Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) Is Associated with Cervical Stromal Involvement in Endometrial Cancer Patients: A Cross-Sectional Study in South China
Abstract
1. Introduction
2. Materials and Methods
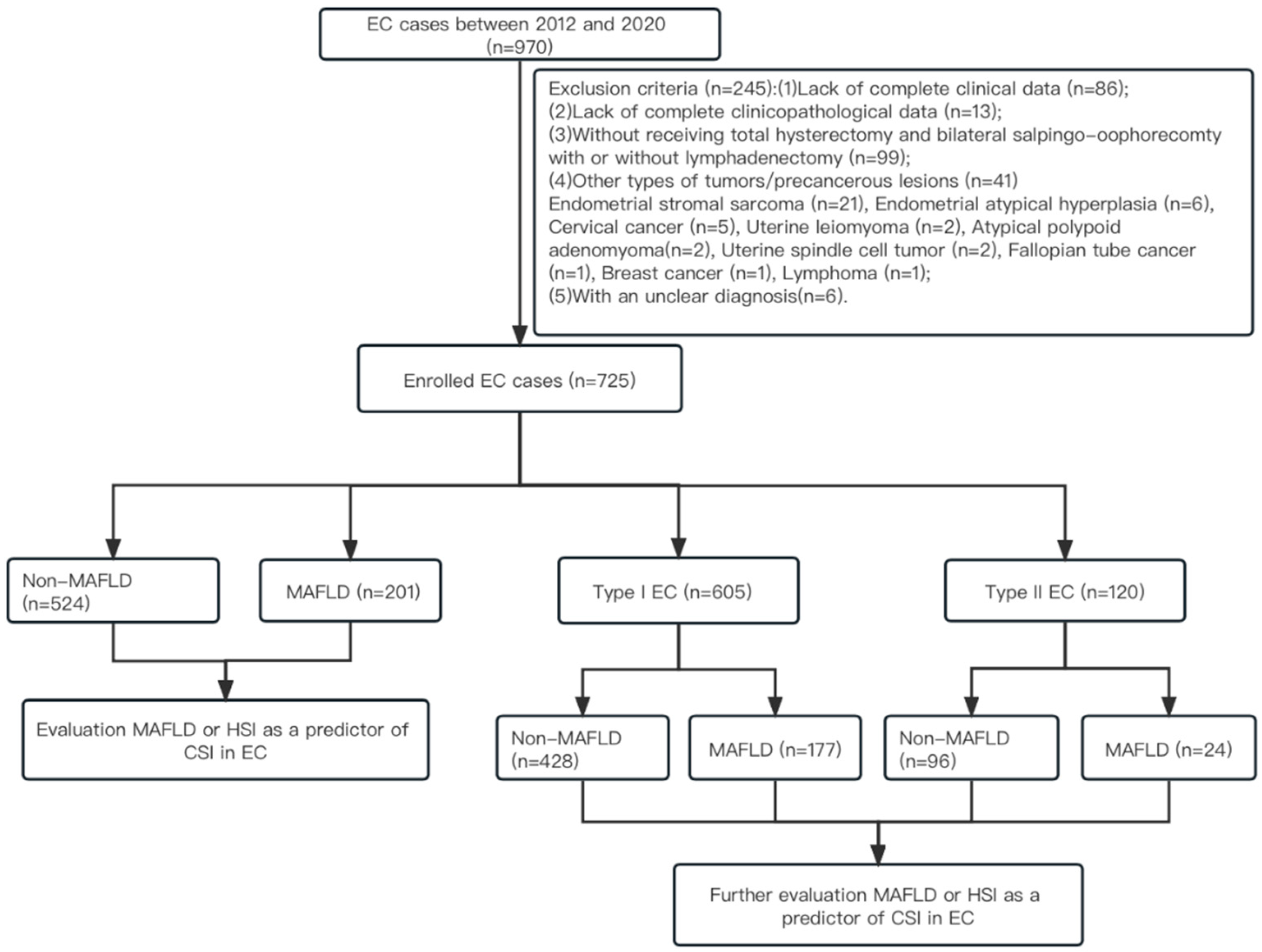
2.1. Study Population and Selection of Patients for Analysis
2.2. Data Collection
2.3. Diagnosis of MAFLD
2.4. Definition of Hepatic Steatosis Index (HSI)
2.5. Subgroup Analysis
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Subjects
3.2. Survival Analyses
3.3. Independent Risk Factors for CSI in EC Patients
3.4. Independent Risk Factors for CSI in Type I/Type II EC Patients
3.5. Relationship between CSI and HSI in Type I EC Patients without T2DM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Saed, L.; Varse, F.; Baradaran, H.R.; Moradi, Y.; Khateri, S.; Friberg, E.; Khazaei, Z.; Gharahjeh, S.; Tehrani, S.; Sioofy-Khojine, A.B.; et al. The effect of diabetes on the risk of endometrial Cancer: An updated a systematic review and meta-analysis. BMC Cancer 2019, 19, 527. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Aune, D.; Rosenblatt, D.A.N.; Chan, D.S.M.; Vingeliene, S.; Abar, L.; Vieira, A.R.; Greenwood, D.C.; Bandera, E.V.; Norat, T. Anthropometric factors and endometrial cancer risk: A systematic review and dose-response meta-analysis of prospective studies. Ann. Oncol. 2015, 26, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef]
- Arem, H.; Chlebowski, R.; Stefanick, M.L.; Anderson, G.; Wactawski-Wende, J.; Sims, S.; Gunter, M.J.; Irwin, M.L. Body mass index, physical activity, and survival after endometrial cancer diagnosis: Results from the Women’s Health Initiative. Gynecol. Oncol. 2013, 128, 181–186. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Umene, K.; Yanokura, M.; Banno, K.; Irie, H.; Adachi, M.; Iida, M.; Nakamura, K.; Nogami, Y.; Masuda, K.; Kobayashi, Y.; et al. Aurora kinase A has a significant role as a therapeutic target and clinical biomarker in endometrial cancer. Int. J. Oncol. 2015, 46, 1498–1506. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Lewin, S.N.; Herzog, T.J.; Medel, N.I.B.; Deutsch, I.; Burke, W.M.; Sun, X.M.; Wright, J.D. Comparative Performance of the 2009 International Federation of Gynecology and Obstetrics’ Staging System for Uterine Corpus Cancer. Obstet. Gynecol. 2010, 116, 1141–1149. [Google Scholar] [CrossRef]
- Fotiou, S.; Vlahos, N.; Kondi-Pafiti, A.; Zarganis, P.; Papakonstantinou, K.; Creatsas, G. Intraoperative gross assessment of myometrial invasion and cervical involvement in endometrial cancer: Role of tumor grade and size. Gynecol. Oncol. 2009, 112, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; et al. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 170–199. [Google Scholar] [CrossRef] [PubMed]
