Abstract
GIST (gastrointestinal stromal tumors) represent 20% of sarcomatous tumors and 1–2% of primary gastrointestinal cancers. They have an excellent prognosis when localized and resectable, though their prognosis is poor in the metastatic setting, with limited options after the second line until recently. Four lines are now standard in KIT-mutated GIST and one in PDGFRA-mutated GIST. An exponential growth of new treatments is expected in this era of molecular diagnostic techniques and systematic sequencing. Currently, the main challenge remains the emergence of resistance linked to secondary mutations caused by selective pressure induced by TKIs. Repeating biopsies to tailor treatments might be a step in the right direction, and liquid biopsies at progression may offer a non-invasive alternative. New molecules with wider KIT inhibition are under investigation and could change the catalog and the sequence of existing treatments. Combination therapies may also be an approach to overcome current resistance mechanisms. Here, we review the current epidemiology and biology of GIST and discuss future management options, with an emphasis on genome-oriented therapies.
1. Introduction
1.1. Epidemiology
GIST (gastrointestinal stromal tumors) represent 20% of sarcomatous tumors and 1–2% of primary gastrointestinal cancers. They typically present in older individuals and are most common in the stomach (60–70%), followed by the small intestine (20–25%), colon and rectum (5%), and esophagus (<5%) [1]. GIST can also occur in children and young adults, especially when a genetic predisposition is involved [2]. They are equally common in male and female patients [3].
1.2. Clinical Presentation
GIST are often asymptomatic and an incidental finding on imaging. However, the clinical presentation can include abdominal pain or digestive bleeding. Endoscopic and radiologic findings are variable and often show a rather homogeneous lesion if the tumor is small, but if larger, one with heterogeneous enhancement, irregular margins, central necrosis, and signs of hemorrhage [4,5].
1.3. Histological Diagnosis
GIST cells derive from Cajal’s interstitial cells, or its precursors, and constitutively express the KIT protein/receptor. GIST occur nearly always in the gastrointestinal tract. While many of the very heterogeneous soft-tissue tumors are difficult to diagnose, GIST are well defined by a combination of morphologic, immunohistochemical, and molecular features.
Histologically, there are two main types, spindle-cell GIST, mainly found in mutated KIT or BRAF GIST (in 70% of cases), and epithelioid-cell GIST (20%), mainly found in platelet-derived growth factor receptor A (PDGFRA) or succinate dehydrogenase (SDH) GIST. Ten percent harbor a mixed morphology [6,7].
1.4. Immunohistochemistry
DOG-1 (discovered on GIST-1) has both high sensitivity and specificity and is found in 88% of cases [8]. KIT is found in 95% of GIST and is very sensitive but not specific. However, in GIST with a PDGFRA mutation, the sensitivity of these markers decreases to 9% and 79%, respectively [9]. These two markers are classically expressed in a diffuse and intense manner.
1.5. Molecular Pathogenesis
KIT is a proto-oncogene that encodes the KIT tyrosine kinase (TK) receptor. It includes two main regions. The first is the receptor regulatory domain with the extracellular region, the transmembrane region, and the juxta-membrane domain. The second is the cytoplasmic region with a TK domain that includes a TK1 domain that anchors ATP (encoded by exons 13 and 14) and a TK2 domain that binds and phosphorylates downstream substrates. An activation loop (encoded by exon 17) is located on the TK2 domain and stabilizes the activated KIT receptor in a permanently active state. Binding of the ligand to KIT results in the activation of tyrosine kinase activity and stimulation of downstream pathways, including RAS/RAF/MAPK, PI3K/Akt/mTOR, and Src kinase pathways, resulting in cellular proliferation and inhibition of apoptosis. Activating mutations in KIT leads to constitutive activation of KIT in a ligand-independent manner. PDGFRA is structurally similar [7,10] (Figure 1).
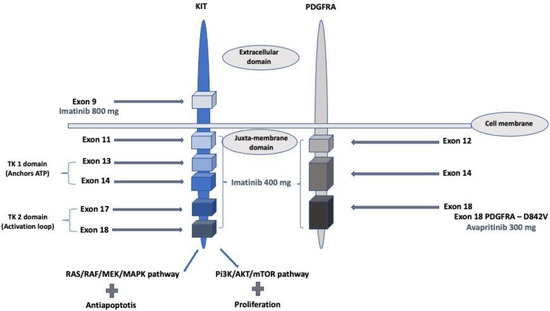
Figure 1.
KIT and PDGFRA gain-of-function mutations and related treatment with recommended dosage (imatinib and avapritinib).
The majority of these gain-of-function mutations are found in the KIT gene, with two-thirds and the most frequent within exon 11. Exon 9 mutations are often found in small or large bowel tumors and represent approximately 10% of GIST. Deletions, deletion–insertion, point mutations, duplications, insertions, and inversion have been identified in KIT. Deletions in exon 11, especially involving codons 557 and 558, are associated with a poorer natural prognosis compared to exon 11 point mutations [11]. Mutations in exons 13, 17, and 18 are rare [12].
Furthermore, 5 to 10% of mutations are related to the PDGFRA oncogene, more specifically to exons 12, 14, and 18. PDGFRA mutations are more commonly found in the stomach. Exon 18 of PDGFRA is the most frequently mutated region, with exon 18 D842V mutations accounting for 70% of PDGFRA-mutant cases, while exons 12 or 14 are rarely mutated. [12] KIT and PDGFRA mutations are considered mutually exclusive [13].
Despite extensive sequencing, some GIST remain “wild-type” GIST, but a number have been identified nowadays with the modern techniques to carry low-frequency KIT or PDGFRA mutations, while others were associated with fusions of NTRK (NTRK3-ETV6) and FGFR1 (FGFR1-HOOK3, FGFR1-TACC1), alterations in the RAS-MAPK pathway with BRAF mutations, NF1 mutations, or SDHA deficiency caused by a germline mutation in the suppressor genes encoding the SDH complex or by SDHC promotor methylation [13,14].
2. Management of Localized GIST
Surgery is the treatment of choice for resectable GIST if no major functional losses are to be expected. The tumor should be resected completely, and rupture needs to be avoided [5]. Patients at high risk of recurrence need post-operative imatinib for a duration of a minimum of 3 years if well tolerated [15].
Different scores are used to determine the risk:
The “Miettinen classification”, also known as the Armed Forces Institute of Pathology (AFIP) classification, includes the mitotic index, the size of the tumor, and the location.
Joensuu’s classification, also known as modified NIH classification, uses the parameters above and integrates the pejorative nature of a perforation. It aims to better split the GIST between intermediate and high risk [16].
All classifications stratify patients into very low, low, intermediate, and high-risk categories of recurrence. The indication for adjuvant treatment depends on the risk score, but also on the mutational status [17]. As an example, patients with the PDGFRA D842V mutation do not receive adjuvant treatment as this is a resistance mutation to imatinib [18].
Molecular prognostic factors such as the level of tumor genome rearrangement have been investigated and are currently evaluated in intermediate-risk GIST in the GI-GIST trial (NCT02576080).
3. Management of Metastatic GIST
3.1. First-Line Treatment in the Metastatic Setting: Imatinib
Imatinib is an inhibitor of KIT, PDGFRA, and BCR-ABL tyrosine kinase and is used to treat inoperable or metastatic GIST. Standard dosage is 400 mg for all sensitive mutations. Indeed, sensitivity to imatinib for exon 9 and exon 11 mutated GIST was demonstrated in the lead trial evaluating imatinib in GIST [19], while KIT exon 9 mutations are treated with 800 mg/d as this provides longer progression-free survival (PFS) [20,21,22].
