Psychosocial Wellbeing among Patients with Breast Cancer during COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Study Sample
2.3. Study Measures
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Correlations between Psychosocial Wellbeing Measures
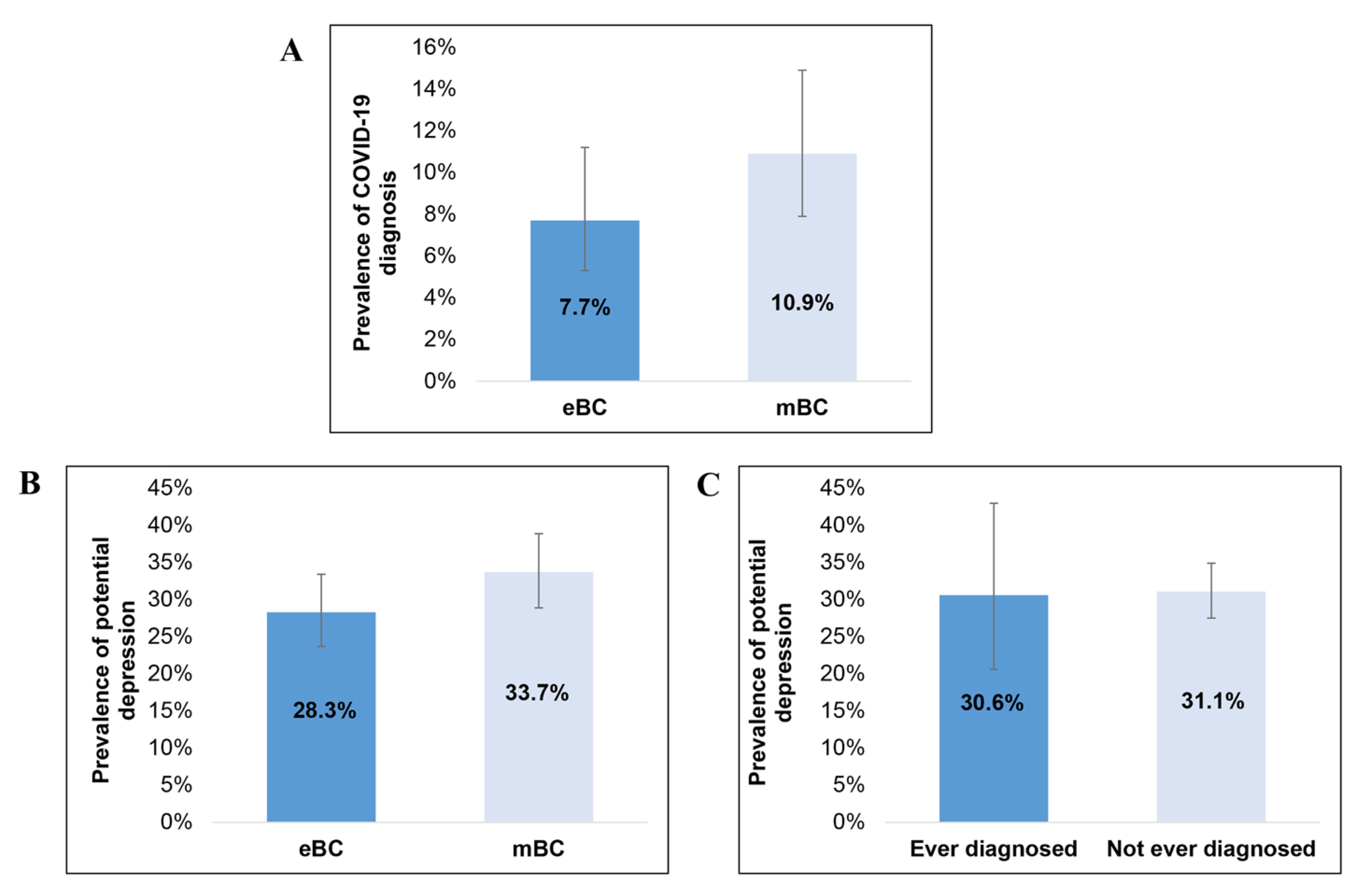
3.3. Prevalence of COVID-19 and Potential Depression
3.4. Psychosocial Wellbeing
3.5. COVID-19-Related Stress and Associated Factors
4. Discussion
4.1. Summary and Contributions
4.2. Limitations and Strengths
4.3. Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Acronyms and Abbreviations
| BC | Breast cancer |
| BMI | Body mass index |
| CCI | Charlson Comorbidity Index |
| CI | Confidence interval |
| COST | Comprehensive Score for Financial Toxicity |
| COVID-19 | Coronavirus disease 2019 |
| CSS | COVID Stress Scale |
| DCIS | Ductal carcinoma in situ |
| eBC | Early-stage breast cancer |
| FACT-B | Functional Assessment of Cancer Therapy-Breast |
| GLM | Generalized linear model |
| HIPAA | Health Insurance Portability and Accountability Act |
| HIV/AIDS | Human immunodeficiency virus/acquired immunodeficiency syndrome |
| HRQoL | Health-related quality of life |
| mBC | Metastatic breast cancer |
| PHQ-8 | 8-Item Patient Health Questionnaire |
| PIC | Psychological Impact of Cancer Scale |
| SD | Standard deviation |
| SE | Standard error |
| US | United States |
| vs. | Versus |
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 24 October 2022).
