Clinicopathological Characteristics, Treatment Patterns, and Outcomes in Patients with Laryngeal Cancer
Abstract
1. Introduction
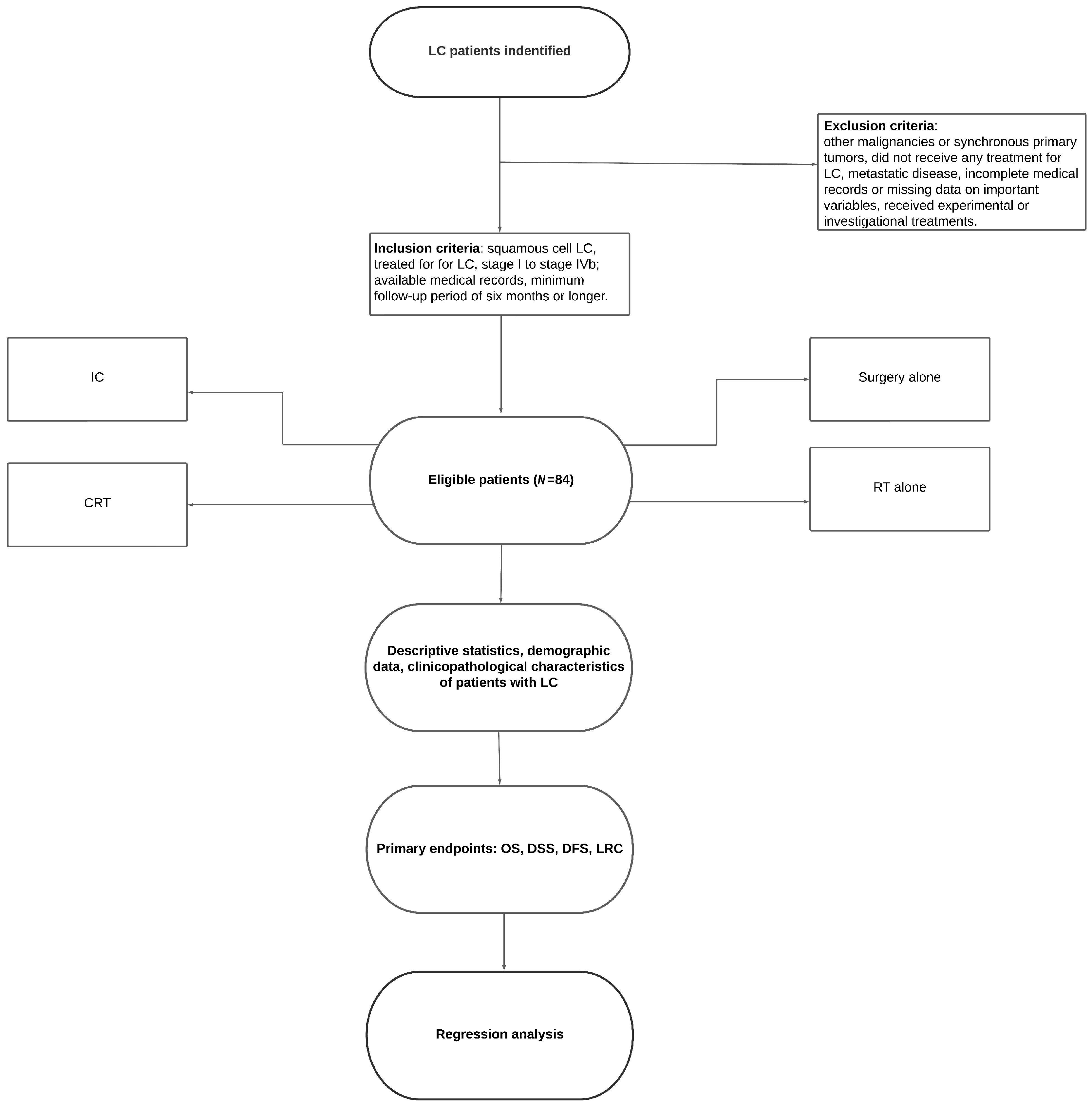
2. Materials and Methods
3. Results
3.1. Sample Characterisrtics
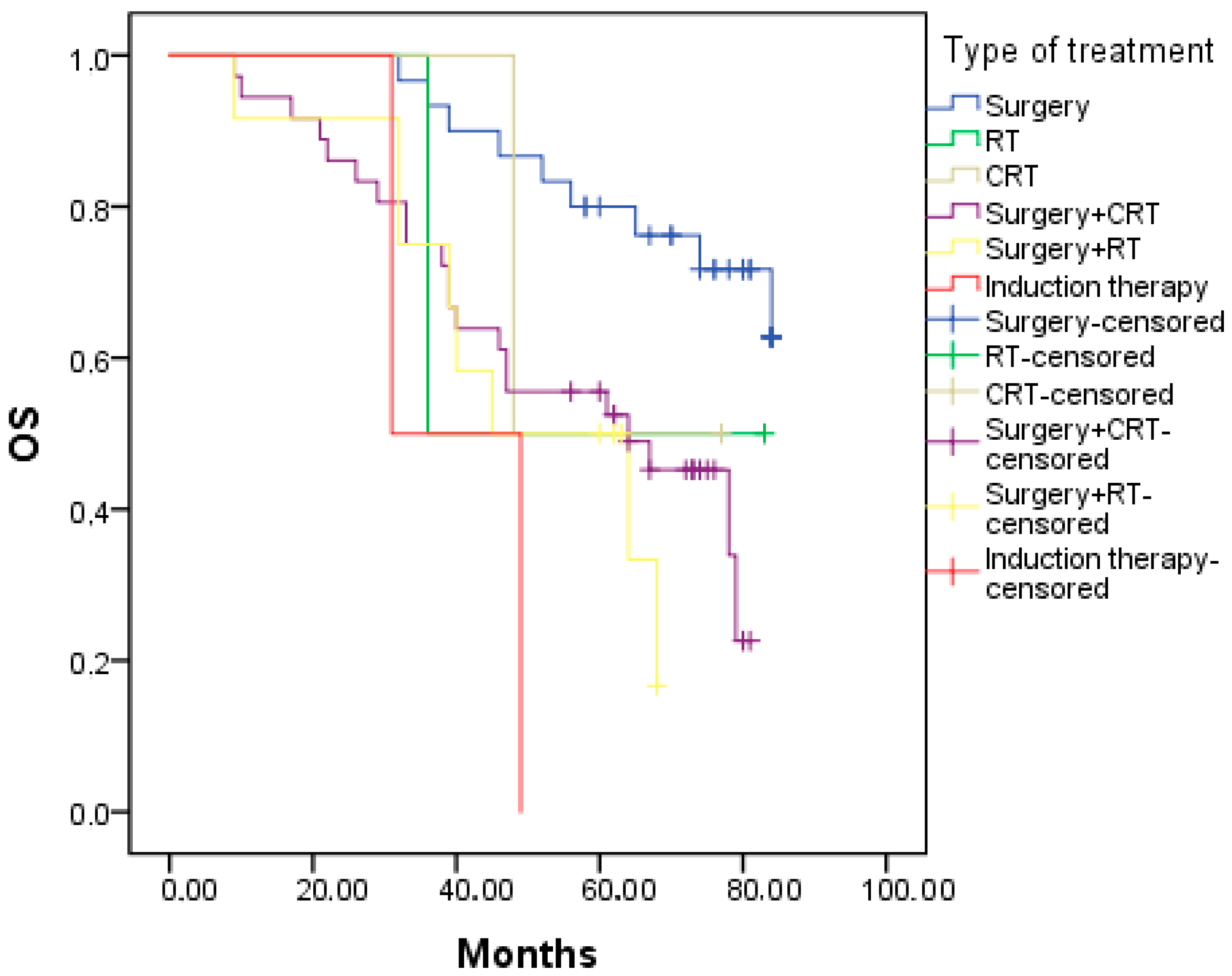
3.2. Survival
3.3. Regression Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; Abdulle, A.S.M.; et al. Global Burden of Disease Cancer Collaboration Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768, Erratum in: JAMA Oncol. 2020, 6, 444. Erratum in: JAMA Oncol. 2020, 6, 789; Erratum in JAMA Oncol. 2021, 7, 466. [Google Scholar] [CrossRef]
- Bradley, P.J. Laryngeal cancer in nondrinker nonsmoker young patients: A distinct pathological entity? Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Aupérin, A. Epidemiology of head and neck cancers: An update. Curr. Opin. Oncol. 2020, 32, 178–186. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Groome, P.A.; O’Sullivan, B.; Irish, J.C.; Rothwell, D.M.; Schulze, K.; Warde, P.R.; Schneider, K.M.; Mackenzie, R.G.; Hodson, D.I.; Hammond, J.A.; et al. Management and outcome differences in supraglottic cancer between Ontario, Canada, and the Surveillance, Epidemiology, and End Results areas of the United States. J. Clin. Oncol. 2003, 21, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Long-term results of RTOG 91-11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J. Clin. Oncol. 2013, 31, 845–852. [Google Scholar] [CrossRef]
- Forastiere, A.A.; Goepfert, H.; Maor, M.; Pajak, T.F.; Weber, R.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; Chao, C.; et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N. Engl. J. Med. 2003, 349, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Cancer of the Glottic Larynx and Cancer of the Supraglottic Larynx in the NCCN Guidelines for Head and Neck Cancers. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 15 January 2023).