Currently, imatinib is continued until progression or intolerance in the metastatic setting.
The exon 13 and 17 activation loop mutations are essentially secondary mutations that occur on imatinib therapy. Primary exon 13 mutations exist and are often sensitive to imatinib in vitro. Given the rarity of this mutation, in vivo sensitivity is not clear as little evidence exists to date [23].
Currently, imatinib is continued until progression or intolerance in the metastatic setting.
Primary PDGFRA mutations occur mainly in exon 18 and exon 12, which, respectively, encode the activation loop in the juxta-membrane domain, but more rarely in exon 14, which encodes the ATP-binding domain [24]. While D842V, the most common exon 18 mutation, confers a primary mutation to imatinib, other types of PDGFRA mutations are sensitive to imatinib [25].
3.2. Primary Resistance to Treatment
3.2.1. Pseudo-Resistance: Imatinib Plasma-Levels and Pharmacokinetics
Demetri et al. showed in a small group of patients that imatinib plasma levels above 1100 ng/mL were associated with clinical benefit and a longer time to disease progression [26]. Pointing in the same direction, in chronic myeloid leukemia, Gotta et al. proved in a prospective randomized controlled trial that imatinib dose monitoring helps in achieving efficient plasma concentrations [27]. In another trial, durable effective imatinib concentrations were reached only by 33.3% [28], raising the question if imatinib should be dosed individually.
Several treatments such as proton pump inhibitors can have interactions with oral oncology treatments. Indeed, the concomitant use of TKIs and proton pump inhibitors can reduce TKI absorption, thus potentially reducing the effectiveness of TKIs [29].
Genetic polymorphisms of cytochrome P450 can participate in interpatient variability in imatinib blood levels [30]. At progression, the use of high-dose imatinib (800 mg daily) has shown benefit to patients with advanced or metastatic GIST that progressed on the standard dose [31].
Compliance issues are also a major factor to consider when suspecting pseudo-progression. Indeed, in a study made on imatinib-treated patients who had a diagnosis of CML or GIST, compliance with imatinib was about 75%, with 30% of patients interrupting therapy for at least 30 consecutive days in the first year [32].
3.2.2. PDGFRA Exon 18 D842V Mutation (75% of PDGFRA Mutations)
Most PDGFRA mutations are sensitive to imatinib, with the exception of the frequent PDGFRA exon 18 D842V mutation, which confers primary resistance to imatinib [33].
Avapritinib has been specifically developed to target this mutation and the NAVIGATOR study showed high efficacy of this drug with above 90% overall response rate (ORR) and a duration of response of 70% at 1 year [34,35,36,37].
To note, among 167 patients starting on 300 mg of avapritinib, 37.0% of all patients and 52.0% of patients older than 65 years showed cognitive dysfunction. This toxicity decreased faster to a lower grade with dose modification (1.3–3.1 weeks) than without (4.9–7.6 weeks). Median PFS was 11.4 months with dose modification and 7.2 months without. As a result, early recognition of neuro-toxicity and adapted dose modification can help maintain patients on this treatment. It can be recommended to assess cognitive function at baseline and monitored [38].
3.2.3. Rare Non-KIT/PDGFRA Molecular Subtypes
In the past, GIST have been classified as KIT or PDGFRA mutated according to testing that included the frequent mutations. GIST missing these mutations have been classified as “wild type” (WT). Later, the concept of quadruple negative WT-GIST was coined, and additional subgroups were identified. Recently, new techniques have detected low-frequency mutations [39]. The latter probably explains why some GIST considered to be WT respond to TKI [40,41].
Within the WT-GIST category, the following “new” subtypes have been identified.
SDH-Deficient (5 to 8%) [42]
About 20–40% of WT-GIST show defects in succinate dehydrogenase (SDH) complexes. Some mutations can be germline [43] and involve genetic testing. Few data are available in SDH-deficient GIST due to their rarity. The early, large trials did not classify WT-GIST further [44].
A retrospective analysis of 87 patients with SDH-deficient GIST found better response rates with sunitinib than with imatinib, which had very little activity in this cohort. Nilotinib and vandetanib have also been used, but limited data do not support the use of these drugs [45].
The dysfunction of the SDH complex in these tumors leads to a pseudo-hypoxic phenotype suggesting a potential benefit of TKIs with anti-angiogenic activity. In the phase II REGISTRI trial, an ORR of nearly 20% was achieved with sunitinib, and regorafenib was associated with an 87% disease control rate [46]. Other TKIs such as regorafenib and pazopanib showed limited efficacy.
A novel third-generation TKI, olverembatinib, has shown antitumor activity in patients with TKI-resistant SDH-deficient GIST in a phase Ib/II study with 2 partial responses in 6 evaluable patients and 1 with stable disease for 36 cycles, calling for its further investigation [47].
As SDH-loss causes succinate accumulation and activation of pseudohypoxia signaling [48] via overexpression of HIF-proteins, specific HIFa inhibitors such as belzutifan are under development in different types of tumors associated with overexpression of HIF (NCT04895748, NCT04924075) and could be good candidates for SDH-deficient GIST in the future.
Furthermore, temozolomide has potential interest in this subgroup with promising results in five patients (100% disease control rate, 40% ORR), motivating an ongoing phase II study (NCT03556384). Overexpression of FGF/FGFR signaling pathways has also been reported in SDH-deficient GIST, and a new pan-FGFR inhibitor, rogaratinib, is being evaluated in a phase II trial (NCT04595747).
Non-SDH Deficient
NTRK fusions
NTRK fusions are agnostic molecular alterations that render tumors sensitive to TRK inhibitors. Larotrectinib or entrectinib demonstrated activity against solid tumors harboring NTRK fusions [49,50,51,52] and can be used in this setting.
BRAF V600E mutations: (0.6 to 3.9%)
Just like NTRK fusions, BRAF V600E mutations are considered tumor-agnostic features predictive to response to BRAF inhibitors. In a study of GIST, BRAF V600E mutations were detected in two of 28 KIT and PDGFRA wild-type patients. [53]. BRAF mutations confer resistance to imatinib and sunitinib [54].
NF1 mutations (0.1 to 2.4%)
Neurofibromatosis 1 (NF1) is an autosomal dominant disorder caused by germline mutations in the NF1 tumor suppressor gene causing its inactivation. There is an estimated 7% of individuals with NF1 who will develop GIST during their life [42].
To date, there is no standard treatment for NF1-mutated GIST, which do not respond to TKIs, and surgery remains the main option for these patients. However, these tumors appear to be more indolent [55].
MEK inhibitors might have clinical efficacy in other NF1-associated tumors, given the role of the RAF/MEK/ERK pathway in neurofibromas. A phase II trial with mirdametinib, a MEK inhibitor, for adolescents and young adults with NF1-associated plexiform neurofibromas showed a 42% partial response and a significant decrease in pain ratings [56].
3.3. Secondary Resistance
3.3.1. KIT and PDGFRA Secondary Mutations
In the second line, 67% of patients have one or more secondary mutations involving KIT exon 17, exon 13, and exon 14, causing resistance to imatinib [57].
Point mutations associated with imatinib resistance are usually located in the drug/ATP-binding pocket of the receptor (encoded by exons 13 and 14) or in the activation loop (encoded by exon 17) [58].
For avapritinib, mechanisms of secondary resistance in PDGFRA-mutant GIST involve compound mutations of exons 13, 14, and 15 of PDGFRA with codon 658 and 680 mutations representing a recurrent cause of resistance [59].
