The Potential Role of Adjuvant Chemoradiotherapy in Patients with Microscopically Positive (R1) Surgical Margins after Resection of Cholangiocarcinoma
Abstract
1. Introduction
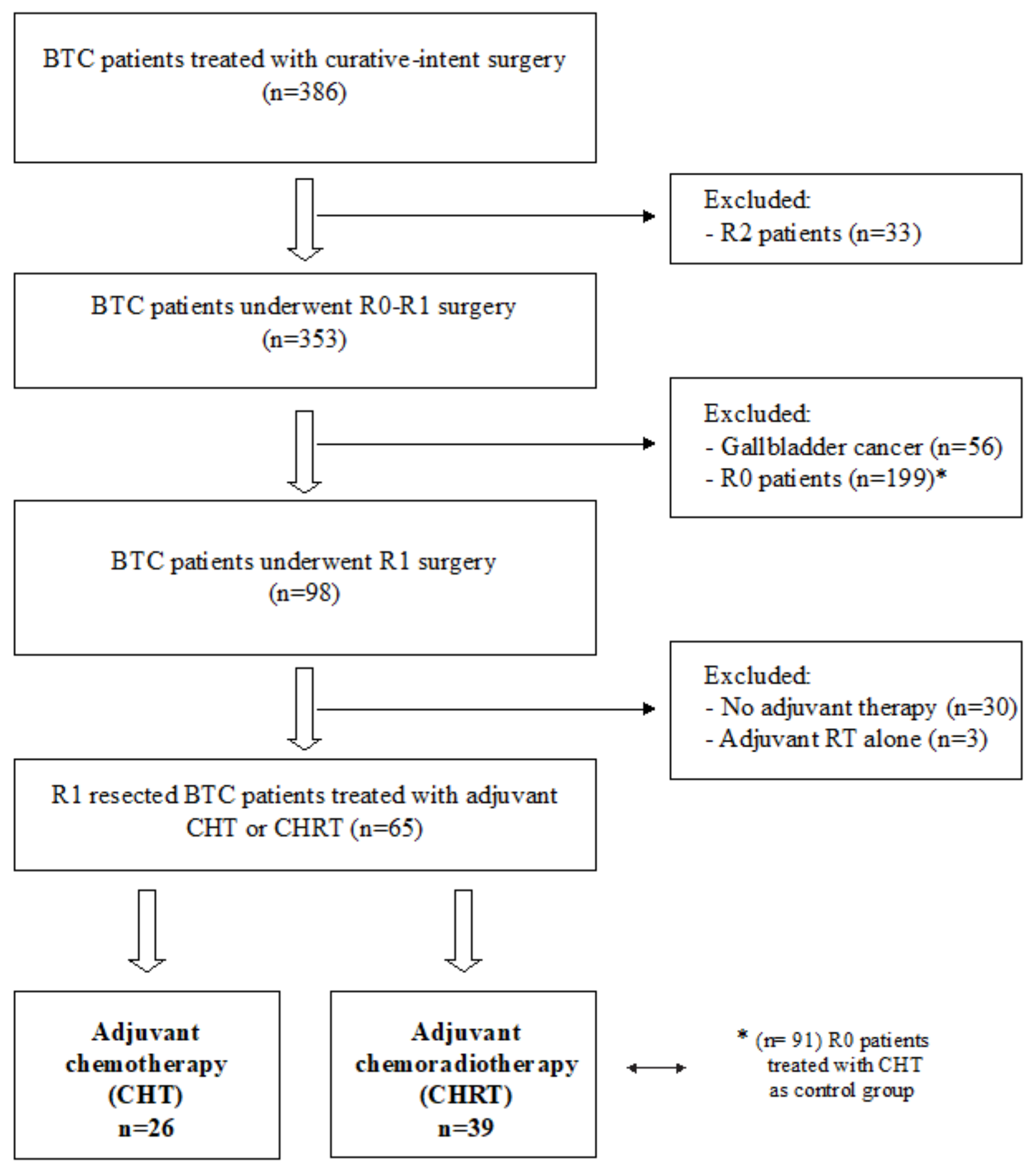
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| iCCA | intrahepatic cholangiocarcinoma |
| dCCA | distal cholangiocarcinoma |
| pCCA | perihilar cholangiocarcinoma |
| CHRT | chemoradiotherapy |
| CHT | chemotherapy |
| BTC | biliary tract cancer |
| GBC | gallbladder cancer |
| OS | overall survival |
| RFS | recurrence-free survival |
References
- Blechacz, B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver 2017, 11, 13–26. [Google Scholar] [CrossRef]
- Doherty, B.; Nambudiri, V.E.; Palmer, W.C. Update on the Diagnosis and Treatment of Cholangiocarcinoma. Curr. Gastroenterol. Rep. 2017, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Jarnagin, W.R.; Ruo, L.; Little, S.A.; Klimstra, D.; D’Angelica, M.; DeMatteo, R.P.; Wagman, R.; Blumgart, L.H.; Fong, Y. Patterns of Initial Disease Recurrence after Resection of Gallbladder Carcinoma and Hilar Cholangiocarcinoma: Implications for Adjuvant Therapeutic Strategies. Cancer 2003, 98, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Groot Koerkamp, B.; Wiggers, J.K.; Allen, P.J.; Besselink, M.G.; Blumgart, L.H.; Busch, O.R.C.; Coelen, R.J.; D’Angelica, M.I.; DeMatteo, R.P.; Gouma, D.J.; et al. Recurrence Rate and Pattern of Perihilar Cholangiocarcinoma after Curative Intent Resection. J. Am. Coll. Surg. 2015, 221, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Spolverato, G.; Kim, Y.; Alexandrescu, S.; Marques, H.P.; Lamelas, J.; Aldrighetti, L.; Gamblin, T.C.; Maithel, S.K.; Pulitano, C.; Bauer, T.W.; et al. Management and Outcomes of Patients with Recurrent Intrahepatic Cholangiocarcinoma Following Previous Curative-Intent Surgical Resection. Ann. Surg. Oncol. 2016, 23, 235–243. [Google Scholar] [CrossRef]
- Farges, O.; Fuks, D.; Boleslawski, E.; Le Treut, Y.-P.; Castaing, D.; Laurent, A.; Ducerf, C.; Rivoire, M.; Bachellier, P.; Chiche, L.; et al. Influence of Surgical Margins on Outcome in Patients with Intrahepatic Cholangiocarcinoma: A Multicenter Study by the AFC-IHCC-2009 Study Group. Ann. Surg. 2011, 254, 824–829; discussion 830. [Google Scholar] [CrossRef]
- DeOliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Choti, M.A.; Yeo, C.J.; Schulick, R.D. Cholangiocarcinoma: Thirty-One-Year Experience with 564 Patients at a Single Institution. Ann. Surg. 2007, 245, 755–762. [Google Scholar] [CrossRef]
- De Jong, M.C.; Nathan, H.; Sotiropoulos, G.C.; Paul, A.; Alexandrescu, S.; Marques, H.; Pulitano, C.; Barroso, E.; Clary, B.M.; Aldrighetti, L.; et al. Intrahepatic Cholangiocarcinoma: An International Multi-Institutional Analysis of Prognostic Factors and Lymph Node Assessment. J. Clin. Oncol. 2011, 29, 3140–3145. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine Compared with Observation in Resected Biliary Tract Cancer (BILCAP): A Randomised, Controlled, Multicentre, Phase 3 Study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Malka, D.; Edeline, J. Adjuvant Capecitabine in Biliary Tract Cancer: A Standard Option? Lancet Oncol. 2019, 20, 606–608. [Google Scholar] [CrossRef]