- Barnes, L.; Tse, L.L.Y.; Hunt, J.L. Tumors of the Hypopharynx, Larynx, and Trachea: Introduction. In Pathology and Genetics of Head and Neck Tumors, World Health Organization Classification of Tumors; Barnes, E.L., Eveson, J.W., Reichart, P., Sidransky, D., Kleihues, P., Sobin, L.H., Eds.; IARC: Lyon, France, 2005. [Google Scholar]
- Ramroth, H.; Schoeps, A.; Rudolph, E.; Dyckhoff, G.; Plinkert, P.; Lippert, B.; Feist, K.; Delank, K.W.; Scheuermann, K.; Baier, G.; et al. Factors predicting survival after diagnosis of laryngeal cancer. Oral Oncol. 2011, 47, 1154–1158. [Google Scholar] [CrossRef]
- Piccirillo, J.F.; Lacy, P.D.; Basu, A.; Spitznagel, E.L. Development of a new head and neck cancer-specific comorbidity index. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 1172–1179. [Google Scholar] [CrossRef]
- Nguyen-Tan, P.F.; Le, Q.T.; Quivey, J.M.; Singer, M.; Terris, D.J.; Goffinet, D.R.; Fu, K.K. Treatment results and prognostic factors of advanced T3–4 laryngeal carcinoma: The University of California, San Francisco (UCSF) and Stanford University Hospital (SUH) experience. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 1172–1180. [Google Scholar] [CrossRef]
- Haapaniemi, A.; Väisänen, J.; Atula, T.; Alho, O.P.; Mäkitie, A.; Koivunen, P. Predictive factors and treatment outcome of laryngeal carcinoma recurrence. Head Neck 2017, 39, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, F.; Bussani, R.; Stanta, G.; Cosatti, C.; Ferlito, A. Supraglottic versus glottic laryngeal cancer: Epidemiological and pathological aspects. ORL J. Otorhinolaryngol. Relat. Spec. 1992, 54, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Raitiola, H.; Pukander, J.; Laippala, P. Glottic and supraglottic laryngeal carcinoma: Differences in epidemiology, clinical characteristics and prognosis. Acta Otolaryngol. 1999, 119, 847–851. [Google Scholar] [PubMed]
- Haapaniemi, A.; Koivunen, P.; Saarilahti, K.; Kinnunen, I.; Laranne, J.; Aaltonen, L.M.; Närkiö, M.; Lindholm, P.; Grénman, R.; Mäkitie, A.; et al. Laryngeal cancer in Finland: A 5-year follow-up study of 366 patients. Head Neck 2016, 38, 36–43. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Patel, S.G.; Shah, J.P. Changes in the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging of Head and Neck Cancer: Rationale and Implications. Curr. Oncol. Rep. 2019, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Cîrstea, A.I.; Berteșteanu, Ș.V.G.; Scăunașu, R.V.; Popescu, B.; Bejenaru, P.L.; Simion-Antonie, C.B.; Berteșteanu, G.S.; Diaconu, T.E.; Taher, P.B.; Rujan, S.A.; et al. Management of Locally Advanced Laryngeal Cancer-From Risk Factors to Treatment, the Experience of a Tertiary Hospital from Eastern Europe. Int. J. Environ. Res. Public Health 2023, 20, 4737. [Google Scholar] [CrossRef]