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, S.; Falzone, L.; Grillo, C.M.; Scandurra, G.; Torino, F.; Libra, M. Cancer management during COVID-19 Pandemic: Is immune checkpoint inhibitors-based immunotherapy harmful or beneficial? Cancers 2020, 12, 2237. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, Y.; Okwan-Duodu, D.; Cui, X. COVID-19 in cancer patients: Risk, clinical features, and management. Cancer Biol. Med. 2020, 17, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Sachdeva, S.; Parekh, T.; Desai, R. COVID-19 and cancer: Lessons from a pooled meta-analysis. JCO Glob. Oncol. 2020, 6, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.M.; Ferguson, J.M.; Kurian, A.; Bondy, M.; Patel, M.I. The impact of COVID-19 on patients with cancer: A national study of patient experiences. Am. J. Clin. Oncol. 2021, 44, 580–587. [Google Scholar]
- Riera, R.; Bagattini, Â.M.; Pacheco, R.L.; Pachito, D.V.; Roitberg, F.; Ilbawi, A. Delays and disruptions in cancer health care due to COVID-19 pandemic: Systematic review. JCO Glob. Oncol. 2021, 7, 311–323. [Google Scholar] [CrossRef]
- Lai, A.G.; Pasea, L.; Banerjee, A.; Hall, G.; Denaxas, S.; Chang, W.H.; Katsoulis, M.; Williams, B.; Pillay, D.; Noursadeghi, M.; et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: Near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open 2020, 10, e043828. [Google Scholar] [CrossRef]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020, 21, 1023–1034. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar]
- Mathelin, C.; Ame, S.; Anyanwu, S.; Avisar, E.; Boubnider, W.M.; Breitling, K.; Anie, H.A.; Conceição, J.C.; Dupont, V.; Elder, E.; et al. Breast cancer management during the COVID-19 pandemic: The Senologic International Society Survey. Eur. J. Breast Health 2021, 17, 188–196. [Google Scholar] [CrossRef]
- Alagoz, O.; Lowry, K.P.; Kurian, A.W.; Mandelblatt, J.S.; Ergun, M.A.; Huang, H.; Lee, S.J.; Schechter, C.B.; Tosteson, A.N.A.; Miglioretti, D.L.; et al. Impact of the COVID-19 pandemic on breast cancer mortality in the US: Estimates from collaborative simulation modeling. J. Natl. Cancer Inst. 2021, 113, 1484–1494. [Google Scholar] [CrossRef]
- Song, H.; Bergman, A.; Chen, A.T.; Ellis, D.; David, G.; Friedman, A.B.; Bond, A.M.; Bailey, J.M.; Brooks, R.; Smith-McLallen, A. Disruptions in preventive care: Mammograms during the COVID-19 pandemic. Health Serv. Res. 2021, 56, 95–101. [Google Scholar] [CrossRef]
- Papautsky, E.L.; Hamlish, T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res. Treat. 2020, 184, 249–254. [Google Scholar] [CrossRef]
