Integrating Tobacco Use Assessment and Treatment in the Oncology Setting: Quality Improvement Results from the Georgetown Lombardi Smoking Treatment and Recovery Program
Abstract
1. Introduction
1.1. Implementation Strategies
1.1.1. Opt-Out Identification of Eligible Patients
1.1.2. Centralized Delivery of Tobacco Treatment
1.1.3. Staff Training and Audit/Feedback
2. Materials and Methods
2.1. Overview of Program Development
2.2. Implementation Strategies and Teams
2.3. The STAR Clinical Program
2.3.1. Patient-Level Procedures
2.3.2. Patient-Level Measures
2.3.3. Patient-Level Data Analyses
3. Results
3.1. Implementation Results
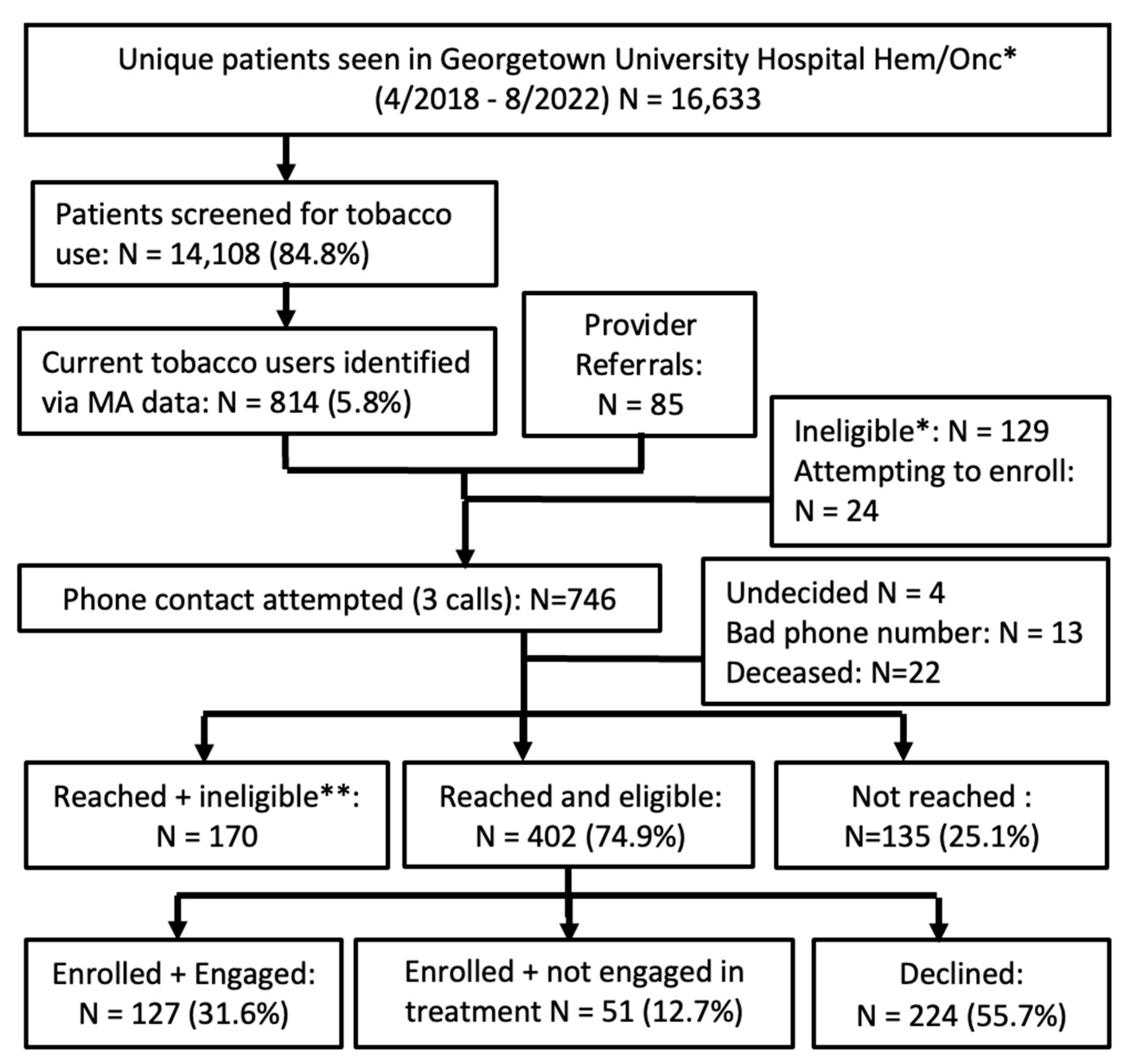
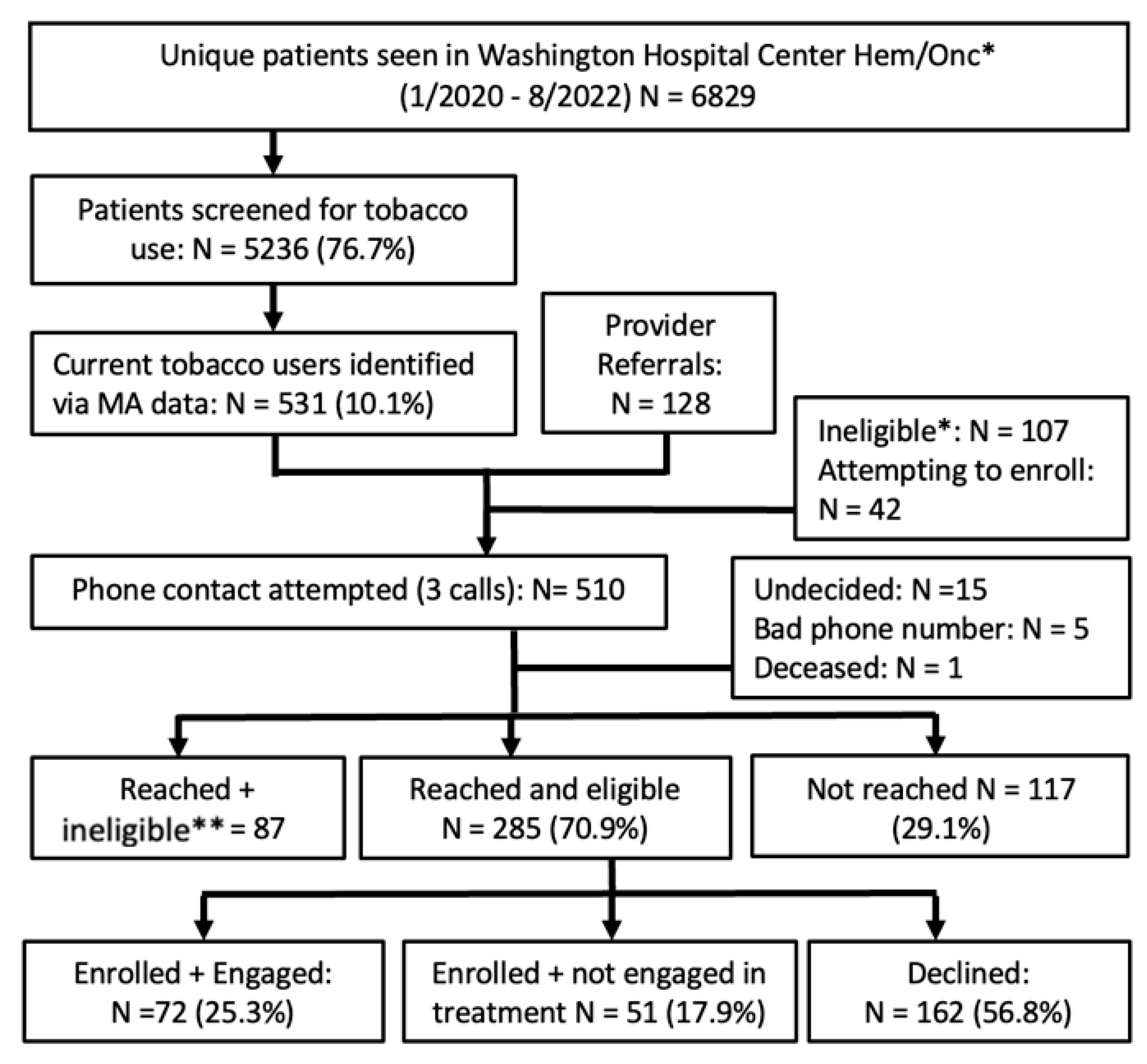