3.3.2. Second-Line Sunitinib
Sunitinib was approved after a phase III trial in patients with GIST failing or intolerant to imatinib and showed a longer time to progression (27.3 versus 6.4 weeks, p < 0.0001) in patients with sunitinib than placebo.
Of interest, higher response rates were observed among GIST with a primary KIT exon 9 mutation [60].
Antiangiogenic effects of sunitinib treatment may contribute to its effectiveness. Indeed, sunitinib selectively inhibits PDGFRB and VEGFR in addition to KIT and PDGFRA, whereas imatinib inhibits PDGFRB but not VEGFR. Sunitinib has, however, little activity against secondary mutations involving the KIT activation loop (exons 17 and 18) [61].
3.3.3. Third-Line Regorafenib
Regorafenib is a multikinase inhibitor and also inhibits VEGFR and showed a significant improvement in median PFS (4.8 vs. 0.9 months, HR 0.27, p < 0·0001) over placebo in patients already treated with imatinib and sunitinib, leading to its approval in the third line [62]. It has become the treatment of choice in patients with an exon 17 mutation, as these do not respond to sunitinib [63].
3.3.4. Third-Line Pazopanib
Pazopanib has been tested in the third line, following imatinib and sunitinib failure, and showed modest benefit with a median PFS of 3.4 months (95% CI 2.4–5.6) vs. 2.3 months in best supportive care only [64].
3.3.5. Fourth-Line Ripretinib
Ripretinib is an anti-KIT anti-PDGFRA TKI, also active against PDGFRB, TIE2, VEGFR2, and BRAF. The INVICTUS trial enrolled 129 participants with advanced GIST who progressed after imatinib, sunitinib, and regorafenib. Ripretinib improved PFS and OS significantly (PFS 6.3 vs. 1 month, HR 0.15, p < 0.0001, OS 15.1 months vs. 6.6 months HR 0.36, p = 0.0004) [65]. A further gain in PFS of 3.7 months was obtained by doubling the dose (2 × 150 mg) upon first progression on the standard dose [66].
In this setting, ripretinib demonstrated a PFS benefit regardless of the primary mutation [67].
Ripretinib did not improve PFS when tested in the second-line INTRIGUE trial against sunitinib, but improved response rates (23.9 vs. 14.6%). OS data are immature. Sunitinib showed better PFS in the exon 9 subgroup [68].
The failure of ripretinib in the second line compared to sunitinib might be explained by the emergence of secondary exon 13 mutations, which decrease ripretinib efficacy compared to sunitinib [68].
Interestingly, a Chinese phase II study, testing ripretinib in the second line, showed a benefit in PFS for ripretinib [69].
3.3.6. Third-Line Avapritinib
VOYAGER, a randomized, phase III trial, tested avapritinib versus regorafenib in the third line. In molecularly unselected patients, the primary end point was not met. There was no significant difference in median PFS between avapritinib and regorafenib, but in the selected subgroup of patients with the PDGFRA exon 18 D842V mutation, which is resistant to the other TKIs, avapritinib showed high response rates [70] (Table 1).

Table 1.
Gain-of-function mutations and related treatment with recommended dosage.
PDGFRA: platelet-derived growth factor receptor A; SDH: succinate dehydrogenase; NTRAK: neurotrophic tyrosine receptor kinase, BRAF: v-raf murine sarcoma viral oncogene homolog B1; NF1: neurofibromin 1
4. Future Perspectives (Table 2)
4.1. Immunotherapy
The activity of anti-PD-1/PD-L1 and CTLA-4 drugs and combinations have been investigated in GIST. In a randomized phase II trial with 40 patients, nivolumab with or without ipilimumab showed only modest response rates. Other clinical trials based on the same combination are ongoing [71]. No or little clinical efficacy was seen with ipilimumab plus dasatinib [72], epacadostat with pembrolizumab [73], and in the subgroup of 31 patients with GIST of the PEMBROSARC trial treated with pembrolizumab and metronomic cyclophosphamide, which showed a low 2.3% ORR [74].

Table 2.
Ongoing studies evaluating drugs for metastatic or advanced unresectable GIST after standard treatment, and first line for SDH mutant/deficient.
Table 2.
Ongoing studies evaluating drugs for metastatic or advanced unresectable GIST after standard treatment, and first line for SDH mutant/deficient.
| Trials | Phase | Drug | Control Arm | Population | Status |
|---|---|---|---|---|---|
| TKIs | |||||
| NCT05489237 | I | IDRX-42 | Metastatic and/or unresectable GIST. After at least 1 line including imatinib | Recruiting | |
| NCT03594422 | I | Olverembatinib | Metastatic and/or unresectable GIST with primary resistance to imatinib. | Recruiting | |
| NCT05160168 | I/II | THE 630 | Metastatic and/or unresectable GIST. After at least 1 line including imatinib | Recruiting | |
| NCT04595747 | II | Rogaratinib | Metastatic and/or unresectable SDH-deficient GIST. No prior treatment required. | Recruiting | |
| NCT04193553 (LENVAGIST) | II | Lenvatinib | Metastatic and/or unresectable GIST. After at least 2 lines including imatinib, and sunitinib | Recruiting | |
| NCT04409223 | III | Famitinib | Sunitnib | Metastatic and/or unresectable GIST. Second line after imatinib failure | Recruiting |
| NCT05208047 | III |
| Sunitinib | Metastatic and/or unresectable GIST. After at least 1 line including imatinib | Recruiting |
| Immunotherapy | |||||
| NCT05152472 (ATEZOGIST) | II | Atezolizumab + imatinib | Imatinib | Metastatic and/or unresectable GIST. After at least 3 lines including imatinib, sunitinib and regorafenib | Recruiting |
| NCT03609424 | Ib/II | Spartalizumab (PDR001) + imatinib | Metastatic and/or unresectable GIST. After at least 3 lines including imatinib, sunitinib and regorafenib | Completed | |
| NCT04000529 | Ib | TNO155 + Spartalizumab | Metastatic and/or unresectable solid tumor, after failure of standard therapies. | Recruiting | |
| NCT03475953 (REGOMUNE) | I/II | Regorafenib + avelumab | Metastatic and/or unresectable solid tumor, after failure of standard of care | Recruiting | |
| NCT04258956 (AXAGIST) | II | Axitinib + avelumab | Metastatic and/or unresectable GIST. After at least 2 lines including imatinib, and sunitinib | Recruiting | |
| NCT02834013 | II | Ipilimumab + nivolumab | Metastatic and/or unresectable solid tumor, after failure of standard therapies. | Recruiting | |
| Chemotherapy | |||||
| NCT03556384 | II | Temozolomide | Metastatic and/or unresectable SDH-deficient GIST. No prior treatment required | Recruiting | |
| NCT03944304 | II | Paclitaxel | Metastatic and/or unresectable GIST. After at least 3 lines including imatinib, sunitinib and regorafenib | Recruiting | |
| Other | |||||
| NCT05245968 (CHAPTERGIST-101) | I | Pimitespib (TAS-116) in Combination with Imatinib | Metastatic and/or unresectable GIST. After imatinib. | Recruiting | |
| NCT04006769 | I | Entacapone + imatinib | Metastatic and/or unresectable GIST. After at least 2 lines including imatinib, and sunitinib | Active, not recruiting | |
| NCT03411915 | I | Tidutamab | Metastatic and/or unresectable GIST. After at least 2 lines including imatinib, and sunitinib | Completed | |
Ongoing trials are investigating avelumab with axitinib (NCT04258956, AXAGIST) or with regorafenib (NCT0347595, REGOMUNE), spartalizumab with imatinib (NCT03609424), or with TNO155 or ribociclib (NCT04000529).