- Patt, D.; Gordan, L.; Diaz, M.; Okon, T.; Grady, L.; Harmison, M.; Markward, N.; Sullivan, M.; Peng, J.; Zhou, A. Impact of COVID-19 on cancer care: How the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin. Cancer Inform. 2020, 4, 1059–1071. [Google Scholar] [CrossRef]
- Dinapoli, L.; Colloca, G.; Di Capua, B.; Valentini, V. Psychological aspects to consider in breast cancer diagnosis and treatment. Curr. Oncol. Rep. 2021, 23, 38. [Google Scholar] [CrossRef]
- Wang, X.; Wang, N.; Zhong, L.; Wang, S.; Zheng, Y.; Yang, B.; Zhang, J.; Lin, Y.; Wang, Z. Prognostic value of depression and anxiety on breast cancer recurrence and mortality: A systematic review and meta-analysis of 282,203 patients. Mol. Psychiatry 2020, 25, 3186–3197. [Google Scholar] [CrossRef]
- Renna, M.E.; Rosie Shrout, M.; Madison, A.A.; Lustberg, M.; Povoski, S.P.; Agnese, D.M.; Reinbolt, R.E.; Wesolowski, R.; Williams, N.O.; Ramaswamy, B.; et al. Worry and rumination in breast cancer patients: Perseveration worsens self-rated health. J. Behav. Med. 2021, 44, 253–259. [Google Scholar] [CrossRef]
- Bartmann, C.; Fischer, L.M.; Hübner, T.; Müller-Reiter, M.; Wöckel, A.; McNeill, R.V.; Schlaiss, T.; Kittel-Schneider, S.; Kämmerer, U.; Diessner, J. The effects of the COVID-19 pandemic on psychological stress in breast cancer patients. BMC Cancer 2021, 21, 1356. [Google Scholar] [CrossRef]
- Swainston, J.; Chapman, B.; Grunfeld, E.A.; Derakshan, N. COVID-19 lockdown and its adverse impact on psychological health in breast cancer. Front. Psychol. 2020, 11, 2033. [Google Scholar]
- Ludwigson, A.; Huynh, V.; Myers, S.; Hampanda, K.; Christian, N.; Ahrendt, G.; Romandetti, K.; Tevis, S. Patient perceptions of changes in breast cancer care and well-being during COVID-19: A mixed methods study. Ann. Surg. Oncol. 2022, 29, 1649–1657. [Google Scholar] [CrossRef]
- Russell, B.; Moss, C.L.; Shah, V.; Ko, T.K.; Palmer, K.; Sylva, R.; George, G.; Monroy-Iglesias, M.J.; Patten, P.; Ceesay, M.M.; et al. Risk of COVID-19 death in cancer patients: An analysis from Guy’s Cancer Centre and King’s College Hospital in London. Br. J. Cancer 2021, 125, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Yousefi Afrashteh, M.; Masoumi, S. Psychological well-being and death anxiety among breast cancer survivors during the COVID-19 pandemic: The mediating role of self-compassion. BMC Womens Health 2021, 21, 387. [Google Scholar] [CrossRef] [PubMed]
