A Clinicopathological Analysis of Asian Patients with Adrenocortical Carcinoma: A Single-Center Experience
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Clinicopathologic Information
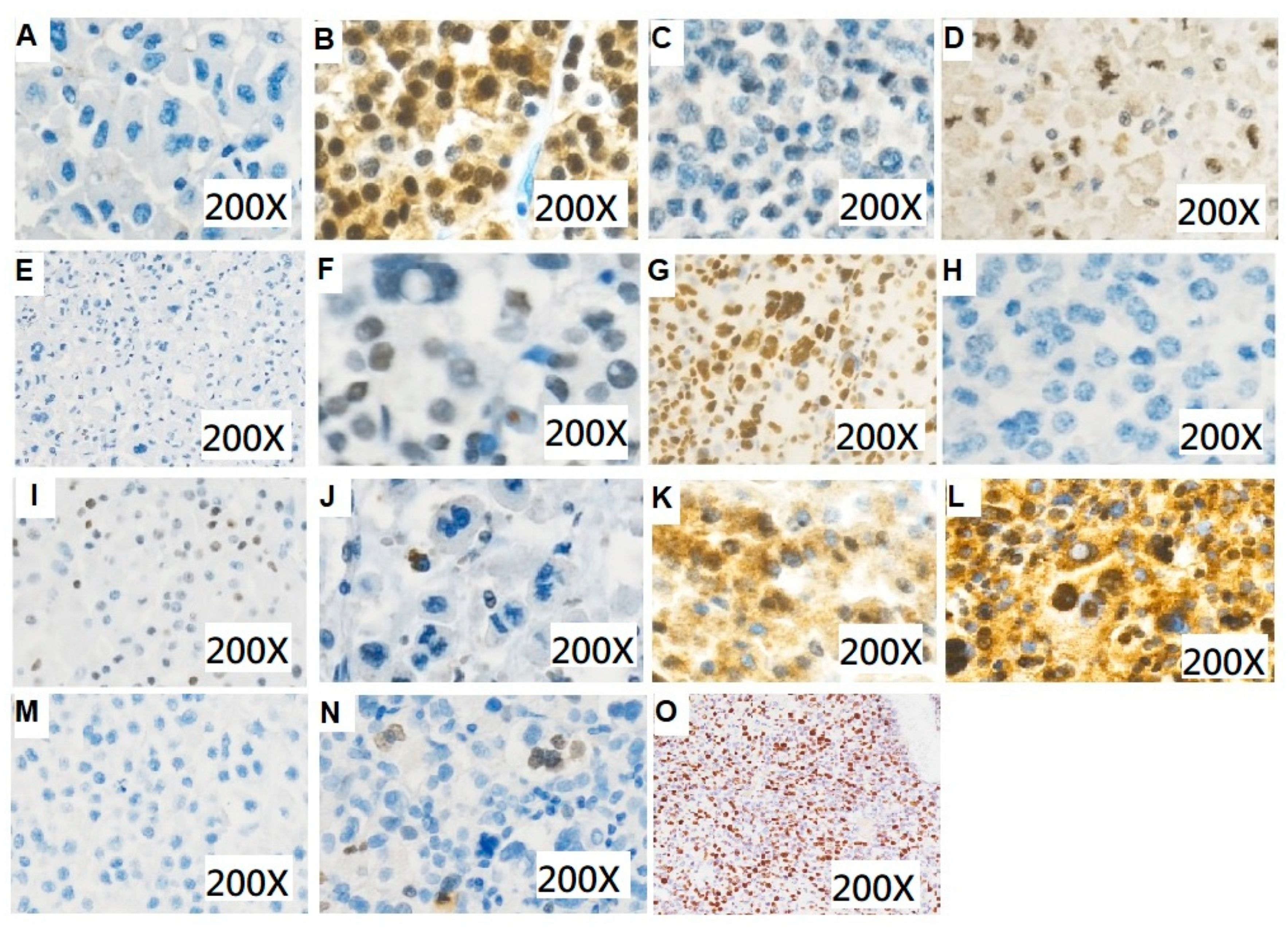
2.3. Immunohistochemistry
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bilimoria, K.Y.; Shen, W.T.; Elaraj, D.; Bentrem, D.J.; Winchester, D.J.; Kebebew, E.; Sturgeon, C. Adrenocortical carcinoma in the United States: Treatment utilization and prognostic factors. Cancer 2008, 113, 3130–3136. [Google Scholar] [CrossRef] [PubMed]
- Kerkhofs, T.; Kerkhofs, T.M.; Verhoeven, R.H.; Van der Zwan, J.M.; Dieleman, J.; Kerstens, M.N.; Links, T.P.; Van de Poll-Franse, L.V.; Haak, H.R. Adrenocortical carcinoma: A population-based study on incidence and survival in the Netherlands since 1993. Eur. J. Cancer 2013, 49, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Kroiss, M.; Allolio, B. Update in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2013, 98, 4551–4564. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; De Krijger, R.R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Lee, S.-E.; Kim, J.H.; Kim, J.H. Characteristics of adrenocortical carcinoma in South Korea: A registry-based nationwide survey. Endocr. Connect. 2020, 9, 519–529. [Google Scholar] [CrossRef]
- Berruti, A.; Baudin, E.; Gelderblom, H.; Haak, H.R.; Porpiglia, F.; Fassnacht, M.; Pentheroudakis, G. Adrenal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23 (Suppl. S7), vii131–vii138. [Google Scholar] [CrossRef]
- Jouinot, A.; Bertherat, J. Management of Endocrine Disease: Adrenocortical carcinoma: Differentiating the good from the poor prognosis tumors. Eur. J. Endocrinol. 2018, 178, R215–R230. [Google Scholar] [CrossRef]
- Wolin, E.M. The expanding role of somatostatin analogs in the management of neuroendocrine tumors. Gastrointest. Cancer Res. 2012, 5, 161–168. [Google Scholar]
- Wasserman, J.D.; Zambetti, G.P.; Malkin, D. Towards an understanding of the role of p53 in adrenocortical carcinogenesis. Mol. Cell. Endocrinol. 2012, 351, 101–110. [Google Scholar] [CrossRef]
