
| Age | 1.04 (1.02–1.06) | 1.04 (1.02–1.07) | 1.05 (1.03–1.07) | 1.04 (1.01–1.07) |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Body mass index | 0.99 (0.93–1.05) | - | 0.97 (0.91–1.03) | - |
| p = 0.78 | p = 0.33 | |||
| Hypertension | 1.00 (0.74–1.36) | - | 0.98 (0.73–1.33) | - |
| p = 0.97 | p = 0.93 | |||
| Diabetes | 1.38 (0.80–2.40) | - | 1.36 (0.80–2.31) | - |
| p = 0.24 | p = 0.25 | |||
| Positive family history for PCa | 1.12 (0.63–2.01) | - | 1.20 (0.68–2.12) | - |
| p = 0.68 | p = 0.52 | |||
| PSA (ng/mL) | 1.02 (0.99–1.05) | - | 1.01 (0.99–1.04) | - |
| p = 0.15 | p = 0.22 | |||
| Positive DRE | 1.91 (1.27–2.86) | 1.47 (0.84–2.59) | 2.15 (1.45–3.20) | 1.75 (1.01–3.02) |
| p = 0.002 | p = 0.17 | p < 0.001 | p = 0.04 | |
| PSA density ≥0.15 | 2.56 (1.78–3.68) | 2.68 (1.73–4.15) | 2.41 (1.70–3.42) | 2.47 (1.62–3.76) |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Previous negative biopsy | 0.95 (0.66–1.36) | - | 0.91 (0.64–1.29) | - |
| p = 0.79 | p = 0.61 | |||
| PIRADS score | - | - | - | - |
| 3 | 2.92 (1.89–4.50) | 2.74 (1.61–4.68) | 3.31 (2.19–5.17) | 3.31 (1.91–5.73) |
| 4 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| 7.87 (4.27–14.48) | 4.02 (1.62–9.96) | 7.81 (4.35–14.02) | 3.56 (1.50–8.45) | |
| 5 | p < 0.001 | p = 0.003 | p < 0.001 | p = 0.004 |
| Size of the lesion (mm) | 1.07 (1.03–1.10) | 1.03 (0.98–1.09) | 1.05 (1.02–1.08) | 1.04 (0.98–1.10) |
| p < 0.001 | p = 0.19 | p < 0.001 | p = 0.12 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oderda, M.; Dematteis, A.; Calleris, G.; Conti, A.; D’Agate, D.; Falcone, M.; Marquis, A.; Montefusco, G.; Marra, G.; Gontero, P. Predictors of Prostate Cancer at Fusion Biopsy: The Role of Positive Family History, Hypertension, Diabetes, and Body Mass Index. Curr. Oncol. 2023, 30, 4957-4965. https://doi.org/10.3390/curroncol30050374
Oderda M, Dematteis A, Calleris G, Conti A, D’Agate D, Falcone M, Marquis A, Montefusco G, Marra G, Gontero P. Predictors of Prostate Cancer at Fusion Biopsy: The Role of Positive Family History, Hypertension, Diabetes, and Body Mass Index. Current Oncology. 2023; 30(5):4957-4965. https://doi.org/10.3390/curroncol30050374
Chicago/Turabian StyleOderda, Marco, Alessandro Dematteis, Giorgio Calleris, Adriana Conti, Daniele D’Agate, Marco Falcone, Alessandro Marquis, Gabriele Montefusco, Giancarlo Marra, and Paolo Gontero. 2023. "Predictors of Prostate Cancer at Fusion Biopsy: The Role of Positive Family History, Hypertension, Diabetes, and Body Mass Index" Current Oncology 30, no. 5: 4957-4965. https://doi.org/10.3390/curroncol30050374
APA StyleOderda, M., Dematteis, A., Calleris, G., Conti, A., D’Agate, D., Falcone, M., Marquis, A., Montefusco, G., Marra, G., & Gontero, P. (2023). Predictors of Prostate Cancer at Fusion Biopsy: The Role of Positive Family History, Hypertension, Diabetes, and Body Mass Index. Current Oncology, 30(5), 4957-4965. https://doi.org/10.3390/curroncol30050374