- Horgan, A.M.; Amir, E.; Walter, T.; Knox, J.J. Adjuvant Therapy in the Treatment of Biliary Tract Cancer: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2012, 30, 1934–1940. [Google Scholar] [CrossRef]
- Edge, S.B.; American Joint Committee on Cancer (Eds.) AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010; ISBN 978-0-387-88440-0. [Google Scholar]
- Jung, S.J.; Woo, S.M.; Park, H.K.; Lee, W.J.; Han, M.A.; Han, S.-S.; Kim, S.H.; Park, S.-J.; Kim, T.H.; Koh, Y.H.; et al. Patterns of Initial Disease Recurrence after Resection of Biliary Tract Cancer. Oncology 2012, 83, 83–90. [Google Scholar] [CrossRef]
- Uchiyama, K.; Yamamoto, M.; Yamaue, H.; Ariizumi, S.; Aoki, T.; Kokudo, N.; Ebata, T.; Nagino, M.; Ohtsuka, M.; Miyazaki, M.; et al. Impact of Nodal Involvement on Surgical Outcomes of Intrahepatic Cholangiocarcinoma: A Multicenter Analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J. Hepatobiliary Pancreat. Sci. 2011, 18, 443–452. [Google Scholar] [CrossRef]
- Ribero, D.; Pinna, A.D.; Guglielmi, A.; Ponti, A.; Nuzzo, G.; Giulini, S.M.; Aldrighetti, L.; Calise, F.; Gerunda, G.E.; Tomatis, M.; et al. Surgical Approach for Long-Term Survival of Patients With Intrahepatic Cholangiocarcinoma: A Multi-Institutional Analysis of 434 Patients. Arch. Surg. 2012, 147, 1107–1113. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Fong, Y.; DeMatteo, R.P.; Gonen, M.; Burke, E.C.; Bodniewicz, B.S.J.; Youssef, B.A.M.; Klimstra, D.; Blumgart, L.H. Staging, Resectability, and Outcome in 225 Patients With Hilar Cholangiocarcinoma. Ann. Surg. 2001, 234, 507–519. [Google Scholar] [CrossRef]
- Ueda, J.; Yoshida, H.; Mamada, Y.; Taniai, N.; Yoshioka, M.; Hirakata, A.; Kawano, Y.; Mizuguchi, Y.; Shimizu, T.; Kanda, T.; et al. Evaluation of Positive Ductal Margins of Biliary Tract Cancer in Intraoperative Histological Examination. Oncol. Lett. 2018, 16, 6677–6684. [Google Scholar] [CrossRef]
- Nakachi, K.; Ikeda, M.; Konishi, M.; Nomura, S.; Katayama, H.; Kataoka, T.; Todaka, A.; Yanagimoto, H.; Morinaga, S.; Kobayashi, S.; et al. Adjuvant S-1 Compared with Observation in Resected Biliary Tract Cancer (JCOG1202, ASCOT): A Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet 2023, 401, 195–203. [Google Scholar] [CrossRef]
- Ebata, T.; Hirano, S.; Konishi, M.; Uesaka, K.; Tsuchiya, Y.; Ohtsuka, M.; Kaneoka, Y.; Yamamoto, M.; Ambo, Y.; Shimizu, Y.; et al. Randomized Clinical Trial of Adjuvant Gemcitabine Chemotherapy versus Observation in Resected Bile Duct Cancer. Br. J. Surg. 2018, 105, 192–202. [Google Scholar] [CrossRef]