- Wooten, M.D.; King, D.K. Adrenal cortical carcinoma. Epidemiology and treatment with mitotane and a review of the literature. Cancer 1993, 72, 3145–3155. [Google Scholar] [CrossRef]
- Wasserman, J.D.; Novokmet, A.; Eichler-Jonsson, C.; Ribeiro, R.C.; Rodriguez-Galindo, C.; Zambetti, G.P.; Malkin, D. Prevalence and functional consequence of TP53 mutations in pediatric adrenocortical carcinoma: A children’s oncology group study. J. Clin. Oncol. 2015, 33, 602–609. [Google Scholar] [CrossRef]
- Murnyák, B.; Hortobágyi, T. Immunohistochemical correlates of TP53 somatic mutations in cancer. Oncotarget 2016, 7, 64910–64920. [Google Scholar] [CrossRef]
- Leccia, F.; Batisse-Lignier, M.; Sahut-Barnola, I.; Val, P. Mouse Models Recapitulating Human Adrenocortical Tumors: What Is Lacking? Front. Endocrinol. 2016, 7, 93. [Google Scholar] [CrossRef]
- Beuschlein, F.; Weigel, J.; Saeger, W.; Kroiss, M.; Wild, V.; Daffara, F.; Libe, R.; Ardito, A.; Al Ghuzlan, A.; Quinkler, M.; et al. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J. Clin. Endocrinol. Metab. 2015, 100, 841–849. [Google Scholar] [CrossRef]
- Der-Sarkissian, H.; Bacchetti, S.; Cazes, L.; Londoño-Vallejo, J.A. The shortest telomeres drive karyotype evolution in transformed cells. Oncogene 2004, 23, 1221–1228. [Google Scholar] [CrossRef]
- Watson, L.A.; Goldberg, H.; Bérubé, N.G. Emerging roles of ATRX in cancer. Epigenomics 2015, 7, 1365–1378. [Google Scholar] [CrossRef]
- Brondani, V.; Lacombe, A.; Mariani, B.; Montenegro, L.; Soares, I.; Bezerra-Neto, J.; Tanno, F.; Srougi, V.; Chambo, J.; Mendonca, B.; et al. Low Protein Expression of both ATRX and ZNRF3 as Novel Negative Prognostic Markers of Adult Adrenocortical Carcinoma. Int. J. Mol. Sci. 2021, 22, 1238. [Google Scholar] [CrossRef]
- Mariniello, B.; Finco, I.; Sartorato, P.; Patalano, A.; Iacobone, M.; Guzzardo, V.; Fassina, A.; Mantero, F. Somatostatin receptor expression in adrenocortical tumors and effect of a new somatostatin analog SOM230 on hormone secretion in vitro and in ex vivo adrenal cells. J. Endocrinol. Invest. 2011, 34, e131–e138. [Google Scholar] [CrossRef]