- Calkovsky, V.; Wallenfels, P.; Calkovska, A.; Hajtman, A. Laryngeal Cancer: 12-Year Experience of a Single Center. Adv. Exp. Med. Biol. 2016, 911, 9–16. [Google Scholar] [CrossRef]
- Islami, F.; Tramacere, I.; Rota, M.; Bagnardi, V.; Fedirko, V.; Scotti, L.; Garavello, W.; Jenab, M.; Corrao, G.; Straif, K.; et al. Alcohol drinking and laryngeal cancer: Overall and dose-risk relation—A systematic review and meta-analysis. Oral Oncol. 2010, 46, 802–810. [Google Scholar] [CrossRef]
- Chirila, M.; Bolboaca, S.D.; Muresan, M.; Tomescu, E.; Cosgarea, M. Die lymphatische und vaskuläre Ausbreitung von Larynx- und Sinus piriformis Plattenepithelkarzinomen [Lymphatic and vascular invasion in laryngeal and pyriform sinus carcinomas]. Laryngorhinootologie 2011, 90, 358–363. [Google Scholar] [CrossRef]
- Yilmaz, T.; Hosal, A.S.; Gedikoğlu, G.; Onerci, M.; Gürsel, B. Prognostic significance of vascular and perineural invasion in cancer of the larynx. Am. J. Otolaryngol. 1998, 19, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Devaney, K.O.; Hunter, B.C.; Ferlito, A.; Rinaldo, A. Pretreatment pathologic prognostic factors in head and neck squamous cell carcinoma. Ann. Otol. Rhinol. Laryngol. 1997, 106, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Fagan, J.J.; Collins, B.; Barnes, L.; D’Amico, F.; Myers, E.N.; Johnson, J.T. Perineural invasion in squamous cell carcinoma of the head and neck. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Altumbabić, H.; Salkić, A.; Ramas, A.; Burgić, M.; Kasumović, M.; Brkić, F. Pattern of head and neck malignant tumours in a Tuzla ENT clinic—A five-year experience. Bosn. J. Basic Med. Sci. 2008, 8, 377–380. [Google Scholar] [CrossRef]
- Chatenoud, L.; Garavello, W.; Pagan, E.; Bertuccio, P.; Gallus, S.; La Vecchia, C.; Negri, E.; Bosetti, C. Laryngeal cancer mortality trends in European countries. Int. J. Cancer 2016, 138, 833–842. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Amdur, R.J.; Morris, C.G.; Hinerman, R.W. T1-T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. J. Clin. Oncol. 2001, 19, 4029–4036. [Google Scholar] [CrossRef]
- Warde, P.; O’Sullivan, B.; Bristow, R.G.; Panzarella, T.; Keane, T.J.; Gullane, P.J.; Witterick, I.P.; Payne, D.; Liu, F.F.; McLean, M.; et al. T1/T2 glottic cancer managed by external beam radiotherapy: The influence of pretreatment hemoglobin on local control. Int. J. Radiat. Oncol. Biol. Phys. 1998, 41, 347–353. [Google Scholar] [CrossRef]
- Dechaphunkul, T. Epidemiology, risk factors, and overall survival rate of laryngeal cancer in Songklanagarind Hospital. J. Med. Assoc. Thail. 2011, 94, 355–360. [Google Scholar]
- Chedid, H.M.; Lehn, C.N.; Rapoport, A.; Amar, A.; Franzi, S.A. Assessment of disease-free survival in patients with laryngeal squamous cell carcinoma treated with radiotherapy associated or not with chemotherapy. Braz. J. Otorhinolaryngol. 2010, 76, 225–230. [Google Scholar] [CrossRef]