- Bargon, C.; Batenburg, M.; van Stam, L.; van der Molen, D.R.M.; van Dam, I.E.; van der Leij, F.; Baas, I.O.; Ernst, M.F.; Maarse, W.; Vermulst, N.; et al. The impact of the COVID-19 pandemic on quality of life, physical and psychosocial wellbeing in breast cancer patients—A prospective, multicenter cohort study. Eur. J. Cancer 2020, 138, S17. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Liu, L.; Jiang, M.; Wang, W.; Zhou, X.; Shao, J. Factors associated with psychological distress among patients with breast cancer during the COVID-19 pandemic: A cross-sectional study in Wuhan, China. Support. Care Cancer 2021, 29, 4773–4782. [Google Scholar] [CrossRef] [PubMed]
- Choobin, M.H.; Mirabolfathi, V.; Chapman, B.; Moradi, A.R.; Grunfeld, E.A.; Derakshan, N. The impact of COVID-19 outbreak on emotional and cognitive vulnerability in Iranian women with breast cancer. Front. Psychol. 2021, 12, 663310. [Google Scholar] [CrossRef] [PubMed]
- Pigozzi, E.; Tregnago, D.; Costa, L.; Insolda, J.; Turati, E.; Rimondini, M.; Donisi, V.; Madera, P.; Fiorica, F.; Giuliani, J.; et al. Psychological impact of COVID-19 pandemic on oncological patients: A survey in Northern Italy. PLoS ONE 2021, 16, e0248714. [Google Scholar] [CrossRef]
- Soriano, E.C.; Perndorfer, C.; Otto, A.K.; Fenech, A.L.; Siegel, S.D.; Dickson-Witmer, D.; Clements, L.; Laurenceau, J.P. Psychosocial impact of cancer care disruptions in women with breast cancer during the COVID-19 pandemic. Front. Psychol. 2021, 12, 662339. [Google Scholar] [CrossRef]
- Shah, Y.B.; Kjelstrom, S.; Martinez, D.; Leitenberger, A.; Manasseh, D.M.; Bollmann-Jenkins, M.; Partridge, A.; Kaklamani, V.; Chlebowski, R.; Larson, S.; et al. Risk factors for heightened COVID-19-related anxiety among breast cancer patients. Cancer Med. 2023, 12, 3577–3588. [Google Scholar] [CrossRef]
- Desai, A.; Warner, J.; Kuderer, N.; Thompson, M.; Painter, C.; Lyman, G.; Lopes, G. Crowdsourcing a crisis response for COVID-19 in oncology. Nat. Cancer 2020, 1, 473–476. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Taylor, S.; Landry, C.A.; Paluszek, M.M.; Fergus, T.A.; McKay, D.; Asmundson, G.J.G. Development and initial validation of the COVID Stress Scales. J. Anxiety Disord. 2020, 72, 102232. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Strine, T.W.; Spitzer, R.L.; Williams, J.B.; Berry, J.T.; Mokdad, A.H. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord. 2009, 114, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Hulbert-Williams, N.J.; Hulbert-Williams, L.; Whelen, L.; Mulcare, H. The Psychological Impact of Cancer (PIC) Scale: Development and comparative psychometric testing against the Mini-MAC Scale in UK and Australian cancer survivors. J. Psychosoc. Oncol. Res. Pract. 2019, 1, e8. [Google Scholar] [CrossRef]