- Giannini, A.; Bogani, G.; Vizza, E.; Chiantera, V.; Laganà, A.S.; Muzii, L.; Salerno, M.G.; Caserta, D.; D’Oria, O. Advances on Prevention and Screening of Gynecologic Tumors: Are We Stepping Forward? Healthcare 2022, 10, 1605. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.S.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Zhang, X.F.; Li, R.Y.; Chen, Y.J.; Dai, Y.N.; Chen, L.; Qin, L.; Cheng, X.B.; Lu, Y. The Role of Thyroid Hormones and Autoantibodies in Metabolic Dysfunction Associated Fatty Liver Disease: TgAb May Be a Potential Protective Factor. Front Endocrinol. 2020, 11, 598836. [Google Scholar] [CrossRef]
- Marchesini, G.; Brizi, M.; Bianchi, G.; Tomassetti, S.; Bugianesi, E.; Lenzi, M.; McCullough, A.J.; Natale, S.; Forlani, G.; Melchionda, N. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes 2001, 50, 1844–1850. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Lin, C.Q.; Suo, C.; Zhao, R.J.; Jin, L.; Zhang, T.J.; Chen, X.D. Metabolic dysfunction-associated fatty liver disease and the risk of 24 specific cancers. Metab.-Clin. Exp. 2022, 127, 154955. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: A meta-analysis of observational cohort studies. Gut 2022, 71, 778–788. [Google Scholar] [CrossRef]
- Allen, A.M.; Hicks, S.B.; Mara, K.C.; Larson, J.J.; Therneau, T.M. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity—A longitudinal cohort study. J. Hepatol. 2019, 71, 1229–1236. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Chen, S.; Wang, Y.; Cao, L.; Liao, W.; Sun, Y.; Wang, X.; Zheng, Y.; Wu, S.; et al. Associations Between Nonalcoholic Fatty Liver Disease and Cancers in a Large Cohort in China. Clin. Gastroenterol. Hepatol. 2021, 19, 788–796.e4. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Harmsen, S.; St Sauver, J.L.; Charatcharoenwitthaya, P.; Enders, F.B.; Therneau, T.; Angulo, P. Nonalcoholic Fatty Liver Disease Increases Risk of Death Among Patients With Diabetes: A Community-Based Cohort Study. Am. J. Gastroenterol. 2010, 105, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, V.K.; Li, Y.; Gupta, K.; Minacapelli, C.D.; Bhurwal, A.; Catalano, C.; Elsaid, M.I. Bariatric Surgery Reduces Cancer Risk in Adults With Nonalcoholic Fatty Liver Disease and Severe Obesity. Gastroenterology 2021, 161, 171–184.e10. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Pathophysiological Molecular Mechanisms of Obesity: A Link between MAFLD and NASH with Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 11629. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Chung, J.; Park, H.S.; Kim, Y.J.; Yu, M.H.; Park, S.; Jung, S.I. Association of Hepatic Steatosis Index with Nonalcoholic Fatty Liver Disease Diagnosed by Non-Enhanced CT in a Screening Population. Diagnostics 2021, 11, 2168. [Google Scholar] [CrossRef]
- Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [CrossRef]