The retrospective analysis of the Sarc028 study showed the presence of tertiary lymphoid structures (TLSs), found to be associated with better response and longer PFS [75]. Today, immunotherapy in GIST requires further evaluation in prospective trials, possibly guided by new biomarkers.
4.2. New Tyrosine Kinase Inhibitors
Lenvatinib, a broad-spectrum TKI targeting KIT, RET, PDGFRA, VEGFR 1-3, and FGFR 1-4, is investigated in the third line in LENVAGIST, a phase II study (NCT04193553).
Bezuclastinib (CGT9486) showed good clinical benefit and a median PFS of 12 months in an early phase trial and is currently tested with or without sunitinib in a phase 3 clinical trial (NCT05208047).
THE 630, a pan KIT inhibitor is in testing in a phase I/II study and showed good preclinical results (NCT05160168).
4.3. Pimitespib
Heat shock protein 90 is necessary for the stabilization of KIT and PDGFRA. Several HSP90 inhibitors had preclinical activity in GIST.
In a randomized placebo-controlled phase III trial (CHAPTERGIST-301), pimitespib, also known as TAS 116, a novel HSP90 inhibitor tested in the fourth line, showed an improved PFS and OS compared with placebo in patients with previously treated advanced GIST. Exploratory pharmacogenomic analysis showed a benefit irrespective of KIT mutation status. As a result, this therapy has been approved in the fourth line in Japan for the treatment of metastatic GIST. Visual impairment was reported in patients receiving pimitespib, with 13% of night blindness, and two cases of retinal vein occlusion and visual impairment, which resolved with discontinuation [76].
A phase I study evaluating pimitespib in combination with imatinib (NCT05245968, CHAPTERGIST-101) is ongoing.
4.4. Intratumoral Vaccination
Ilixadencel is a cell-based immune primer injected intratumorally that has been clinically investigated in metastatic renal cell carcinoma and hepatocellular carcinoma. It has been evaluated in a phase I study and presented an acceptable safety profile and radiological tumor responses in 33% of treated patients. Further investigation is needed [77].
5. Discussion
Despite the rarity of GIST, multicentric and multinational trials have led to the approval of four lines of treatment, and an exponential growth of new treatments is expected in this era of molecular diagnostic techniques and systematic sequencing.
Currently, the main challenge remains the emergence of resistance linked to secondary mutations caused by selection pressure induced by TKIs [78]. Future studies might select patients according to the secondary mutations, either on the basis of a (repeated) solid or liquid biopsy. Indeed, the failure of ripretinib in the second line compared to sunitinib might be explained by the emergence of secondary exon 13 mutations, which decrease ripretinib efficacy compared to sunitinib [62]. Furthermore, liquid biopsies may overcome the challenge of tumor heterogeneity and aid in detecting relevant resistance clones. An exploratory study of the NAVIGATOR trial for PDGFRA-mutant GIST showed that mutant ctDNA was detected in 63% of patients, and in this same population, the median sum of target lesions was 18.2 cm, suggesting that ctDNA detection might be potentially limited by the tumor burden [59]. Further research is needed to validate the optimal approach [79,80,81,82].
Imatinib has been the first-line standard since the discovery of its efficacy in GIST, and current studies focus mainly on later lines given the excellent tolerance profile of the molecule. New studies might focus on earlier lines to delay the emergence of resistance. Nonetheless, imatinib remains the gold standard, and the use of an experimental first-line TKI does not seem to reduce imatinib effectiveness in the second line [83,84]. Sequencing TKIs is an important challenge in the management of GIST, and a tailored approach based on an identified resistance mechanism will be an important part of future therapies.
6. Conclusions
GIST have a rich molecular landscape that is being unraveled thanks to modern genomic analyses. Four lines of therapy are currently standard, but the prognosis after the second line is poor and optimal management of secondary mutations remains a challenge.
Repeating biopsies to tailor treatments might be a step in the right direction, and liquid biopsies at progression may offer a non-invasive alternative.
New molecules with wider KIT inhibition are being tested and could change the catalog and the sequence of existing treatments. Combination therapies may also be an approach to overcome current resistance mechanisms [85], either by targeting these directly or avoiding their development.
Author Contributions
Conceptualization H.M. and A.F.; methodology, H.M. and A.F..; validation, H.M., A.F. and M.M.; formal analysis, H.M., A.F. and M.M..; resources, H.M., A.F. and M.M.; writing—original draft preparation, H.M.; writing—review and editing, H.M.; supervision, A.F. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miettinen, M.; Lasota, J. Gastrointestinal Stromal Tumors—Definition, Clinical, Histological, Immunohistochemical, and Molecular Genetic Features and Differential Diagnosis. Virchows Arch. 2001, 438, 1–12. [Google Scholar] [CrossRef]
- Benesch, M.; Wardelmann, E.; Ferrari, A.; Brennan, B.; Verschuur, A. Gastrointestinal Stromal Tumors (GIST) in Children and Adolescents: A Comprehensive Review of the Current Literature. Pediatr. Blood Cancer 2009, 53, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Søreide, K.; Sandvik, O.M.; Søreide, J.A.; Giljaca, V.; Jureckova, A.; Bulusu, V.R. Global Epidemiology of Gastrointestinal Stromal Tumours (GIST): A Systematic Review of Population-Based Cohort Studies. Cancer Epidemiol. 2016, 40, 39–46. [Google Scholar] [CrossRef]
- Gong, J.; Kang, W.; Zhu, J.; Xu, J. CT and MR Imaging of Gastrointestinal Stromal Tumor of Stomach: A Pictorial Review. Quant. Imaging Med. Surg. 2012, 2, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Blay, J.Y.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; et al. Gastrointestinal Stromal Tumours: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2021, 33, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Wardelmann, E.; Neidt, I.; Bierhoff, E.; Speidel, N.; Manegold, C.; Fischer, H.-P.; Pfeifer, U.; Pietsch, T. C-Kit Mutations in Gastrointestinal Stromal Tumors Occur Preferentially in the Spindle rather than in the Epithelioid Cell Variant. Mod. Pathol. 2002, 15, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Brčić, I.; Argyropoulos, A.; Liegl-Atzwanger, B. Update on Molecular Genetics of Gastrointestinal Stromal Tumors. Diagnostics 2021, 11, 194. [Google Scholar] [CrossRef]