- Brady, M.J.; Cella, D.F.; Mo, F.; Bonomi, A.E.; Tulsky, D.S.; Lloyd, S.R.; Deasy, S.; Cobleigh, M.; Shiomoto, G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J. Clin. Oncol. 1997, 15, 974–986. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.A.; Yap, B.J.; Wroblewski, K.; Blinder, V.; Araújo, F.S.; Hlubocky, F.J.; Nicholas, L.H.; O’Connor, J.M.; Brockstein, B.; Ratain, M.J.; et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: The validation of the COmprehensive Score for financial Toxicity (COST). Cancer 2017, 123, 476–484. [Google Scholar] [CrossRef]
- de Souza, J.A.; Wroblewski, K.; Proussaloglou, E.; Nicholson, L.; Hantel, A.; Wang, Y. Validation of a financial toxicity (FT) grading system. JCO Oncol. Pract. 2017, 35, 6615. [Google Scholar] [CrossRef]
- Alagizy, H.A.; Soltan, M.R.; Soliman, S.S.; Hegazy, N.N.; Gohar, S.F. Anxiety, depression and perceived stress among breast cancer patients: Single institute experience. Middle East Curr. Psychiatry 2020, 27, 29. [Google Scholar] [CrossRef]
- Naser, A.Y.; Hameed, A.N.; Mustafa, N.; Alwafi, H.; Dahmash, E.Z.; Alyami, H.S.; Khalil, H. Depression and anxiety in patients with cancer: A cross-sectional study. Front. Psychol. 2021, 12, 585534. [Google Scholar] [CrossRef]
- Morlock, R.; Morlock, A.; Downen, M.; Shah, S.N. COVID-19 prevalence and predictors in United States adults during peak stay-at-home orders. PLoS ONE 2021, 16, e0245586. [Google Scholar] [CrossRef]
- Vuagnat, P.; Frelaut, M.; Ramtohul, T.; Basse, C.; Diakite, S.; Noret, A.; Bellesoeur, A.; Servois, V.; Hequet, D.; Laas, E.; et al. COVID-19 in breast cancer patients: A cohort at the Institut Curie hospitals in the Paris area. Breast Cancer Res. 2020, 22, 55. [Google Scholar] [CrossRef]
- Pilevarzadeh, M.; Amirshahi, M.; Afsargharehbagh, R.; Rafiemanesh, H.; Hashemi, S.M.; Balouchi, A. Global prevalence of depression among breast cancer patients: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2019, 176, 519–533. [Google Scholar] [CrossRef]
- Rhee, T.G.; Wilkinson, S.T.; Steffens, D.C.; Rosenheck, R.A.; Olfson, M. Prevalence of treatment for depression among US adults who screen positive for depression, 2007–2016. JAMA Psychiatry 2020, 77, 1193–1195. [Google Scholar] [CrossRef]
- Criscitiello, C.; Spurden, D.; Piercy, J.; Rider, A.; Williams, R.; Mitra, D.; Wild, R.; Corsaro, M.; Kurosky, S.K.; Law, E.H. Health-related quality of life among patients with HR+/HER2- early breast cancer. Clin. Ther. 2021, 43, 1228–1244.e4. [Google Scholar] [CrossRef]