- Qian, Z.R.; Li, T.; Ter-Minassian, M.; Yang, J.; Chan, J.A.; Brais, L.K.; Masugi, Y.; Thiaglingam, A.; Brooks, N.; Nishihara, R.; et al. Association Between Somatostatin Receptor Expression and Clinical Outcomes in Neuroendocrine Tumors. Pancreas 2016, 45, 1386–1393. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, H.S.; Kim, W.H. Clinical significance of protein expression of cyclooxygenase-2 and somatostatin receptors in gastroenteropancreatic neuroendocrine tumors. Cancer Res. Treat. 2011, 43, 181–188. [Google Scholar] [CrossRef]
- Elf, A.-K.; Johanson, V.; Marin, I.; Bergström, A.; Nilsson, O.; Svensson, J.; Wängberg, B.; Bernhardt, P.; Elias, E. Evaluation of SSTR2 Expression in SI-NETs and Relation to Overall Survival after PRRT. Cancers 2021, 13, 2035. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, N.; Xiao, Y.; Zhu, W.; Bai, C.; Zhao, L. Metastatic Adrenal Cortical Carcinoma Responding to Octreotide: A Case Report. Oncologist 2019, 24, e793–e797. [Google Scholar] [CrossRef] [PubMed]
- Roslyakova, A.; Selivanova, L.; Tarasova, A.; Beltsevich, D. Somatostatin receptors 2A and 5 expression in adrenocortical cancer. Endocr. Abstr. 2020, 70, AEP41. [Google Scholar] [CrossRef]
- Grisanti, S.; Filice, A.; Basile, V.; Cosentini, D.; Rapa, I.; Albano, D.; Morandi, A.; Laganà, M.; Volta, A.D.; Bertagna, F.; et al. Treatment With 90Y/177Lu-DOTATOC in Patients with Metastatic Adrenocortical Carcinoma Expressing Somatostatin Receptors. J. Clin. Endocrinol. Metab. 2019, 105, e1–e5. [Google Scholar] [CrossRef]
- Charoenpitakchai, M.; Liu, E.; Zhao, Z.; Koyama, T.; Huh, W.J.; Berlin, J.; Hande, K.; Walker, R.; Shi, C. In liver metastases from small intestinal neuroendocrine tumors, SSTR2A expression is heterogeneous. Virchows Arch. 2017, 470, 545–552. [Google Scholar] [CrossRef]
| No. | Age | Sex | Initial Presentation | Tumor Size (cm) and Laterality | Stage | Metastatic Site | Treatment | Survival Duration (Months) | Survival Status | Hormone Profile | Other Malignancy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | M | penis enlargement, virilization, and acne | 7.4, unilateral | 4 | liver, retroperitoneum | Adrenalectomy and mitotane | 15 | expired | elevated DHEAS | |