- Chen, A.Y.; Matson, L.K.; Roberts, D.; Goepfert, H. The significance of comorbidity in advanced laryngeal cancer. Head Neck 2001, 23, 566–572. [Google Scholar] [CrossRef]
- Du, E.; Mazul, A.L.; Farquhar, D.; Brennan, P.; Anantharaman, D.; Abedi-Ardekani, B.; Weissler, M.C.; Hayes, D.N.; Olshan, A.F.; Zevallos, J.P. Long-term Survival in Head and Neck Cancer: Impact of Site, Stage, Smoking, and Human Papillomavirus Status. Laryngoscope 2019, 129, 2506–2513. [Google Scholar] [CrossRef]
- Baxi, S.S.; Pinheiro, L.C.; Patil, S.M.; Pfister, D.G.; Oeffinger, K.C.; Elkin, E.B. Causes of death in long-term survivors of head and neck cancer. Cancer 2014, 120, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Vukelić, J.; Dobrila-Dintinjana, R.; Marijić, B.; Maržić, D.; Braut, T. Clinical Course of the Disease and Treatment Outcome in Patients with Malignant Laryngeal Tumor: Retrospective Five-Year Analysis. Acta Clin. Croat. 2022, 61, 311–319. [Google Scholar] [CrossRef]
- Cosetti, M.; Yu, G.P.; Schantz, S.P. Five-year survival rates and time trends of laryngeal cancer in the US population. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.T.; Porter, K.; Karnell, L.H.; Cooper, J.S.; Weber, R.S.; Langer, C.J.; Ang, K.K.; Gay, G.; Stewart, A.; Robinson, R.A. Laryngeal cancer in the United States: Changes in demographics, patterns of care, and survival. Laryngoscope 2006, 116 Pt 2 (Suppl. S111), 1–13. [Google Scholar] [CrossRef]
- Koskinen, W.J.; Brøndbo, K.; Mellin Dahlstrand, H.; Luostarinen, T.; Hakulinen, T.; Leivo, I.; Molijn, A.; Quint, W.G.; Røysland, T.; Munck-Wikland, E.; et al. Alcohol, smoking and human papillomavirus in laryngeal carcinoma: A Nordic prospective multicenter study. J. Cancer Res. Clin. Oncol. 2007, 133, 673–678. [Google Scholar] [CrossRef]
- Mayne, S.T.; Cartmel, B.; Kirsh, V.; Goodwin, W.J., Jr. Alcohol and tobacco use prediagnosis and postdiagnosis, and survival in a cohort of patients with early stage cancers of the oral cavity, pharynx, and larynx. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3368–3374. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Faisal, M.; Rashid, A.; Usman, S.; Khaleeq, U.; Abbas, T.; Rehman, A.; Malik, K.; Hussain, R.; Jamshed, A. An Overview of Laryngeal Cancer Treatment at a Tertiary Care Oncological Center in a Developing Country. Cureus 2018, 10, e2730. [Google Scholar] [CrossRef]

| Characteristic | Category | N (%) | OS (%) | DSS (%) | DFS (%) | LRC (%) |
|---|---|---|---|---|---|---|
| Gender | Men | 78 (92.9) | 50 | 70.5 | 64.1 | 70.5 |
| Women | 6 (7.1) | 50 | 100 | 100 | 100 | |
| Age | 50–<60 years | 19 | 42.1 | 63.2 | 68.4 | 84.2 |
| 60–<70 years | 42 | 61.9 | 76.2 | 64.3 | 66.7 | |
| 70+ years | 23 | 34.8 | 73.9 | 69.6 | 73.9 | |
| Subsite | Glottic | 42 (50.0) | 66.7 | 90.5 | 83.3 | 83.3 |
| Supraglottic | 39 (46.4) | 30.8 | 53.8 | 48.7 | 61.5 | |
| Surgery | LPS | 49 (58.3) | 57.1 | 85.7 | 77.6 | 77.6 |