- Edeline, J.; Benabdelghani, M.; Bertaut, A.; Watelet, J.; Hammel, P.; Joly, J.-P.; Boudjema, K.; Fartoux, L.; Bouhier-Leporrier, K.; Jouve, J.-L.; et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J. Clin. Oncol. 2019, 37, 658–667. [Google Scholar] [CrossRef]
- Lamarca, A.; Edeline, J.; McNamara, M.G.; Hubner, R.A.; Nagino, M.; Bridgewater, J.; Primrose, J.; Valle, J.W. Current Standards and Future Perspectives in Adjuvant Treatment for Biliary Tract Cancers. Cancer Treat. Rev. 2020, 84, 101936. [Google Scholar] [CrossRef]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.-J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in Combination with Gemcitabine and Cisplatin Compared with Gemcitabine and Cisplatin Alone for Patients with Advanced Biliary Tract Cancer (KEYNOTE-966): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Ruth He, A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah Lee, M.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, 15. [Google Scholar] [CrossRef]
- Komaya, K.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Mizuno, T.; Yamaguchi, J.; Nagino, M. Recurrence after Curative-Intent Resection of Perihilar Cholangiocarcinoma: Analysis of a Large Cohort with a Close Postoperative Follow-up Approach. Surgery 2018, 163, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Pitt, H.A.; Nakeeb, A.; Abrams, R.A.; Coleman, J.; Piantadosi, S.; Yeo, C.J.; Lillemore, K.D.; Cameron, J.L. Perihilar Cholangiocarcinoma. Postoperative Radiotherapy Does Not Improve Survival. Ann. Surg. 1995, 221, 788–797. [Google Scholar] [CrossRef]
- Todoroki, T.; Ohara, K.; Kawamoto, T.; Koike, N.; Yoshida, S.; Kashiwagi, H.; Otsuka, M.; Fukao, K. Benefits of Adjuvant Radiotherapy after Radical Resection of Locally Advanced Main Hepatic Duct Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 581–587. [Google Scholar] [CrossRef]
- Gwak, H.K.; Kim, W.C.; Kim, H.J.; Park, J.H. Extrahepatic Bile Duct Cancers: Surgery Alone versus Surgery plus Postoperative Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 194–198. [Google Scholar] [CrossRef]
- Vern-Gross, T.Z.; Shivnani, A.T.; Chen, K.; Lee, C.M.; Tward, J.D.; MacDonald, O.K.; Crane, C.H.; Talamonti, M.S.; Munoz, L.L.; Small, W. Survival Outcomes in Resected Extrahepatic Cholangiocarcinoma: Effect of Adjuvant Radiotherapy in a Surveillance, Epidemiology, and End Results Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 189–198. [Google Scholar] [CrossRef]
- Cheng, Q.; Luo, X.; Zhang, B.; Jiang, X.; Yi, B.; Wu, M. Predictive Factors for Prognosis of Hilar Cholangiocarcinoma: Postresection Radiotherapy Improves Survival. Eur. J. Surg. Oncol. 2007, 33, 202–207. [Google Scholar] [CrossRef]
- Shinohara, E.T.; Mitra, N.; Guo, M.; Metz, J.M. Radiation Therapy Is Associated with Improved Survival in the Adjuvant and Definitive Treatment of Intrahepatic Cholangiocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1495–1501. [Google Scholar] [CrossRef]