| 2 | 1 | M | penis enlargement, virilization | 12, unilateral | 4 | peritoneum seeding | Adrenalectomy | 14 | expired | ||
| 3 | 70 | M | retroperitoneal tumor | 5, unilateral | 1 | NA | Adrenalectomy | 61 | expired | testis cancer | |
| 4 | 44 | M | Severe abdominal pain for 2 days | 9.5, unilateral | 3 | NA | Adrenalectomy and mitotane | 21 | expired | ||
| 5 | 60 | M | body weight loss about 11 kg in recent 5–6 months | 11, unilateral | 4 | lung | Adrenalectomy, mitotane, chemotherapy with DEP regimen | 144 | alive | ||
| 6 | 1 | F | progressive rapid weight gain for months, hirsutism, pubic hair | NA | 3 | NA | Adrenalectomy | 0.67 | expired | ||
| 7 | 62 | M | enlarged soft tissue mass in left adrenal gland | 11.5, unilateral | 4 | liver, kidney, pancreas, diaphragm, small and large intestine | Adrenalectomy, chemotherapy with doxorubicin | 16 | expired | HCC | |
| 8 | 49 | F | general weakness, nausea, and dizziness for 2 weeks, acne, right adrenal tumor | 7, unilateral | 4 | suspected bone metastasis | Adrenalectomy | 90 | expired | Cushing syndrome, parathyroid adenoma, pituitary tumor, elevated DHEAS | Endometrial mullerian adenosarcoma |
| 9 | 50 | M | progressive abdomen distension for months | 13.1, unilateral | 4 | suspected liver and lung | No treatment | 0.4 | expired | Cushing syndrome, elevated estrogen/DHEAS/17OHP | |
| 10 | 40 | F | amenorrhea at 40 y/o | 11, unilateral | 3 | NA | Adrenalectomy, chemotherapy with unknown regimen | 244 | expired | Cushing syndrome | |
| 11 | 66 | F | suprarenal mass noted via renal echo | 9.5, unilateral | 2 | local recurrence, retroperitoneum | Adrenalectomy | 5 | expired | ||
| 12 | 36 | F | abdomen pain, body weight increase, acne, buffalo hump | 7.7, unilateral | 3 | lung | Adrenalectomy, mitotane, radiotherapy, metastasis resection, chemotherapy with DEP regimen | 24 | alive | Cushing syndrome, elevated testosterone/DHEAS/ASD/17OHP | |
| 13 | 56 | M | abdomen pain and fullness for 2 years | 9.2, unilateral | 2 | NA | Adrenalectomy and mitotane | 15 | alive | ||