| TL | 29 (34.5) | 41.4 | 58.6 | 55.2 | 69 | |
| Inoperable | 6 (7.1) | 33.3 | 33.3 | 33.3 | 50.0 | |
| Surgical margin | Negative | 56 (66.7) | 55.4 | 80.4 | 73.2 | 75 |
| Positive | 21 (25.0) | 38.1 | 61.9 | 57.1 | 71.4 | |
| Not evaluable | 7 (8.3) | |||||
| Tumor grade | Grade 1 | 15 (17.9) | 73.3 | 73.3 | 66.7 | 73.3 |
| Grade 2 | 58 (69.0) | 81.0 | 74.1 | 67.2 | 74.1 | |
| Grade 3 | 11 (13.1) | 72.7 | 63.6 | 63.6 | 63.6 | |
| Vascular invasion | Not present | 44 (52.4) | 61.4 | 77.3 | 75 | 79.5 |
| Present | 33 (39.3) | 36.4 | 60.6 | 51.5 | 60.6 | |
| Unknown | 7 (8.3) | |||||
| Lymph invasion | Not present | 38 (45.2) | 61.4 | 77.3 | 72.7 | 77.3 |
| Present | 39 (46.4) | 36.4 | 60.6 | 54.5 | 63.6 | |
| Unknown | 7 (8.3) | |||||
| Perineural invasion | Not present | 42 (50.0) | 63.2 | 56.4 | 78.9 | 78.9 |
| Present | 35 (41.5) | 38.5 | 45.2 | 51.3 | 64.1 | |
| Unknown | 7 (8.5) | |||||
| Smoking | Yes | 74 (88.1) | 52.7 | 71.6 | 74.3 | 78.5 |
| No | 10 (11.9) | 30.0 | 80.0 | 60.0 | 55.6 | |
| Alcohol | Yes | 51 (60.7) | 52.9 | 76.5 | 74.5 | 78.4 |
| No | 33 (39.3) | 45.5 | 66.7 | 54.5 | 63.6 | |
| T stage | T1 | 14 (16.7) | 78.6 | 92.9 | 78.6 | 78.6 |
| T2 | 36 (42.9) | 47.2 | 72.2 | 75.0 | 77.8 | |
| T3 | 16 (19) | 37.5 | 75.0 | 62.5 | 62.5 | |
| T4 | 18 (21.4) | 44.4 | 55.6 | 44.4 | 66.7 | |
| N stage | N0 | 56 (66.7) | 55.4 | 78.6 | 73.2 | 76.8 |
| N1-3 | 28 (33.3) | 39.3 | 60.7 | 53.6 | 64.3 | |
| Disease stage | I | 14 (16.7) | 78.6 | 100.0 | 78.6 | 78.6 |
| II | 23 (27.4) | 47.8 | 73.9 | 82.6 | 82.6 | |
| III | 22 (26.2) | 50.0 | 86.4 | 77.3 | 77.3 | |
| IVa, IVb | 25 (29.8) | 36.0 | 44.0 | 36.0 | 56.0 | |
| Therapy | Surgery + CRT | 36 (42.9) | 41.7 | 61.1 | 52.8 | 63.9 |
| Surgery | 30 (35.7) | 70.0 | 96.7 | 86.7 | 86.7 | |
| Surgery + RT | 12 (14.3) | 33.3 | 66.7 | 75.0 | 75.0 | |
| RT | 2 (2.4) | 50.0 | 50.0 | 50.0 | 50.0 | |
| CRT | 2 (2.4) | 50.0 | 50.0 | 50.0 | 50.0 | |
| IC | 2 (2.4) | 0.0 | 0.0 | 0.0 | 50.0 |
| Site of Recurrence | N | % |
|---|---|---|
| Local recurrence | 18 | 21.4 |
| Lung | 15 | 17.9 |
| Lymph nodes | 13 | 15.5 |
| Bones | 4 | 4.8 |
| Liver | 2 | 2.4 |
| Incidence of second primary malignancy | 4 | 4.8 |
| Lung cancer | 2 | 2.4 |
| Rectal cancer | 1 | 1.2 |
| Acute myeloid leukemia | 1 | 1.2 |
| Statistics | Surgery | RT | CRT | Surgery + CRT | Surgery + RT | IC | Overall |
|---|---|---|---|---|---|---|---|
| OS (months) | NR | 36 | 48 | 64 | 45 | 31 | 78 |
| N | 30 | 2 | 2 | 36 | 12 | 2 | 84 |
| N of events | 9 | 1 | 1 | 21 | 8 | 2 | 42 |
| 95% CI | 40.1:87.9 | 17.8–72.1 | 64.3–91.7 | ||||
| p value | 0.009 | ||||||
| DSS | NR | 36 | 48 | 78 | NR | 31 | NR |
| N | 20 | 2 | 2 | 36 | 12 | 2 | 84 |
| N of events | 1 | 1 | 1 | 14 | 4 | 2 | 23 |
| 95% CI | 56.3:94.8 | ||||||
| p value | 0.001 | ||||||
| DFS | NR | 35 | 42 | NR | NR | 13 | NR |
| N | 30 | 2 | 2 | 36 | 12 | 2 | 84 |