- Kiśluk, J.; Zińczuk, J.; Kemona, A.; Guzińska-Ustymowicz, K.; Żurawska, J.; Kędra, B. Expression of CD117, DOG-1, and IGF-1R in Gastrointestinal Stromal Tumours—An Analysis of 70 Cases from 2004 to 2010. Gastroenterol. Rev. 2016, 2, 115–122. [Google Scholar] [CrossRef]
- Espinosa, I.; Lee, C.-H.; Kim, M.K.; Rouse, B.-T.; Subramanian, S.; Montgomery, K.; Varma, S.; Corless, C.L.; Heinrich, M.C.; Smith, K.S.; et al. A Novel Monoclonal Antibody against DOG1 Is a Sensitive and Specific Marker for Gastrointestinal Stromal Tumors. Am. J. Surg. Pathol. 2008, 32, 210–218. [Google Scholar] [CrossRef]
- Ksienski, D. Imatinib Mesylate: Past Successes and Future Challenges in the Treatment of Gastrointestinal Stromal Tumors. Clin. Med. Insights Oncol. 2011, 5, CMO.S4259. [Google Scholar] [CrossRef]
- Martín, J.; Poveda, A.; Llombart-Bosch, A.; Ramos, R.; López-Guerrero, J.A.; del Muro, J.G.; Maurel, J.; Calabuig, S.; Gutierrez, A.; de Sande, J.L.G.; et al. Deletions Affecting Codons 557-558 of the C-KIT Gene Indicate a Poor Prognosis in Patients with Completely Resected Gastrointestinal Stromal Tumors: A Study by the Spanish Group for Sarcoma Research (GEIS). J. Clin. Oncol. 2005, 23, 6190–6198. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.M.; Gutierrez Sainz, L.; Chi, P. The Management of Metastatic GIST: Current Standard and Investigational Therapeutics. J. Hematol. Oncol. 2021, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Bannon, A.E.; Klug, L.R.; Corless, C.L.; Heinrich, M.C. Using Molecular Diagnostic Testing to Personalize the Treatment of Patients with Gastrointestinal Stromal Tumors. Expert Rev. Mol. Diagn. 2017, 17, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Brenca, M.; Rossi, S.; Polano, M.; Gasparotto, D.; Zanatta, L.; Racanelli, D.; Valori, L.; Lamon, S.; Dei Tos, A.P.; Maestro, R. Transcriptome Sequencing IdentifiesETV6-NTRK3as a Gene Fusion Involved in GIST. J. Pathol. 2016, 238, 543–549. [Google Scholar] [CrossRef]
- Joensuu, H.; Eriksson, M.; Sundby Hall, K.; Hartmann, J.T.; Pink, D.; Schütte, J.; Ramadori, G.; Hohenberger, P.; Duyster, J.; Al-Batran, S.-E.; et al. One vs Three Years of Adjuvant Imatinib for Operable Gastrointestinal Stromal Tumor. JAMA 2012, 307, 1265. [Google Scholar] [CrossRef]
- Joensuu, H. Risk Stratification of Patients Diagnosed with Gastrointestinal Stromal Tumor. Hum. Pathol. 2008, 39, 1411–1419. [Google Scholar] [CrossRef]
- Joensuu, H.; Wardelmann, E.; Sihto, H.; Eriksson, M.; Sundby Hall, K.; Reichardt, A.; Hartmann, J.T.; Pink, D.; Cameron, S.; Hohenberger, P.; et al. Effect of KIT and PDGFRA Mutations on Survival in Patients with Gastrointestinal Stromal Tumors Treated with Adjuvant Imatinib. JAMA Oncol. 2017, 3, 602. [Google Scholar] [CrossRef]
- Wozniak, A.; Rutkowski, P.; Schöffski, P.; Ray-Coquard, I.; Hostein, I.; Schildhaus, H.-U.; Le Cesne, A.; Bylina, E.; Limon, J.; Blay, J.-Y.; et al. Tumor Genotype Is an Independent Prognostic Factor in Primary Gastrointestinal Stromal Tumors of Gastric Origin: A European Multicenter Analysis Based on ConticaGIST. Clin. Cancer Res. 2014, 20, 6105–6116. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Blanke, C.D.; Van den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and Safety of Imatinib Mesylate in Advanced Gastrointestinal Stromal Tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef]
- Verweij, J.; Casali, P.G.; Zalcberg, J.; LeCesne, A.; Reichardt, P.; Blay, J.-Y.; Issels, R.; van Oosterom, A.; Hogendoorn, P.C.; Van Glabbeke, M.; et al. Progression-Free Survival in Gastrointestinal Stromal Tumours with High-Dose Imatinib: Randomised Trial. Lancet 2004, 364, 1127–1134. [Google Scholar] [CrossRef]
- Blanke, C.D.; Rankin, C.; Demetri, G.D.; Ryan, C.W.; von Mehren, M.; Benjamin, R.S.; Raymond, A.K.; Bramwell, V.H.C.; Baker, L.H.; Maki, R.G.; et al. Phase III Randomized, Intergroup Trial Assessing Imatinib Mesylate at Two Dose Levels in Patients with Unresectable or Metastatic Gastrointestinal Stromal Tumors Expressing the Kit Receptor Tyrosine Kinase: S0033. J. Clin. Oncol. 2008, 26, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Comparison of Two Doses of Imatinib for the Treatment of Unresectable or Metastatic Gastrointestinal Stromal Tumors: A Meta-Analysis of 1640 Patients. J. Clin. Oncol. 2010, 28, 1247–1253. [CrossRef] [PubMed]
- McAuliffe, J.C.; Wang, W.-L.; Pavan, G.M.; Pricl, S.; Yang, D.; Chen, S.S.; Lazar, A.J.F.; Pollock, R.E.; Trent, J.C. Unlucky Number 13? Differential Effects of KIT Exon 13 Mutation in Gastrointestinal Stromal Tumors. Mol. Oncol. 2008, 2, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Martin-Broto, J.; Martinez-Marín, V.; Serrano, C.; Hindi, N.; López-Guerrero, J.A.; Ramos-Asensio, R.; Vallejo-Benítez, A.; Marcilla-Plaza, D.; González-Cámpora, R. Gastrointestinal Stromal Tumors (GISTs): SEAP–SEOM Consensus on Pathologic and Molecular Diagnosis. Clin. Transl. Oncol. 2016, 19, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Ryu, M.-H.; Jo, J.; Park, I.; Ryoo, B.-Y.; Kang, Y.-K. Efficacy of Imatinib in Patients with Platelet-Derived Growth Factor Receptor Alpha–Mutated Gastrointestinal Stromal Tumors. Cancer Res. Treat. 2016, 48, 546–552. [Google Scholar] [CrossRef]
- Demetri, G.D.; Wang, Y.; Wehrle, E.; Racine, A.; Nikolova, Z.; Blanke, C.D.; Joensuu, H.; von Mehren, M. Imatinib Plasma Levels Are Correlated with Clinical Benefit in Patients with Unresectable/Metastatic Gastrointestinal Stromal Tumors. J. Clin. Oncol. 2009, 27, 3141–3147. [Google Scholar] [CrossRef]
- Gotta, V.; Widmer, N.; Decosterd, L.A.; Chalandon, Y.; Heim, D.; Gregor, M.; Benz, R.; Leoncini-Franscini, L.; Baerlocher, G.M.; Duchosal, M.A.; et al. Clinical Usefulness of Therapeutic Concentration Monitoring for Imatinib Dosage Individualization: Results from a Randomized Controlled Trial. Cancer Chemother. Pharmacol. 2014, 74, 1307–1319. [Google Scholar] [CrossRef]
- Farag, S.; Verheijen, R.B.; Martijn Kerst, J.; Cats, A.; Huitema, A.D.R.; Steeghs, N. Imatinib Pharmacokinetics in a Large Observational Cohort of Gastrointestinal Stromal Tumour Patients. Clin. Pharmacokinet. 2016, 56, 287–292. [Google Scholar] [CrossRef]
- Sharma, M.; Holmes, H.M.; Mehta, H.B.; Chen, H.; Aparasu, R.R.; Shih, Y.T.; Giordano, S.H.; Johnson, M.L. The Concomitant Use of Tyrosine Kinase Inhibitors and Proton Pump Inhibitors: Prevalence, Predictors, and Impact on Survival and Discontinuation of Therapy in Older Adults with Cancer. Cancer 2019, 125, 1155–1162. [Google Scholar] [CrossRef]
- Pena, M.Á.; Muriel, J.; Saiz-Rodríguez, M.; Borobia, A.M.; Abad-Santos, F.; Frías, J.; Peiró, A.M. Effect of Cytochrome P450 and ABCB1 Polymorphisms on Imatinib Pharmacokinetics after Single-Dose Administration to Healthy Subjects. Clin. Drug Investig. 2020, 40, 617–628. [Google Scholar] [CrossRef]