- Matsuo, K.; Opper, N.R.; Ciccone, M.A.; Garcia, J.; Tierney, K.E.; Baba, T.; Muderspach, L.I.; Roman, L.D. Time interval between endometrial biopsy and surgical staging for type I endometrial cancer: Association between tumor characteristics and survival outcome. Obstet. Gynecol. 2015, 125, 424–433. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1991. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, X.; Li, G.; Wong, G.L.; Wong, V.W. Epidemiology and Clinical Outcomes of Metabolic (Dysfunction)-associated Fatty Liver Disease. J. Clin. Transl. Hepatol. 2021, 9, 972–982. [Google Scholar] [CrossRef]
- Toprak, S.; Sahin, E.A.; Sahin, H.; Tohma, Y.A.; Yilmaz, E.; Meydanli, M.M. Risk factors for cervical stromal involvement in endometrioid-type endometrial cancer. Int. J. Gynaecol. Obstet. 2021, 153, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Vanni, E.; Marengo, A.; Mezzabotta, L.; Bugianesi, E. Systemic Complications of Nonalcoholic Fatty Liver Disease: When the Liver Is Not an Innocent Bystander. Semin. Liver Dis. 2015, 35, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Diehl, A.M. NAFLD and extrahepatic cancers: Have a look at the colon. Gut 2011, 60, 745–746. [Google Scholar] [CrossRef] [PubMed]
- Nseir, W.; Abu-Rahmeh, Z.; Tsipis, A.; Mograbi, J.; Mahamid, M. Relationship between Non-Alcoholic Fatty Liver Disease and Breast Cancer. Israel Med. Assoc. J. 2017, 19, 242–245. [Google Scholar]
- Kwak, M.S.; Yim, J.Y.; Yi, A.; Chung, G.E.; Yang, J.I.; Kim, D.; Kim, J.S.; Noh, D.Y. Nonalcoholic fatty liver disease is associated with breast cancer in nonobese women. Dig. Liver Dis. 2019, 51, 1030–1035. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, D.H.; Han, K.D.; Yoon, H.; Shin, C.M.; Park, Y.S.; Kim, N. Is nonalcoholic fatty liver disease associated with the development of prostate cancer? A nationwide study with 10,516,985 Korean men. PLoS ONE 2018, 13, e0201308. [Google Scholar] [CrossRef]
- Moeini, A.; Machida, H.; Takiuchi, T.; Blake, E.A.; Hom, M.S.; Miki, T.; Matsuo, O.; Matsuo, K. Association of Nonalcoholic Fatty Liver Disease and Venous Thromboembolism in Women With Endometrial Cancer. Clin. Appl. Thromb. Hemost. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Matsuo, K.; Gualtieri, M.R.; Cahoon, S.S.; Jung, C.E.; Paulson, R.J.; Shoupe, D.; Muderspach, L.I.; Wakatsuki, A.; Wright, J.D.; Roman, L.D. Surgical menopause and increased risk of nonalcoholic fatty liver disease in endometrial cancer. Menopause 2016, 23, 189–196. [Google Scholar] [CrossRef]
- Onstad, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef]
- Ko, E.M.; Walter, P.; Clark, L.; Jackson, A.; Franasiak, J.; Bolac, C.; Havrilesky, L.; Secord, A.A.; Moore, D.T.; Gehrig, P.A.; et al. The complex triad of obesity, diabetes and race in Type I and II endometrial cancers: Prevalence and prognostic significance. Gynecol. Oncol. 2014, 133, 28–32. [Google Scholar] [CrossRef]