- Hamer, J.; McDonald, R.; Zhang, L.; Verma, S.; Leahey, A.; Ecclestone, C.; Bedard, G.; Pulenzas, N.; Bhatia, A.; Chow, R.; et al. Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Support. Care Cancer 2017, 25, 409–419. [Google Scholar] [CrossRef]
- Sinkar, S.; Too, F.; Carr, K.; Jelinek, J.; Saylor, E.; Bacon, J.; Stearns, V.; Smith, K.L. Assessing financial toxicity in patients with metastatic breast cancer: A single institution experience during the COVID-19 pandemic. J. Clin. Oncol. 2021, 39, 176. [Google Scholar] [CrossRef]
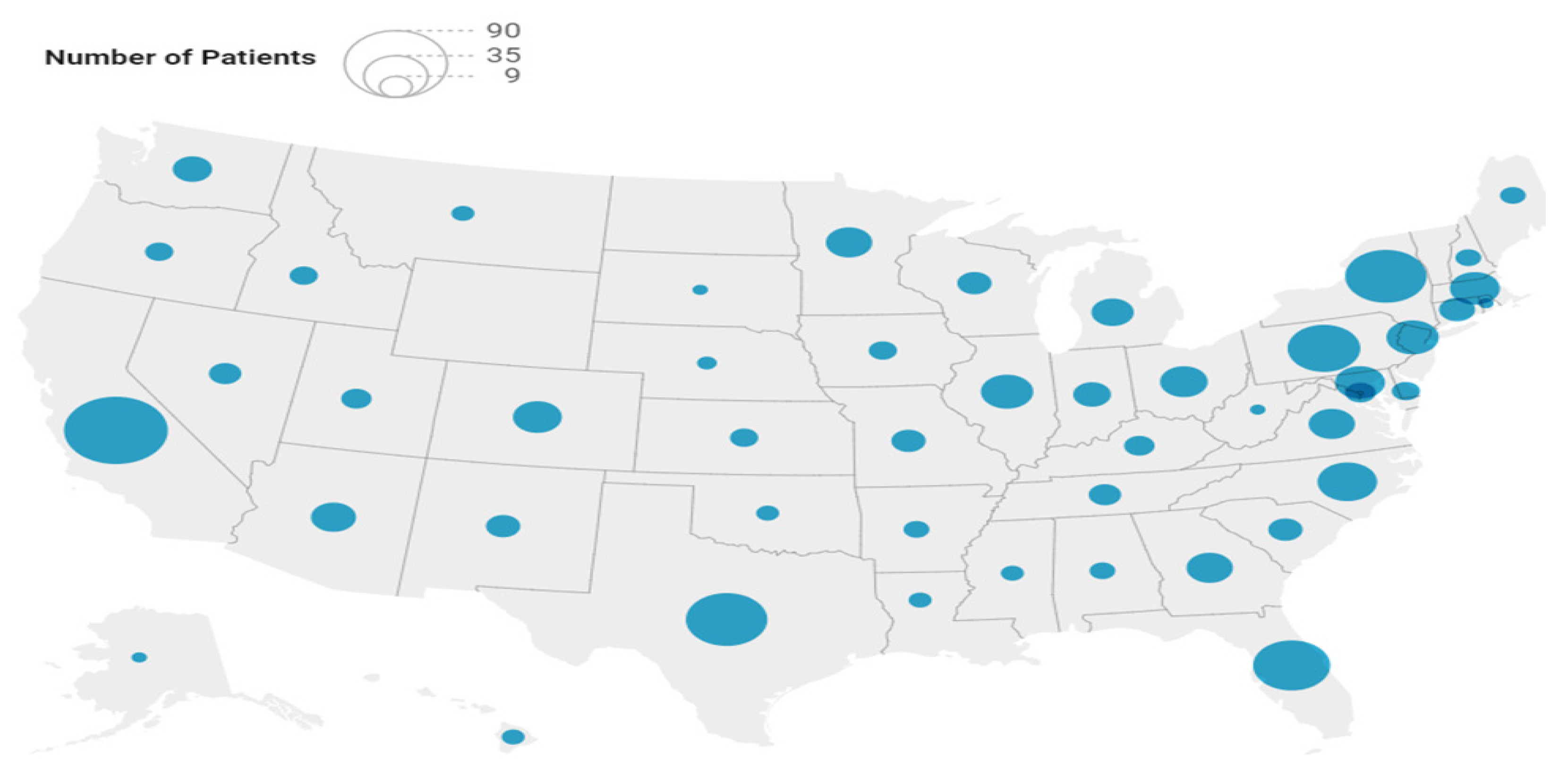

| Characteristics | N = 669 |
|---|---|
| Age, mean (SD) | 51.6 (11.3) |
| Sex, n (%) | |
| Male | 7 (1.0) |
| Female | 662 (99.0) |
| BMI Category *, n (%) | |
| Underweight | 22 (3.3) |
| Normal weight | 234 (35.6) |
| Overweight | 179 (27.2) |
| Obese | 223 (33.9) |
| Race, n (%) | |
| White | 561 (83.9) |
| Black | 60 (9.0) |
| Any other race | 41 (6.1) |
| Prefer not to answer | 7 (1.0) |
| Region of Residence, n (%) | |
| Northeast | 156 (23.3) |
| Midwest | 106 (15.8) |
| South | 233 (34.8) |
| West | 174 (26.0) |
| Community Type, n (%) | |
| Rural area | 68 (10.2) |
| Small city or town | 170 (25.4) |
| Suburb near a large city | 297 (44.4) |
| Large city | 134 (20.0) |
| Number of Adults in Household, mean (SD) | 2.09 (0.89) |
| Number of Children in Household, mean (SD) | 0.62 (1.02) |
| Education, n (%) | |
| Less than college degree | 203 (30.3) |
| College degree or higher | 465 (69.5) |
| Prefer not to answer | 1 (0.1) |
| Marital Status, n (%) | |
| Not Married/Living with Partner | 208 (31.1) |
| Married/Living with Partner | 461 (68.9) |
| Employment, n (%) | |
| Not Employed | 359 (53.7) |
| Employed | 303 (45.3) |
| Prefer not to answer | 7 (1.0) |
| Income, n (%) | |
| <$75k | 275 (41.1) |
| $75k+ | 366 (54.7) |
| Prefer not to answer | 28 (4.2) |
| Current BC Type, n (%) | |
| eBC | 325 (48.6) |
| mBC | 344 (51.4) |
| Stage at Initial Diagnosis, n (%) | |
| DCIS | 36 (5.4) |
| Stage 0 | 11 (1.6) |
| Stage 1, 2, or 3 | 465 (69.5) |
| Stage 4 | 153 (22.9) |
| Don’t know | 4 (0.6) |
| Disease Duration in Years, mean (SD) | 5.50 (5.83) |
| Diagnosed Comorbidities †, n (%) | |
| Depression | 142 (21.2) |
| Anxiety | 120 (17.9) |
| Another type of cancer, including leukemia/lymphoma | 22 (3.3) |
| Chronic pulmonary disease | 11 (1.6) |
| Mild liver disease | 9 (1.3) |
| Kidney disease | 5 (0.7) |
| Congestive heart failure | 4 (0.6) |
| Chronic complications from diabetes | 3 (0.4) |
| Moderate/severe liver disease | 2 (0.3) |
| CCI score, mean (SD) | 3.27 (2.40) |
| Current Treatments, n (%) | |
| Hormone therapy | 393 (58.7) |
| Targeted therapy | 257 (38.4) |