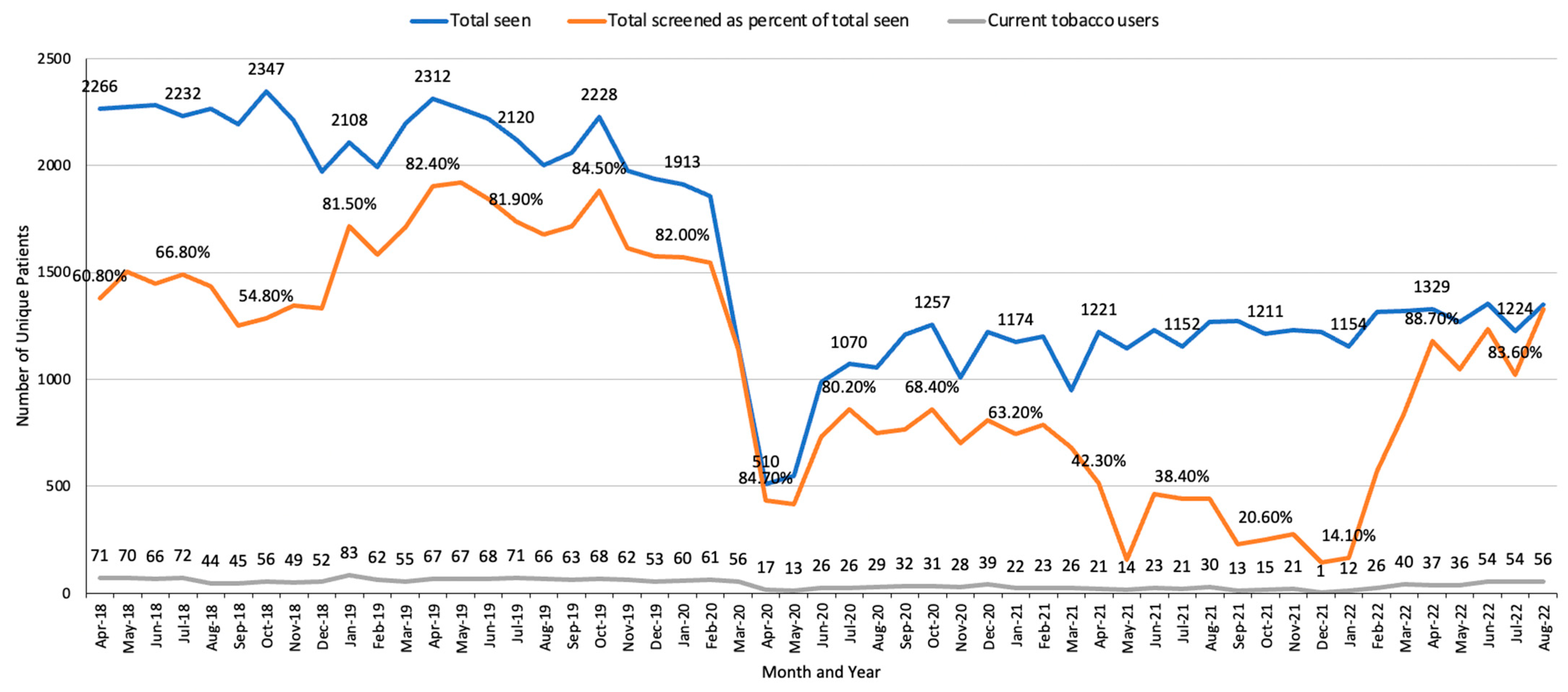
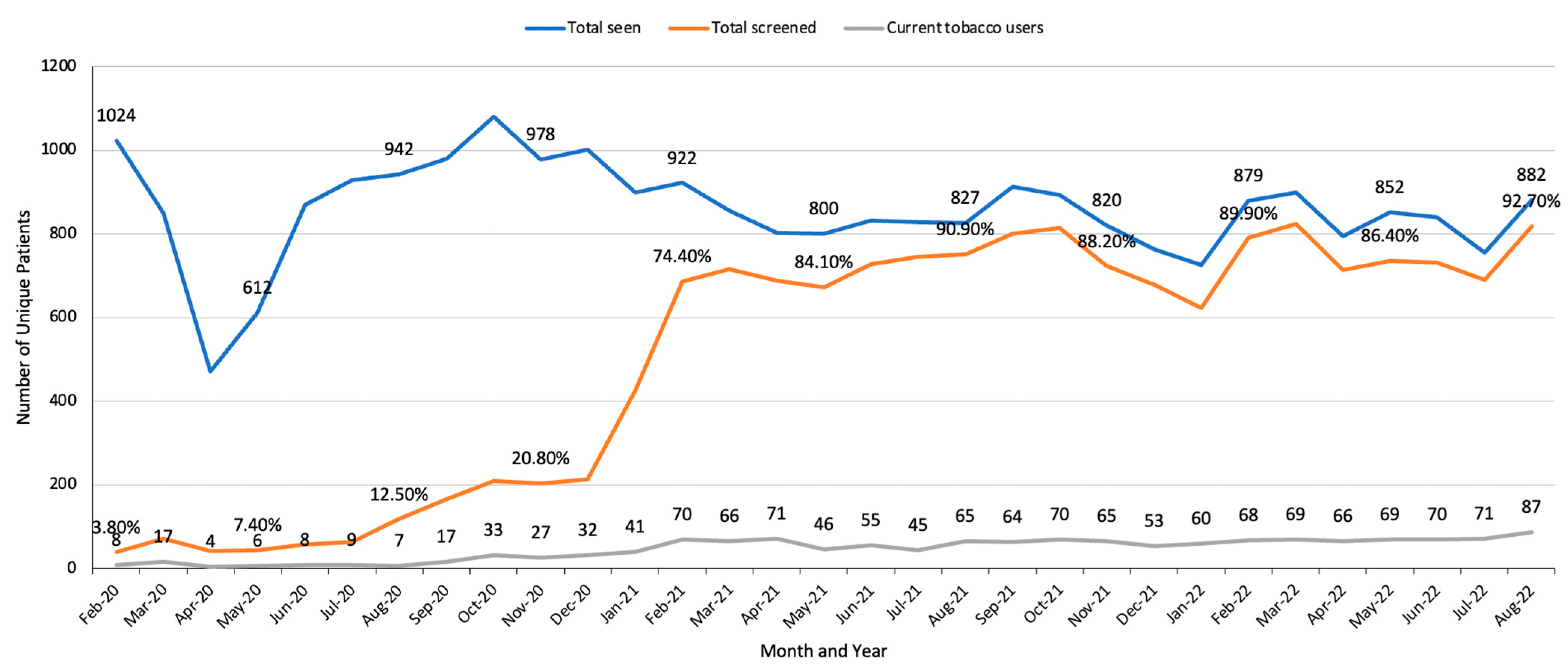
3.2. Patient-Level Results
3.2.1. MedStar Georgetown University Hospital (MGUH)
3.2.2. Baseline Predictors of Enrollment
3.2.3. Baseline Predictors of Treatment Engagement
3.2.4. Baseline Predictors of Abstinence at Six Months Post-Enrollment
3.2.5. MedStar Washington Hospital Center (MWHC)
3.2.6. Baseline Predictors of Enrollment and Engagement (Table 5)
| Not Enrolled (n = 279) | Enrolled (n = 123) | Enrolled/Not Engaged 1 (n = 51) | Engaged (n = 72) | ||
|---|---|---|---|---|---|
| Demographic Characteristics | |||||
| Age | Mean (SD) | 60.73 (12.1) | 60.28 (9.8) | 59.59 (10.6) | 60.76 (9.2) |
| ≤60 | N (%) | 112 (40.1) * | 61 (49.6) | 26 (51.0) | 35 (48.6) |