- Gronchi, A.; Blay, J.-Y.; Trent, J.C. The Role of High-Dose Imatinib in the Management of Patients with Gastrointestinal Stromal Tumor. Cancer 2010, 116, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Henk, H.; Thomas, S.; Baladi, J.; Hatfield, A.; Goldberg, G.A.; Cortes, J. Compliance and Persistency with Imatinib. J. Clin. Oncol. 2006, 24 (Suppl. S18), 6038. [Google Scholar] [CrossRef]
- Cassier, P.A.; Fumagalli, E.; Rutkowski, P.; Schöffski, P.; Van Glabbeke, M.; Debiec-Rychter, M.; Emile, J.-F.; Duffaud, F.; Martin-Broto, J.; Landi, B.; et al. Outcome of Patients with Platelet-Derived Growth Factor Receptor Alpha–Mutated Gastrointestinal Stromal Tumors in the Tyrosine Kinase Inhibitor Era. Clin. Cancer Res. 2012, 18, 4458–4464. [Google Scholar] [CrossRef] [PubMed]
- Farag, S.; Somaiah, N.; Choi, H.; Heeres, B.; Wang, W.-L.; van Boven, H.; Nederlof, P.; Benjamin, R.; van der Graaf, W.; Grunhagen, D.; et al. Clinical Characteristics and Treatment Outcome in a Large Multicentre Observational Cohort of PDGFRA Exon 18 Mutated Gastrointestinal Stromal Tumour Patients. Eur. J. Cancer 2017, 76, 76–83. [Google Scholar] [CrossRef]
- Joseph, C.P.; Abaricia, S.N.; Angelis, M.A.; Polson, K.; Jones, R.L.; Kang, Y.; Riedel, R.F.; Schöffski, P.; Serrano, C.; Trent, J.; et al. Optimal Avapritinib Treatment Strategies for Patients with Metastatic or Unresectable Gastrointestinal Stromal Tumors. Oncologist 2021, 26, e622–e631. [Google Scholar] [CrossRef]
- Jones, R.L.; Serrano, C.; von Mehren, M.; George, S.; Heinrich, M.C.; Kang, Y.-K.; Schöffski, P.; Cassier, P.A.; Mir, O.; Chawla, S.P.; et al. Avapritinib in Unresectable or Metastatic PDGFRA D842V-Mutant Gastrointestinal Stromal Tumours: Long-Term Efficacy and Safety Data from the NAVIGATOR Phase I Trial. Eur. J. Cancer 2021, 145, 132–142. [Google Scholar] [CrossRef]
- Trullas-Jimeno, A.; Delgado, J.; Garcia-Ochoa, B.; Wang, I.; Sancho-Lopez, A.; Payares-Herrera, C.; Dalhus, M.L.; Strøm, B.O.; Egeland, E.J.; Enzmann, H.; et al. The EMA Assessment of Avapritinib in the Treatment of Gastrointestinal Stromal Tumours Harbouring the PDGFRA D842V Mutation. ESMO Open 2021, 6, 100159. [Google Scholar] [CrossRef]
- von Mehren, M.; Heinrich, M.C.; Shi, H.; Iannazzo, S.; Mankoski, R.; Dimitrijević, S.; Hoehn, G.; Chiroli, S.; George, S. Clinical Efficacy Comparison of Avapritinib with Other Tyrosine Kinase Inhibitors in Gastrointestinal Stromal Tumors with PDGFRA D842V Mutation: A Retrospective Analysis of Clinical Trial and Real-World Data. BMC Cancer 2021, 21, 291. [Google Scholar] [CrossRef]
- Nannini, M.; Astolfi, A.; Urbini, M.; Indio, V.; Santini, D.; Heinrich, M.C.; Corless, C.L.; Ceccarelli, C.; Saponara, M.; Mandrioli, A.; et al. Integrated Genomic Study of Quadruple-WT GIST (KIT/PDGFRA/SDH/RAS Pathway Wild-Type GIST). BMC Cancer 2014, 14, 685. [Google Scholar] [CrossRef]
- Unk, M.; Bombač, A.; Jezeršek Novaković, B.; Stegel, V.; Šetrajčič, V.; Blatnik, O.; Klančar, G.; Novaković, S. Correlation of Treatment Outcome in Sanger/RT-QPCR KIT/PDGFRAWild-Type Metastatic Gastrointestinal Stromal Tumors with Next-Generation Sequencing Results: A Single-Center Report. Oncol. Rep. 2022, 48, 167. [Google Scholar] [CrossRef]
- Gheysen, M.; Vander Borght, S.; Lehnert, S.; Vanslembrouck, R.; Vanden Bempt, I.; Schöffski, P. An Unexpected Response to Imatinib in a “Wild-Type” Gastrointestinal Stromal Tumor. Oncol. Res. Treat. 2020, 43, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Mathias-Machado, M.C.; de Jesus, V.H.F.; de Carvalho Oliveira, L.J.; Neumann, M.; Peixoto, R.D. Current Molecular Profile of Gastrointestinal Stromal Tumors and Systemic Therapeutic Implications. Cancers 2022, 14, 5330. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, M.A.; Urbini, M.; Schipani, A.; Nannini, M.; Indio, V.; De Leo, A.; Vincenzi, B.; Brunello, A.; Grignani, G.; Casagrande, M.; et al. SDHA Germline Variants in Adult Patients with SDHA-Mutant Gastrointestinal Stromal Tumor. Front. Oncol. 2022, 11, 5400. [Google Scholar] [CrossRef] [PubMed]
- Boikos, S.A.; Pappo, A.S.; Killian, J.K.; LaQuaglia, M.P.; Weldon, C.B.; George, S.; Trent, J.C.; von Mehren, M.; Wright, J.A.; Schiffman, J.D.; et al. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report from the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol. 2016, 2, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Nannini, M.; Rizzo, A.; Indio, V.; Schipani, A.; Astolfi, A.; Pantaleo, M.A. Targeted Therapy in SDH-Deficient GIST. Ther. Adv. Med. Oncol. 2021, 13, 175883592110232. [Google Scholar] [CrossRef]
- Martin Broto, J.; Valverde, C.; Hindi, N.; Vincenzi, B.; Martinez-Trufero, J.; Grignani, G.; Italiano, A.; Lavernia, J.; Gonzalez-Campora, R.; Vallejo, A.; et al. 1520O REGISTRI: Regorafenib in First-Line of KIT/PDGFR Wild Type Advanced GIST: Capatalize the a Spanish (GEIS), Italian (ISG) and French Sarcoma Group (FSG) Phase II Trial. Ann. Oncol. 2021, 32, S1111. [Google Scholar] [CrossRef]
- Qiu, H.; Zhou, Z.; Zhou, Y.; Wan, X.; Tao, K.; Li, Y.; Wu, X.; Liu, L.; Chen, Z.; Men, L.; et al. Promising Antitumor Activity of Olverembatinib (HQP1351) in Patients (Pts) with Tyrosine Kinase Inhibitor- (TKI-) Resistant Succinate Dehydrogenase- (SDH-) Deficient Gastrointestinal Stromal Tumor (GIST). J. Clin. Oncol. 2022, 40 (Suppl. S16), 11513. [Google Scholar] [CrossRef]
- Indio, V.; Schipani, A.; Nannini, M.; Urbini, M.; Rizzo, A.; De Leo, A.; Altimari, A.; Di Scioscio, V.; Messelodi, D.; Tarantino, G.; et al. Gene Expression Landscape of SDH-Deficient Gastrointestinal Stromal Tumors. J. Clin. Med. 2021, 10, 1057. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, S.-J.; Choe, E.-A.; Kim, J.; Hyung, W.J.; Kim, H.S.; Jung, M.; Beom, S.-H.; Kim, T.I.; Ahn, J.B.; et al. Correction: Lee et al. Tropomyosin-Related Kinase Fusions in Gastrointestinal Stromal Tumors. Cancers 2022, 14, 2659. Cancers 2022, 14, 5658. [Google Scholar] [CrossRef]
- D’Alpino Peixoto, R.; Medeiros, B.A.; Cronemberger, E.H. Resected High-Risk Rectal GIST Harboring NTRK1 Fusion: A Case Report and Review of the Literature. J. Gastrointest. Cancer 2020, 52, 316–319. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in Patients with TRK Fusion-Positive Solid Tumours: A Pooled Analysis of Three Phase 1/2 Clinical Trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Nathenson, M.; Demetri, G.; Lassen, U.; Hong, D.; Boni, V.; Deeken, J.; Dowlati, A.; Cox, M.; Ku, N.; Cruickshank, S.; et al. Activity of Larotrectinib in Patients with TRK Fusion GI Malignancies. Ann. Oncol. 2018, 29, v107. [Google Scholar] [CrossRef]