- Kasamatsu, T.; Onda, T.; Sawada, M.; Kato, T.; Ikeda, S. Radical hysterectomy for FIGO stage IIB cervical cancer: Clinicopathological characteristics and prognostic evaluation. Gynecol. Oncol. 2009, 114, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sartori, E.; Gadducci, A.; Landoni, F.; Lissoni, A.; Maggino, T.; Zola, P.; Zanagnolo, V. Clinical behavior of 203 stage II endometrial cancer cases: The impact of primary surgical approach and of adjuvant radiation therapy. Int. J. Gynecol. Cancer 2001, 11, 430–437. [Google Scholar] [CrossRef]
- Dogan Altunpulluk, M.; Kir, G.; Topal, C.S.; Cetiner, H.; Gocmen, A. The association of the microcystic, elongated and fragmented (MELF) invasion pattern in endometrial carcinomas with deep myometrial invasion, lymphovascular space invasion and lymph node metastasis. J. Obstet. Gynaecol. 2015, 35, 397–402. [Google Scholar] [CrossRef]
- Yura, Y.; Tauchi, K.; Koshiyama, M.; Konishi, I.; Yura, S.; Mori, T.; Matsushita, K.; Hayashi, M.; Yoshida, M. Parametrial involvement in endometrial carcinomas: Its incidence and correlation with other histological parameters. Gynecol. Oncol. 1996, 63, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Schmandt, R.E.; Iglesias, D.A.; Co, N.N.; Lu, K.H. Understanding obesity and endometrial cancer risk: Opportunities for prevention. Am. J. Obstet. Gynecol. 2011, 205, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Sanna, C.; Rosso, C.; Marietti, M.; Bugianesi, E. Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. Int. J. Mol. Sci. 2016, 17, 717. [Google Scholar] [CrossRef]
- Choi, D.S.; Kim, H.J.; Yoon, J.H.; Yoo, S.C.; Jo, H.; Lee, S.Y.; Min, C.K.; Ryu, H.S. Endometrial cancer invasion depends on cancer-derived tumor necrosis factor-alpha and stromal derived hepatocyte growth factor. Int. J. Cancer 2009, 124, 2528–2538. [Google Scholar] [CrossRef]
- Chu, Y.; Wang, Y.; Peng, W.; Xu, L.; Liu, M.; Li, J.; Hu, X.; Li, Y.; Zuo, J.; Ye, Y. STAT3 activation by IL-6 from adipose-derived stem cells promotes endometrial carcinoma proliferation and metastasis. Biochem. Biophys. Res. Commun. 2018, 500, 626–631. [Google Scholar] [CrossRef]
- Jaffe, T.; Schwartz, B. Leptin promotes motility and invasiveness in human colon cancer cells by activating multiple signal-transduction pathways. Int. J. Cancer 2008, 123, 2543–2556. [Google Scholar] [CrossRef]
- Gilbert, C.A.; Slingerland, J.M. Cytokines, obesity, and cancer: New insights on mechanisms linking obesity to cancer risk and progression. Annu. Rev. Med. 2013, 64, 45–57. [Google Scholar] [CrossRef]
- Mu, N.; Zhu, Y.; Wang, Y.; Zhang, H.; Xue, F. Insulin resistance: A significant risk factor of endometrial cancer. Gynecol. Oncol. 2012, 125, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Friberg, E.; Orsini, N.; Mantzoros, C.S.; Wolk, A. Diabetes mellitus and risk of endometrial cancer: A meta-analysis. Diabetologia 2007, 50, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, O.; Corrado, G.; Laganà, A.S.; Chiantera, V.; Vizza, E.; Giannini, A. New Advances in Cervical Cancer: From Bench to Bedside. Int. J. Environ. Res. Public Health 2022, 19, 7094. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Delair, D.F.; Bean, S.M.; Abu-Rustum, N.R.; Soslow, R.A. Evolving Roles of Histologic Evaluation and Molecular/Genomic Profiling in the Management of Endometrial Cancer. J. Natl. Compr. Cancer Netw. 2018, 16, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Chiappa, V.; Lopez, S.; Salvatore, C.; Interlenghi, M.; D’Oria, O.; Giannini, A.; Maggiore, U.L.R.; Chiarello, G.; Palladino, S.; et al. Radiomics and Molecular Classification in Endometrial Cancer (The ROME Study): A Step Forward to a Simplified Precision Medicine. Healthcare 2022, 10, 2464. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | CSI (−) | CSI (+) | p |
|---|---|---|---|
| n | 619 | 106 | |
| Age, median (IQR) | 54 (49, 59) | 53.5 (49, 58.8) | 0.612 |
| BMI, median (IQR) | 24.1 (22.2, 26.6) | 23.7 (22.0, 26.3) | 0.448 |
| Menopause status, n (%) | 0.265 | ||
| Premenopausal | 285 (46%) | 55 (51.9%) | |
| Postmenopausal | 334 (54%) | 51 (48.1%) | |
| History of diabetes, n (%) | 0.608 | ||
| No | 498 (80.5%) | 83 (78.3%) | |
| Yes | 121 (19.5%) | 23 (21.7%) | |
| History of hypertension, n (%) | 0.528 | ||
| No | 389 (62.8%) | 83 (64.8%) | |
| Yes | 230 (37.2%) | 45 (35.2%) | |
| FIGO stage, n (%) | <0.001 * | ||
| I | 576 (93.1%) | 0 (0%) | |
| II–IV | 43 (6.9%) | 106 (100%) | |
| Histologic grade, n (%) | <0.001 * | ||
| G1 | 322 (52.0%) | 29 (27.4%) | |
| G2 | 192 (31.0%) | 40 (37.7%) | |
| G3 | 45 (7.3%) | 17 (16.0%) | |
| ER expression, n (%) | <0.001 * | ||
| Low | 57 (9.2%) | 31 (29.2%) | |
| High | 478 (77.2%) | 67 (63.2%) | |
| PR expression, n (%) | <0.001 * | ||
| Low | 89 (14.4%) | 37 (34.9%) | |
| High | 446 72.1%) | 61 (57.5%) | |
| Ki67 %, median (IQR) | 50 (30, 70) | 40 (30, 60) | 0.013 * |
| Characteristic | CSI (−) | CSI (+) | p |
|---|---|---|---|
| n | 619 | 106 | |
| FPG, median (IQR) | 5.23 (4.85, 5.85) | 5.18 (4.80, 5.88) | 0.586 |
| TG, median (IQR) | 1.36 (1.00, 1.93) | 1.31 (0.95, 1.73) | 0.455 |
| HDL, median (IQR) | 1.31 (1.13, 1.54) | 1.3 (1.11, 1.53) | 0.526 |
| ALT, median (IQR) | 19.60 (13.40, 27.54) | 15.95 (12.40, 24.50) | 0.017 * |
| AST, median (IQR) | 18.80 (15.30, 24.00) | 18.20 (14.25, 24.00) | 0.360 |
| HSI, median (IQR) | 35.35 (31.69, 39.24) | 34.18 (30.82, 37.79) | 0.058 |
| Ultrasound | 0.043* | ||
| Non-fatty liver | 399 (64.5%) | 79 (74.5%) | |
| Fatty liver | 220 (35.5%) | 27 (25.5%) | |
| MAFLD, n (%) | 0.027 * | ||
| Non-MAFLD | 438 (70.8%) | 86 (81.1%) | |
| MAFLD | 181 (29.2%) | 20 (18.9%) |
| Characteristic | Type I EC | Type II EC | ||||
|---|---|---|---|---|---|---|
| CSI (−) | CSI (+) | p-Value | CSI (−) | CSI (+) | p-Value | |
| n | 532 | 73 | 87 | 33 | ||
| FPG | 5.25 (4.85, 5.87) | 5.08 (4.74, 5.96) | 0.251 | 5.17 (4.82, 5.66) | 5.26 (5.00, 5.78) | 0.313 |
| TG | 1.40 (1.04, 1.96) | 1.23 (0.91, 1.73) | 0.139 | 1.10 (0.81, 1.80) | 1.42 (1.08, 1.74) | 0.062 |
| HDL | 1.31 (1.12, 1.54) | 1.32 (1.20, 1.57) | 0.362 | 1.39 ± 0.30 | 1.22 ± 0.32 | 0.010 * |