- Nakeeb, A.; Tran, K.Q.; Black, M.J.; Erickson, B.A.; Ritch, P.S.; Quebbeman, E.J.; Wilson, S.D.; Demeure, M.J.; Rilling, W.S.; Dua, K.S.; et al. Improved Survival in Resected Biliary Malignancies. Surgery 2002, 132, 555–563; discussion 563–564. [Google Scholar] [CrossRef]
- Nelson, J.W.; Ghafoori, A.P.; Willett, C.G.; Tyler, D.S.; Pappas, T.N.; Clary, B.M.; Hurwitz, H.I.; Bendell, J.C.; Morse, M.A.; Clough, R.W.; et al. Concurrent Chemoradiotherapy in Resected Extrahepatic Cholangiocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 148–153. [Google Scholar] [CrossRef]
- Bonet Beltrán, M.; Allal, A.S.; Gich, I.; Solé, J.M.; Carrió, I. Is Adjuvant Radiotherapy Needed after Curative Resection of Extrahepatic Biliary Tract Cancers? A Systematic Review with a Meta-Analysis of Observational Studies. Cancer Treat. Rev. 2012, 38, 111–119. [Google Scholar] [CrossRef]
- Kim, H.; Heo, M.H.; Kim, J.Y. Comparison of the Effects of Adjuvant Concurrent Chemoradiotherapy and Chemotherapy for Resected Biliary Tract Cancer. BMC Gastroenterol. 2020, 20, 20. [Google Scholar] [CrossRef]
- Ben-Josef, E.; Guthrie, K.A.; El-Khoueiry, A.B.; Corless, C.L.; Zalupski, M.M.; Lowy, A.M.; Thomas, C.R.; Alberts, S.R.; Dawson, L.A.; Micetich, K.C.; et al. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J. Clin. Oncol. 2015, 33, 2617–2622. [Google Scholar] [CrossRef]
- Gholami, S.; Colby, S.; Horowitz, D.P.; Guthrie, K.A.; Ben-Josef, E.; El-Khoueiry, A.B.; Blanke, C.D.; Philip, P.A.; Kachnic, L.A.; Ahmad, S.A.; et al. ASO Visual Abstract: Adjuvant Chemoradiation in Patients with Lymph Node-Positive Biliary Tract Cancers-Secondary Analysis of a Single-Arm Clinical Trial (SWOG 0809). Ann. Surg. Oncol. 2023, 30, 1364–1365. [Google Scholar] [CrossRef]
- Shroff, R.T.; Kennedy, E.B.; Bachini, M.; Bekaii-Saab, T.; Crane, C.; Edeline, J.; El-Khoueiry, A.; Feng, M.; Katz, M.H.G.; Primrose, J.; et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1015–1027. [Google Scholar] [CrossRef]
- Valle, J.W.; Palmer, D.; Jackson, R.; Cox, T.; Neoptolemos, J.P.; Ghaneh, P.; Rawcliffe, C.L.; Bassi, C.; Stocken, D.D.; Cunningham, D.; et al. Optimal Duration and Timing of Adjuvant Chemotherapy after Definitive Surgery for Ductal Adenocarcinoma of the Pancreas: Ongoing Lessons from the ESPAC-3 Study. J. Clin. Oncol. 2014, 32, 504–512. [Google Scholar] [CrossRef]
- Parsons, M.; Lloyd, S.; Johnson, S.; Scaife, C.; Soares, H.; Kim, R.; Kim, R.; Garrido-Laguna, I.; Tao, R. The Implications of Treatment Delays in Adjuvant Therapy for Resected Cholangiocarcinoma Patients. J. Gastrointest. Cancer 2022, 1–9. [Google Scholar] [CrossRef]


| Total (n = 65) | Adjuvant CHT (n = 26) | Adjuvant CHRT (n = 39) | p Value | |
|---|---|---|---|---|
| Age, years | ||||
| ≥70 (%) | 21 (32.2) | 10 (38.4) | 11 (28.2) | 0.39 |
| <70 (%) | 44 (67.7) | 16 (61.6) | 28 (71.8) | |
| Median | 65.8 | 67 | 64.4 | |