| 14 | 59 | F | abdomen pain and fullness | 23, unilateral ovary (ectopic ACC) | 4 | lung, liver, peritoneum | Laparotomy optimal cytoreduction, mitotane and chemotherapy with etoposide+ cisplatin | 6 | expired |
| No. | Beta-Catenin (Nuclear Stain) | CDK4 (Nuclear Expression) | ATRX (Nuclear Expression) | p53 (Aberrant Expression) | SSTR2 | SSTR2 Expression Score | Ki-67 | Mitotic Count | Fuhrman Nuclear Grade | Resection Margin Status and Tumor Rupture |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | − | + | + | Diffuse (+) | + | 100 | 10 | NA | 3 | R0, no rupture |
| 2 | − | + | + | Diffuse (+) | + | 155 | 5 | >31/50HPF | 4 | R0, no rupture |
| 3 | − | + | + | Total (−) | − | 0 | <1 | NA | 4 | R0, no rupture |
| 4 | − | + | − | Mosaic | + | 70 | >4 | >5/50 HPF | 2 | R0, no rupture, with renal vein invasion |
| 5 | − | − | − | Total (−) | + | 45 | 15–50 | NA | 4 | R0, no rupture |
| 6 | − | + | + | Diffuse (+) | + | 155 | 80 | NA | 4 | R0, no rupture |
| 7 | − | − | − | Total (−) | + | 50 | 5 | NA | 4 | R1, no rupture |
| 8 | − | − | − | Total (−) | − | 0 | <1 | >5/50 HPF | 4 | R0, no rupture |
| 9 | − | − | − | Total (−) | + | 270 | 50 | >2/10 HPF | 2 | No operation |
| 10 | − | − | − | Total (−) | − | 0 | <1 | NA | 3 | R0, no rupture |
| 11 | + | − | + | Total (−) | + | 155 | >4 | >5/50 HPF | 3 | R0, no rupture |
| 12 | − | + | + | Mosaic | + | 105 | 15 | 10/50HPF | 2 | R0, no rupture |
| 13 | − | + | − | Total (−) | − | 0 | 15 | NA | 4 | R0, no rupture |
| 14 | − | + | + | Mosaic | + | 96 | >80 | NA | 2 | R1, tumor rupture |
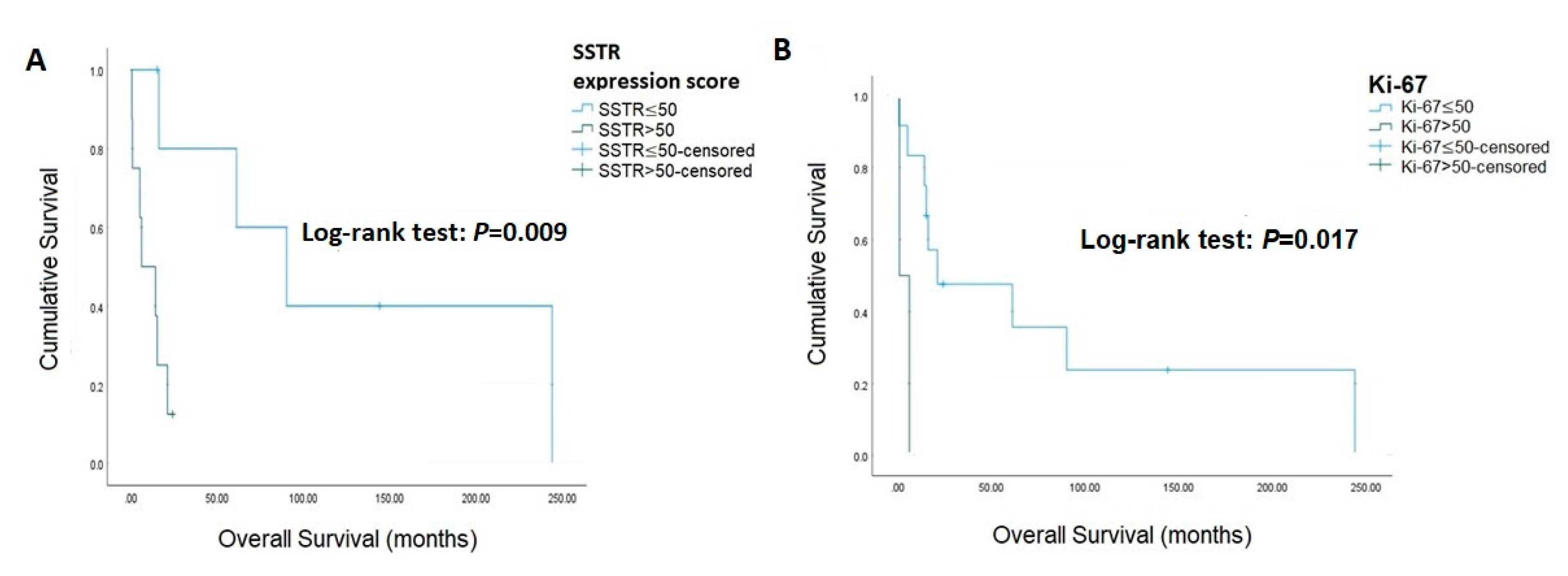
| Variables | No. of Patients | Log-Rank Test (p Value) |
|---|---|---|
| Ki-67 | 0.017 | |
| ≤50% | 12 | |
| >50% | 2 | |
| SSTR2 | 0.099 | |
| Positive | 10 | |
| Negative | 4 | |
| SSTR2 expression score | 0.009 | |
| ≤50 | 6 | |
| >50 | 8 | |
| P53 | 0.994 | |
| Diffuse positive | 3 | |
| Total negative | 8 | |
| Mosaic | 3 | |
| Beta-catenin | 0.097 | |
| Positive | 1 | |
| Negative | 13 | |
| CDK4 | 0.388 | |
| Positive | 8 | |
| Negative | 6 | |
| ATRX | 0.095 | |
| Positive | 7 | |
| Negative | 7 |