| ALT | 19.73 (13.53, 27.85) | 16.50 (12.94, 25.20) | 0.088 | 18.00 (12.75, 26.00) | 15.5 (11.26, 21.00) | 0.210 |
| AST | 18.70 (15.20, 23.93) | 18.50 (14.20, 24.00) | 0.624 | 19.4 (16.15, 25.85) | 17.50 (14.40, 22.00) | 0.273 |
| HSI | 35.65 (31.97, 39.88) | 33.66 (30.60, 38.40) | 0.049 * | 33.70 ± 4.72 | 34.42 ± 4.52 | 0.451 |
| Ultrasound | 0.047 * | 0.779 | ||||
| Non-fatty liver | 338 (63.5%) | 55 (75.3%) | 61 (70.1%) | 24 (72.7%) | ||
| Fatty liver | 194 (36.5%) | 18 (24.7%) | 26 (29.9%) | 9 (27.3%) | ||
| MAFLD, n (%) | 0.022 * | 0.838 | ||||
| Non-MAFLD | 368 (69.2%) | 60 (82.2%) | 70 (80.5%) | 26 (78.8%) | ||
| MAFLD | 164 (30.8%) | 13 (17.8%) | 17 (19.5%) | 7 (21.2%) | ||
| Ki67 | 40 (20, 50) | 40 (30, 60) | 0.210 | 60 (40, 80) | 55 (50, 72.5) | 0.813 |
| Characteristics | Total (N) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | ||
| MAFLD | 484 | ||||
| Non-MAFLD | 362 | Reference | Reference | ||
| MAFLD | 122 | 2.572 (1.752–3.392) | 0.024 * | 2.718 (1.089–6.783) | 0.032 * |
| HSI | 484 | 1.079 (1.020–1.139) | 0.012 * | 0.873 (0.778–0.979) | 0.020 * |
| Ultrasound, n (%) | 484 | ||||
| Non-fatty liver | 327 | Reference | |||
| Fatty liver | 157 | 1.879 (1.210–2.548) | 0.065 | ||
| Age | 484 | 1.009 (0.976–1.041) | 0.599 | ||
| BMI | 484 | 1.058 (0.973–1.143) | 0.190 | ||
| Histologic grade | 468 | ||||
| G1 | 170 | Reference | |||
| G2 | 298 | 2.251 (1.670–2.833) | 0.006 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Chen, C.; Jiang, T.; Ma, J.; Huang, L.; Huang, L.; Lei, H.; Tong, Y.; Huang, G.; Mao, X.; et al. Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) Is Associated with Cervical Stromal Involvement in Endometrial Cancer Patients: A Cross-Sectional Study in South China. Curr. Oncol. 2023, 30, 3787-3799. https://doi.org/10.3390/curroncol30040287
Lin X, Chen C, Jiang T, Ma J, Huang L, Huang L, Lei H, Tong Y, Huang G, Mao X, et al. Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) Is Associated with Cervical Stromal Involvement in Endometrial Cancer Patients: A Cross-Sectional Study in South China. Current Oncology. 2023; 30(4):3787-3799. https://doi.org/10.3390/curroncol30040287
Chicago/Turabian StyleLin, Xite, Chunxia Chen, Tingting Jiang, Jincheng Ma, Lixiang Huang, Leyi Huang, Huifang Lei, Yao Tong, Guanxiang Huang, Xiaodan Mao, and et al. 2023. "Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) Is Associated with Cervical Stromal Involvement in Endometrial Cancer Patients: A Cross-Sectional Study in South China" Current Oncology 30, no. 4: 3787-3799. https://doi.org/10.3390/curroncol30040287
APA StyleLin, X., Chen, C., Jiang, T., Ma, J., Huang, L., Huang, L., Lei, H., Tong, Y., Huang, G., Mao, X., & Sun, P. (2023). Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) Is Associated with Cervical Stromal Involvement in Endometrial Cancer Patients: A Cross-Sectional Study in South China. Current Oncology, 30(4), 3787-3799. https://doi.org/10.3390/curroncol30040287