- Huss, S.; Pasternack, H.; Ihle, M.A.; Merkelbach-Bruse, S.; Heitkötter, B.; Hartmann, W.; Trautmann, M.; Gevensleben, H.; Büttner, R.; Schildhaus, H.-U.; et al. Clinicopathological and Molecular Features of a Large Cohort of Gastrointestinal Stromal Tumors (GISTs) and Review of the Literature: BRAF Mutations in KIT/PDGFRA Wild-Type GISTs Are Rare Events. Hum. Pathol. 2017, 62, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Terracciano, L.M.; Dirnhofer, S.; Tornillo, L.; Foerster, A.; Hartmann, A.; Bihl, M.P. V600E BRAF Mutations Are Alternative Early Molecular Events in a Subset of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumours. J. Clin. Pathol. 2009, 62, 613–616. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Kang, Y.-K.; Nishida, T.; von Mehren, M. Gastrointestinal Stromal Tumours. Nat. Rev. Dis. Prim. 2021, 7, 22. [Google Scholar] [CrossRef]
- Weiss, B.D.; Wolters, P.L.; Plotkin, S.R.; Widemann, B.C.; Tonsgard, J.H.; Blakeley, J.; Allen, J.C.; Schorry, E.; Korf, B.; Robison, N.J.; et al. NF106: A Neurofibromatosis Clinical Trials Consortium Phase II Trial of the MEK Inhibitor Mirdametinib (PD-0325901) in Adolescents and Adults with NF1-Related Plexiform Neurofibromas. J. Clin. Oncol. 2021, 39, 797–806. [Google Scholar] [CrossRef]
- Antonescu, C.R. Acquired Resistance to Imatinib in Gastrointestinal Stromal Tumor Occurs through Secondary Gene Mutation. Clin. Cancer Res. 2005, 11, 4182–4190. [Google Scholar] [CrossRef]
- Gajiwala, K.S.; Wu, J.C.; Christensen, J.; Deshmukh, G.D.; Diehl, W.; DiNitto, J.P.; English, J.M.; Greig, M.J.; He, Y.-A.; Jacques, S.L.; et al. KIT Kinase Mutants Show Unique Mechanisms of Drug Resistance to Imatinib and Sunitinib in Gastrointestinal Stromal Tumor Patients. Proc. Natl. Acad. Sci. USA 2009, 106, 1542–1547. [Google Scholar] [CrossRef]
- Grunewald, S.; Klug, L.R.; Mühlenberg, T.; Lategahn, J.; Falkenhorst, J.; Town, A.; Ehrt, C.; Wardelmann, E.; Hartmann, W.; Schildhaus, H.-U.; et al. Resistance to Avapritinib in PDGFRA-Driven GIST Is Caused by Secondary Mutations in the PDGFRA Kinase Domain. Cancer Discov. 2021, 11, 108–125. [Google Scholar] [CrossRef]
- Demetri, G.D.; van Oosterom, A.T.; Garrett, C.R.; Blackstein, M.E.; Shah, M.H.; Verweij, J.; McArthur, G.; Judson, I.R.; Heinrich, M.C.; Morgan, J.A.; et al. Efficacy and Safety of Sunitinib in Patients with Advanced Gastrointestinal Stromal Tumour after Failure of Imatinib: A Randomised Controlled Trial. Lancet 2006, 368, 1329–1338. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Maki, R.G.; Corless, C.L.; Antonescu, C.R.; Harlow, A.; Griffith, D.; Town, A.; Mckinley, A.; Ou, W.-B.; Fletcher, J.A.; et al. Primary and Secondary Kinase Genotypes Correlate with the Biological and Clinical Activity of Sunitinib in Imatinib-Resistant Gastrointestinal Stromal Tumor. J. Clin. Oncol. 2008, 26, 5352–5359. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; Reichardt, P.; Kang, Y.-K.; Blay, J.-Y.; Rutkowski, P.; Gelderblom, H.; Hohenberger, P.; Leahy, M.; von Mehren, M.; Joensuu, H.; et al. Efficacy and Safety of Regorafenib for Advanced Gastrointestinal Stromal Tumours after Failure of Imatinib and Sunitinib (GRID): An International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2013, 381, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-N.; Chen, M.-H.; Chen, Y.-Y.; Yang, C.-Y.; Yen, C.-C.; Tzen, C.-Y.; Chen, L.-T.; Chen, J.-S. A Phase II Trial of Regorafenib in Patients with Metastatic And/or a Unresectable Gastrointestinal Stromal Tumor Harboring Secondary Mutations of Exon 17. Oncotarget 2017, 8, 44121–44130. [Google Scholar] [CrossRef] [PubMed]
- Mir, O.; Cropet, C.; Toulmonde, M.; Cesne, A.L.; Molimard, M.; Bompas, E.; Cassier, P.; Ray-Coquard, I.; Rios, M.; Adenis, A.; et al. Pazopanib plus Best Supportive Care versus Best Supportive Care Alone in Advanced Gastrointestinal Stromal Tumours Resistant to Imatinib and Sunitinib (PAZOGIST): A Randomised, Multicentre, Open-Label Phase 2 Trial. Lancet Oncol. 2016, 17, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.-Y.; Serrano, C.; Heinrich, M.C.; Zalcberg, J.; Bauer, S.; Gelderblom, H.; Schöffski, P.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Ripretinib in Patients with Advanced Gastrointestinal Stromal Tumours (INVICTUS): A Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2020, 21, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Zalcberg, J.R.; Heinrich, M.C.; George, S.; Bauer, S.; Schöffski, P.; Serrano, C.; Gelderblom, H.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Clinical Benefit of Ripretinib Dose Escalation after Disease Progression in Advanced Gastrointestinal Stromal Tumor: An Analysis of the INVICTUS Study. Oncologist 2021, 26, e2053–e2060. [Google Scholar] [CrossRef]
- Bauer, S.; Heinrich, M.C.; George, S.; Zalcberg, J.R.; Serrano, C.; Gelderblom, H.; Jones, R.L.; Attia, S.; D’Amato, G.; Chi, P.; et al. Clinical Activity of Ripretinib in Patients with Advanced Gastrointestinal Stromal Tumor Harboring Heterogeneous KIT/PDGFRA Mutations in the Phase III INVICTUS Study. Clin. Cancer Res. 2021, 27, 6333–6342. [Google Scholar] [CrossRef]
- Bauer, S.; Jones, R.L.; Blay, J.-Y.; Gelderblom, H.; George, S.; Schöffski, P.; von Mehren, M.; Zalcberg, J.R.; Kang, Y.-K.; Razak, A.A.; et al. Ripretinib versus Sunitinib in Patients with Advanced Gastrointestinal Stromal Tumor after Treatment with Imatinib (INTRIGUE): A Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2022, 40, 3918–3928. [Google Scholar] [CrossRef]
- Li, J.; Cai, S.; Zhou, Y.; Zhang, J.; Zhou, Y.; Cao, H.; Wu, X.; Deng, Y.; Huang, Z.; Dong, J.; et al. Efficacy and Safety of Ripretinib in Chinese Patients with Advanced Gastrointestinal Stromal Tumors as a Fourth- or Later-Line Therapy: A Multicenter, Single-Arm, Open-Label Phase II Study. Clin. Cancer Res. 2022, 28, 3425–3432. [Google Scholar] [CrossRef]
- Kang, Y.-K.; George, S.; Jones, R.L.; Rutkowski, P.; Shen, L.; Mir, O.; Patel, S.; Zhou, Y.; von Mehren, M.; Hohenberger, P.; et al. Avapritinib versus Regorafenib in Locally Advanced Unresectable or Metastatic GI Stromal Tumor: A Randomized, Open-Label Phase III Study. J. Clin. Oncol. 2021, 39, 3128–3139. [Google Scholar] [CrossRef]
- Singh, A.S.; Chmielowski, B.; Hecht, J.R.; Rosen, L.S.; Chow, W.A.; Wang, X.; Brackert, S.; Adame, C.; Bovill, J.; Schink, E.; et al. A Randomized Phase II Study of Nivolumab Monotherapy versus Nivolumab Combined with Ipilimumab in Advanced Gastrointestinal Stromal Tumor (GIST). J. Clin. Oncol. 2019, 37 (Suppl. S15), 11017. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). Phase I Study of Dasatinib in Combination with Ipilimumab for Patients with Advanced Gastrointestinal Stromal Tumor and Other Sarcomas. Available online: https://clinicaltrials.gov/ct2/show/NCT01643278?term=Ipilimumab%2Cdasatinib&cond=gist&draw=2&rank=1) (accessed on 10 April 2023).