| N of events | 4 | 1 | 1 | 17 | 3 | 2 | 28 |
| 95% CI | |||||||
| p value | 0.022 | ||||||
| LRC | NR | 35 | 42 | NR | NR | 13 | |
| N | 30 | 2 | 2 | 36 | 12 | 2 | 84 |
| N of events | 4 | 1 | 1 | 13 | 3 | 1 | 23 |
| 95% CI | |||||||
| p value | 0.297 |
| OS | DSS | |||||||||||
| Characteristics | HR (a) | 95% CI | p Value | HR (b) | 95% CI | p Value | HR (a) | 95% CI | p Value | HR (b) | 95% CI | p Value |
| Surgery | 3.2 | 1.3:8.2 | 0.014 | |||||||||
| Subsite | 3.1 | 1.6:5.9 | 0.001 | 7.1 | 2.4:21.2 | <0.001 | ||||||
| Recurrences | 5.0 | 2.7:9.3 | <0.001 | 5.6 | 2.8:11.2 | <0.001 | 35.5 | 8.3:152 | <0.001 | 22.5 | 5.1:99.6 | <0.001 |
| Vascular invasion | 2.1 | 1.1:3.9 | 0.026 | |||||||||
| Lymph invasion | 2.2 | 1.1:4.2 | 0.015 | 2.1 | 1.1:4 | 0.031 | 2.3 | 1:5.3 | 0.048 | |||
| Perineural invasion | 1.2 | 1.02:3.8 | 0.043 | 3.2 | 1.3:8.2 | 0.013 | ||||||
| Advanced disease stage | 2.7 | 1.1:7.0 | 0.033 | |||||||||
| Advanced N stage | 2.3 | 1:5.2 | 0.047 | |||||||||
| DFS | LRC | |||||||||||
| Characteristics | HR (a) | 95% CI | p Value | HR (b) | 95% CI | p Value | HR (a) | 95% CI | p Value | HR (b) | 95% CI | p Value |
| Gender | 2.2 | 1.0:5.0 | 0.045 | |||||||||
| Subsite | 4.2 | 1.7:9.9 | 0.001 | 4.5 | 1.8:11.3 | 0.001 | 2.9 | 1.2:7.2 | 0.018 | 3.1 | 1.2:8.2 | 0.017 |
| Vascular invasion | 2.3 | 1.1:5.1 | 0.028 | 2.3 | 1.1:5.0 | 0.031 | ||||||
| Perineural invasion | 2.7 | 1.2:6.2 | 0.018 | 2.6 | 1.1:6.0 | 0.021 | ||||||
| Advanced T stage | 2.1 | 1.0:4.6 | 0.042 | |||||||||
| Advanced stage | 2.8 | 1.2:6.7 | 0.016 | 2.7 | 1.1:6.5 | 0.020 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đokanović, D.; Gajanin, R.; Gojković, Z.; Marošević, G.; Sladojević, I.; Gajanin, V.; Jović-Đokanović, O.; Amidžić, L. Clinicopathological Characteristics, Treatment Patterns, and Outcomes in Patients with Laryngeal Cancer. Curr. Oncol. 2023, 30, 4289-4300. https://doi.org/10.3390/curroncol30040327
Đokanović D, Gajanin R, Gojković Z, Marošević G, Sladojević I, Gajanin V, Jović-Đokanović O, Amidžić L. Clinicopathological Characteristics, Treatment Patterns, and Outcomes in Patients with Laryngeal Cancer. Current Oncology. 2023; 30(4):4289-4300. https://doi.org/10.3390/curroncol30040327
Chicago/Turabian StyleĐokanović, Dejan, Radoslav Gajanin, Zdenka Gojković, Goran Marošević, Igor Sladojević, Vesna Gajanin, Olja Jović-Đokanović, and Ljiljana Amidžić. 2023. "Clinicopathological Characteristics, Treatment Patterns, and Outcomes in Patients with Laryngeal Cancer" Current Oncology 30, no. 4: 4289-4300. https://doi.org/10.3390/curroncol30040327
APA StyleĐokanović, D., Gajanin, R., Gojković, Z., Marošević, G., Sladojević, I., Gajanin, V., Jović-Đokanović, O., & Amidžić, L. (2023). Clinicopathological Characteristics, Treatment Patterns, and Outcomes in Patients with Laryngeal Cancer. Current Oncology, 30(4), 4289-4300. https://doi.org/10.3390/curroncol30040327