| Chemotherapy | 143 (21.4) |
| Supportive/palliative care | 90 (13.5) |
| Radiation | 85 (12.7) |
| Surgery in past 3 months | 74 (11.1) |
| Immunotherapy | 23 (3.4) |
| Not receiving treatment | 76 (11.4) |
| Scale, Mean (SD) | Overall (N = 669) | BC Type | COVID-19 Diagnosis History | |||||
|---|---|---|---|---|---|---|---|---|
| eBC (N = 325) | mBC (N = 344) | p-Value | Ever Diagnosed (N = 62) | Not Ever Diagnosed (N = 599 *) | p-Value | |||
| FACT-B Scores | BC Subscale | 22.8 (6.8) | 23.0 (6.8) | 22.7 (6.7) | 0.534 | 22.0 (7.1) | 23.0 (6.7) | 0.277 |
| Physical Wellbeing | 18.5 (6.3) | 19.8 (6.0) | 17.4 (6.4) | <0.001 | 19.1 (5.6) | 18.5 (6.4) | 0.478 | |
| Social Wellbeing | 17.4 (6.0) | 18.2 (6.1) | 16.6 (5.7) | <0.001 | 17.3 (5.8) | 17.5 (5.9) | 0.822 | |
| Emotional Wellbeing | 15.4 (4.8) | 16.1 (4.6) | 14.7 (4.9) | <0.001 | 15.2 (5.0) | 15.4 (4.8) | 0.830 | |
| Functional Wellbeing | 16.3 (6.1) | 17.2 (6.0) | 15.4 (6.0) | <0.001 | 16.2 (5.5) | 16.3 (6.1) | 0.888 | |
| FACT-General | 67.6 (17.9) | 71.4 (17.5) | 64.0 (17.6) | <0.001 | 67.8 (16.8) | 67.6 (18.0) | 0.943 | |
| FACT-Breast Cancer | 90.4 (22.5) | 94.3 (22.3) | 86.7 (22.1) | <0.001 | 89.8 (21.8) | 90.6 (22.6) | 0.788 | |
| PIC Scores | Cognitive Distress | 5.4 (2.0) | 5.2 (1.7) | 5.6 (2.1) | 0.002 | 5.2 (1.9) | 5.4 (2.0) | 0.412 |
| Cognitive Avoidance | 7.3 (2.2) | 6.9 (2.1) | 7.7 (2.3) | <0.001 | 7.6 (1.7) | 7.3 (2.3) | 0.296 | |
| Emotional Distress | 9.1 (1.8) | 9.0 (1.8) | 9.2 (1.8) | 0.192 | 9.0 (1.7) | 9.1 (1.8) | 0.630 | |
| Fighting Spirit | 10.0 (1.6) | 10.0 (1.6) | 10.0 (1.7) | 0.916 | 10.1 (1.4) | 10.0 (1.7) | 0.546 | |
| COST Score | COST Total Score | 22.7 (10.8) | 24.2 (11.3) | 21.3 (10.2) | <0.001 | 21.3 (9.8) | 22.9 (10.9) | 0.263 |
| CSS Score | Danger | 8.2 (5.6) | 8.1 (5.6) | 8.3 (5.6) | 0.732 | 7.5 (6.0) | 8.2 (5.6) | 0.291 |
| Socioeconomic | 3.7 (4.8) | 3.5 (4.8) | 3.9 (4.9) | 0.339 | 4.6 (5.7) | 3.6 (4.7) | 0.122 | |
| Traumatic Stress | 3.0 (4.2) | 3.0 (4.3) | 2.9 (4.1) | 0.662 | 2.8 (4.3) | 3.0 (4.2) | 0.684 | |
| B | SE | 95% LCL | 95% UCL | p-Value | |
|---|---|---|---|---|---|
| CSS Danger Scale | |||||
| Intercept | 2.24 | 0.24 | 1.77 | 2.72 | <0.001 |
| mBC | −0.04 | 0.09 | −0.21 | 0.14 | 0.696 |
| Rural/small town | 0.11 | 0.09 | −0.06 | 0.29 | 0.205 |
| Potential depression | 0.26 | 0.10 | 0.07 | 0.45 | 0.006 |
| Number of adults in household | 0.06 | 0.05 | −0.04 | 0.15 | 0.219 |
| <$75,000 income | 0.25 | 0.09 | 0.07 | 0.43 | 0.006 |
| Age (years) | −0.01 | 0.00 | −0.01 | 0.00 | 0.164 |
| Overweight/obese BMI | 0.00 | 0.09 | −0.18 | 0.17 | 0.965 |