- Carvajal, R.D.; Richard, D.; Carvajal. A Phase II Study of Epacadostat and Pembrolizumab in Patients with Imatinib Refractory Advanced Gastrointestinal Stromal Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT03291054?term=pembrolizumab&cond=gist&draw=2&rank=1) (accessed on 10 April 2023).
- Toulmonde, M.; Penel, N.; Adam, J.; Chevreau, C.; Blay, J.-Y.; Le Cesne, A.; Bompas, E.; Piperno-Neumann, S.; Cousin, S.; Grellety, T.; et al. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas. JAMA Oncol. 2018, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.-W.; Sun, C.-M.; Calderaro, J.; Jeng, Y.-M.; Hsiao, L.-P.; Lacroix, L.; Bougoüin, A.; et al. B Cells Are Associated with Survival and Immunotherapy Response in Sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Honma, Y.; Sawaki, A.; Naito, Y.; Iwagami, S.; Komatsu, Y.; Takahashi, T.; Nishida, T.; Doi, T. Pimitespib in Patients with Advanced Gastrointestinal Stromal Tumor (CHAPTER-GIST-301): A Randomized, Double-Blind, Placebo-Controlled Phase III Trial. Ann. Oncol. 2022, 33, 959–967. [Google Scholar] [CrossRef]
- Fröbom, R.; Berglund, E.; Berglund, D.; Nilsson, I.-L.; Åhlén, J.; von Sivers, K.; Linder-Stragliotto, C.; Suenaert, P.; Karlsson-Parra, A.; Bränström, R. Phase I Trial Evaluating Safety and Efficacy of Intratumorally Administered Inflammatory Allogeneic Dendritic Cells (Ilixadencel) in Advanced Gastrointestinal Stromal Tumors. Cancer Immunol. Immunother. 2020, 69, 2393–2401. [Google Scholar] [CrossRef]
- Du, J.; Wang, S.; Wang, R.; Wang, S.-Y.; Han, Q.; Xu, H.-T.; Yang, P.; Liu, Y. Identifying Secondary Mutations in Chinese Patients with Imatinib-Resistant Gastrointestinal Stromal Tumors (GISTs) by next Generation Sequencing (NGS). Pathol. Oncol. Res. 2019, 26, 91–100. [Google Scholar] [CrossRef]
- Namløs, H.M.; Boye, K.; Mishkin, S.J.; Barøy, T.; Lorenz, S.; Bjerkehagen, B.; Stratford, E.W.; Munthe, E.; Kudlow, B.A.; Myklebost, O.; et al. Noninvasive Detection of CtDNA Reveals Intratumor Heterogeneity and Is Associated with Tumor Burden in Gastrointestinal Stromal Tumor. Mol. Cancer Ther. 2018, 17, 2473–2480. [Google Scholar] [CrossRef]
- Gómez-Peregrina, D.; García-Valverde, A.; Pilco-Janeta, D.; Serrano, C. Liquid Biopsy in Gastrointestinal Stromal Tumors: Ready for Prime Time? Curr. Treat. Options Oncol. 2021, 22, 32. [Google Scholar] [CrossRef]
- Jilg, S.; Rassner, M.; Maier, J.; Waldeck, S.; Kehl, V.; Follo, M.; Philipp, U.; Sauter, A.; Specht, K.; Mitschke, J.; et al. Circulating CKIT and PDGFRA DNA Indicates Disease Activity in Gastrointestinal Stromal Tumor (GIST). Int. J. Cancer 2019, 145, 2292–2303. [Google Scholar] [CrossRef]
- Boonstra, P.A.; ter Elst, A.; Tibbesma, M.; Gietema, J.A.; Schuuring, E.; Reyners, A.K.L. Diagnosis and Treatment Monitoring of a Patient with Gastrointestinal Stromal Tumor by Next-Generation Sequencing and Droplet Digital Polymerase Chain Reaction Assay of a PDGFRA Mutation in Plasma-Derived Cell-Free Tumor DNA. Oncologist 2019, 24, e387–e390. [Google Scholar] [CrossRef]
- Montemurro, M.; Cioffi, A.; Dômont, J.; Rutkowski, P.; Roth, A.D.; von Moos, R.; Inauen, R.; Toulmonde, M.; Burkhard, R.O.; Knuesli, C.; et al. Long-Term Outcome of Dasatinib First-Line Treatment in Gastrointestinal Stromal Tumor: A Multicenter, 2-Stage Phase 2 Trial (Swiss Group for Clinical Cancer Research 56/07). Cancer 2018, 124, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Boilève, A.; Dufresne, A.; Chamseddine, A.; Nassif, E.; Dumont, S.; Brahmi, M.; Adam, J.; Rouleau, E.; Karanian, M.; Haddad, V.; et al. Outcomes of Patients with Metastatic Gastrointestinal Stromal Tumors (GIST) Treated with Multi-Kinase Inhibitors Other than Imatinib as First-Line Treatment. ESMO Open 2020, 5, e001082. [Google Scholar] [CrossRef] [PubMed]
- Mühlenberg, T.; Ketzer, J.; Heinrich, M.C.; Grunewald, S.; Marino-Enriquez, A.; Trautmann, M.; Hartmann, W.; Wardelmann, E.; Treckmann, J.; Worm, K.; et al. KIT-Dependent and KIT-Independent Genomic Heterogeneity of Resistance in Gastrointestinal Stromal Tumors—TORC1/2 Inhibition as Salvage Strategy. Mol. Cancer Ther. 2019, 18, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).