| range | 39.7–81.8 | 39.7–81.8 | 43.2–80.8 | |
| Sex (%) | ||||
| M | 33 (50.8) | 12 (46.1) | 21 (53.8) | 0.54 |
| F | 32 (49.2) | 14 (53.9) | 18 (46.2) | |
| Site of primary tumor | ||||
| ICC | 31 (47.7) | 15 (57.7) | 16 (41.0) | 0.41 |
| pCC | 21 (32.3) | 7 (26.9) | 14 (35.9) | |
| dCC | 13 (20.0) | 4 (15.4) | 9 (23.1) | |
| Grading (%) | ||||
| G1 | 2 (3.1) | 2 (7.8) | 0 (0.0) | 0.33 |
| G2 | 31 (47.7) | 10 (38.4) | 21 (53.8) | |
| G3 | 19 (29.2) | 7 (26.9) | 12 (30.8) | |
| n.a. | 13 (20.0) | 7 (26.9) | 6 (15.4) | |
| Pathological lymph node status (%) | ||||
| N0 | 25 (38.5) | 10 (38.4) | 15 (38.5) | 0.41 |
| N+ | 25 (38.5) | 8 (30.8) | 17 (43.6) | |
| n.a. | 15 (23.0) | 8 (30.8) | 7 (17.9) | |
| T stage (8th edition AJCC staging system) (%) | ||||
| T1 | 8 (12.3) | 5 (19.2) | 3 (7.7) | 0.17 |
| T2 | 41 (63.1) | 18 (69.2) | 23 (59.0) | |
| T3 | 11 (16.9) | 2 (7.7) | 9 (23.1) | |
| T4 | 5 (7.7) | 1 (3.9) | 4 (10.2) |
| Total (n = 65) | Adjuvant CHT (n = 26) | Adjuvant CHRT (n = 39) | |
|---|---|---|---|
| Postoperative CEA | |||
| Median (ng/mL) | 1.7 | 2.2 | 1.6 |
| Range (ng/mL) | 0.2–6.6 | 0.3–6.1 | 0.2–6.6 |
| Postoperative CA 19.9 | |||
| Median (U/mL) | 17 | 16.8 | 17 |
| Range (U/mL) | 4.0–219.0 | 4.0–62.1 | 5.4–219.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palloni, A.; Bisello, S.; Maggio, I.; Massucci, M.; Galuppi, A.; Di Federico, A.; Rizzo, A.; Ricci, A.D.; Siepe, G.; Morganti, A.G.; et al. The Potential Role of Adjuvant Chemoradiotherapy in Patients with Microscopically Positive (R1) Surgical Margins after Resection of Cholangiocarcinoma. Curr. Oncol. 2023, 30, 4754-4766. https://doi.org/10.3390/curroncol30050358
Palloni A, Bisello S, Maggio I, Massucci M, Galuppi A, Di Federico A, Rizzo A, Ricci AD, Siepe G, Morganti AG, et al. The Potential Role of Adjuvant Chemoradiotherapy in Patients with Microscopically Positive (R1) Surgical Margins after Resection of Cholangiocarcinoma. Current Oncology. 2023; 30(5):4754-4766. https://doi.org/10.3390/curroncol30050358
Chicago/Turabian StylePalloni, Andrea, Silvia Bisello, Ilaria Maggio, Maria Massucci, Andrea Galuppi, Alessandro Di Federico, Alessandro Rizzo, Angela Dalia Ricci, Giambattista Siepe, Alessio Giuseppe Morganti, and et al. 2023. "The Potential Role of Adjuvant Chemoradiotherapy in Patients with Microscopically Positive (R1) Surgical Margins after Resection of Cholangiocarcinoma" Current Oncology 30, no. 5: 4754-4766. https://doi.org/10.3390/curroncol30050358
APA StylePalloni, A., Bisello, S., Maggio, I., Massucci, M., Galuppi, A., Di Federico, A., Rizzo, A., Ricci, A. D., Siepe, G., Morganti, A. G., Brandi, G., & Frega, G. (2023). The Potential Role of Adjuvant Chemoradiotherapy in Patients with Microscopically Positive (R1) Surgical Margins after Resection of Cholangiocarcinoma. Current Oncology, 30(5), 4754-4766. https://doi.org/10.3390/curroncol30050358