| CCI score > 2 | 0.08 | 0.11 | −0.13 | 0.28 | 0.462 |
| Ever diagnosed with COVID-19 | −0.14 | 0.15 | −0.43 | 0.16 | 0.366 |
| CSS Socioeconomic Scale | |||||
| Intercept | 0.64 | 0.28 | 0.10 | 1.18 | 0.021 |
| mBC | −0.09 | 0.10 | −0.29 | 0.11 | 0.384 |
| Less than college degree) | −0.17 | 0.11 | −0.38 | 0.04 | 0.115 |
| Age (years) | 0.00 | 0.00 | 0.00 | 0.01 | 0.324 |
| Currently using hormone therapy | 0.21 | 0.10 | 0.02 | 0.41 | 0.034 |
| Rural/small town | 0.15 | 0.10 | −0.05 | 0.34 | 0.141 |
| White race | 0.23 | 0.13 | −0.02 | 0.48 | 0.066 |
| Potential depression | 0.46 | 0.10 | 0.26 | 0.66 | <0.001 |
| Overweight/obese BMI | 0.32 | 0.10 | 0.12 | 0.52 | 0.001 |
| Currently using chemotherapy | 0.25 | 0.12 | 0.01 | 0.49 | 0.045 |
| <$75,000 income | 0.30 | 0.10 | 0.11 | 0.50 | 0.002 |
| CCI score > 2 | 0.13 | 0.11 | −0.09 | 0.35 | 0.248 |
| Ever diagnosed with COVID-19 | 0.20 | 0.16 | −0.11 | 0.51 | 0.211 |
| CSS Traumatic Stress Scale | |||||
| Intercept | 1.15 | 0.25 | 0.66 | 1.63 | <0.001 |
| mBC | −0.12 | 0.13 | −0.37 | 0.14 | 0.377 |
| CCI score > 2 | 0.27 | 0.11 | 0.05 | 0.50 | 0.018 |
| Potential depression | 0.78 | 0.10 | 0.58 | 0.98 | <0.001 |
| Currently using targeted therapy | −0.09 | 0.13 | −0.34 | 0.16 | 0.488 |
| <$75,000 income | 0.34 | 0.10 | 0.15 | 0.53 | <0.001 |
| Age (years) | 0.00 | 0.00 | −0.01 | 0.00 | 0.335 |
| Overweight/obese BMI | 0.07 | 0.10 | −0.13 | 0.27 | 0.497 |
| Ever diagnosed with COVID-19 | −0.15 | 0.17 | −0.48 | 0.18 | 0.374 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maculaitis, M.C.; Liu, X.; Berk, A.; Massa, A.; Weiss, M.C.; Kurosky, S.K.; Li, B.; McRoy, L. Psychosocial Wellbeing among Patients with Breast Cancer during COVID-19. Curr. Oncol. 2023, 30, 3886-3900. https://doi.org/10.3390/curroncol30040294
Maculaitis MC, Liu X, Berk A, Massa A, Weiss MC, Kurosky SK, Li B, McRoy L. Psychosocial Wellbeing among Patients with Breast Cancer during COVID-19. Current Oncology. 2023; 30(4):3886-3900. https://doi.org/10.3390/curroncol30040294
Chicago/Turabian StyleMaculaitis, Martine C., Xianchen Liu, Alexandra Berk, Angelina Massa, Marisa C. Weiss, Samantha K. Kurosky, Benjamin Li, and Lynn McRoy. 2023. "Psychosocial Wellbeing among Patients with Breast Cancer during COVID-19" Current Oncology 30, no. 4: 3886-3900. https://doi.org/10.3390/curroncol30040294
APA StyleMaculaitis, M. C., Liu, X., Berk, A., Massa, A., Weiss, M. C., Kurosky, S. K., Li, B., & McRoy, L. (2023). Psychosocial Wellbeing among Patients with Breast Cancer during COVID-19. Current Oncology, 30(4), 3886-3900. https://doi.org/10.3390/curroncol30040294