| Variables | Univariate Analysis | Bootstrapping Univariate Analysis | Adjusted Multivariate Analysis | Adjusted Bootstrapping Multivariate Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | B | 95% CI | p Value | HR | 95% CI | p Value | B | 95% CI | p Value | |||||
| Ki-67 | 7.76 | 1.07 | 56.37 | 0.043 | 7.76 | 0.9 | 14 | 0.003 | 7.66 | 1.01 | 58.02 | 0.049 | 7.66 | 0.72 | 14.01 | 0.004 |
| Beta-catenin | 5.98 | 0.54 | 66.05 | 0.144 | 5.98 | 0.78 | 14 | 0.006 | 8.94 | 0.61 | 131.03 | 0.110 | 8.94 | 0.93 | 21.65 | 0.009 |
| CDK4 | 1.86 | 0.45 | 7.76 | 0.394 | 1.86 | −0.99 | 3.83 | 0.396 | 2.62 | 0.51 | 13.55 | 0.250 | 2.62 | −1.00 | 12.94 | 0.304 |
| ATRX | 3.15 | 0.77 | 12.95 | 0.111 | 3.15 | −0.19 | 5.09 | 0.055 | 4.37 | 0.91 | 21.11 | 0.066 | 4.37 | 0.02 | 13.42 | 0.026 |
| P53 | 4.54 | 0.90 | 22.83 | 0.067 | 4.54 | 0.42 | 9.01 | 0.009 | 4.84 | 0.84 | 27.82 | 0.077 | 4.84 | 0.11 | 13.49 | 0.027 |
| SSTR2 | 3.62 | 0.72 | 18.14 | 0.118 | 3.62 | 0.21 | 4.82 | 0.014 | 4.87 | 0.70 | 33.98 | 0.111 | 4.87 | 0.29 | 14.00 | 0.015 |
| SSTR2 score | 1.03 | 1.01 | 1.05 | 0.003 | 1.03 | 0.02 | 0.07 | <0.001 | 1.03 | 1.01 | 1.05 | 0.002 | 1.03 | 0.02 | 0.18 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, W.-H.; Dai, S.-H.; Lee, C.-C.; Chien, M.-N.; Zeng, Y.-H. A Clinicopathological Analysis of Asian Patients with Adrenocortical Carcinoma: A Single-Center Experience. Curr. Oncol. 2023, 30, 4117-4125. https://doi.org/10.3390/curroncol30040313
Tsai W-H, Dai S-H, Lee C-C, Chien M-N, Zeng Y-H. A Clinicopathological Analysis of Asian Patients with Adrenocortical Carcinoma: A Single-Center Experience. Current Oncology. 2023; 30(4):4117-4125. https://doi.org/10.3390/curroncol30040313
Chicago/Turabian StyleTsai, Wen-Hsuan, Shuen-Han Dai, Chun-Chuan Lee, Ming-Nan Chien, and Yi-Hong Zeng. 2023. "A Clinicopathological Analysis of Asian Patients with Adrenocortical Carcinoma: A Single-Center Experience" Current Oncology 30, no. 4: 4117-4125. https://doi.org/10.3390/curroncol30040313
APA StyleTsai, W.-H., Dai, S.-H., Lee, C.-C., Chien, M.-N., & Zeng, Y.-H. (2023). A Clinicopathological Analysis of Asian Patients with Adrenocortical Carcinoma: A Single-Center Experience. Current Oncology, 30(4), 4117-4125. https://doi.org/10.3390/curroncol30040313