| ≥61 | N (%) | 167 (59.9) | 62 (50.4) | 25 (49.0) | 37 (51.4) |
| Sex: Female | N (%) | 155 (55.6) *** | 92 (74.8) | 38 (74.5) | 54 (75.0) |
| Race | |||||
| African American or Black | N (%) | 244 (88.4) | 116 (94.3) | 48 (94.1) | 68 (94.4) |
| White | N (%) | 28 (10.1) | 5 (4.1) | 2 (3.9) | 3 (4.2) |
| Other (Asian, NHPI, Multirace, Other) | N (%) | 4 (1.4) | 2 (1.6) | 1 (2.0) | 1 (1.4) |
| Missing | N | 3 (-) | 0 (-) | 0 (-) | 0 (-) |
| Race | |||||
| African American or Black | N (%) | 244 (87.5) * | 116 (94.3) | 48 (94.1) | 68 (94.4) |
| White or other (Asian, NHPI 2, Multirace, Other) | N (%) | 32 (11.5) | 7 (5.7) | 3 (5.9) | 4 (5.6) |
| Missing | N | 4 (-) | 0 (-) | 0 (-) | 0 (-) |
| Ethnicity | |||||
| Hispanic or Latino | N (%) | 4 (1.5) | 1 (0.8) | 0 (0.0) | 1 (1.4) |
| Non-Hispanic | 268 (98.5) | 120 (99.2) | 49 (100.0) | 71 (98.6) | |
| Missing/unknown | 7 (-) | 2 (-) | 2 (-) | 0 (-) | |
| Insurance Type | |||||
| Private | N (%) | 69 (24.7) | 29 (23.6) | 7 (13.7) | 22 (30.6) |
| Medicare or military | N (%) | 136 (48.7) | 50 (40.7) | 23 (45.1) | 27 (37.5) |
| Medicaid or other government plan | N (%) | 74 (26.5) | 44 (35.8) | 21 (41.2) | 23 (31.9) |
| Clinical Characteristics | |||||
| Tobacco-Related Cancer 3 | |||||
| Tobacco-related cancer | N (%) | 76 (27.2) | 33 (26.8) | 11 (21.6) | 22 (30.6) |
| Non-tobacco-related cancer | N (%) | 139 (49.8) | 61 (49.6) | 26 (51.0) | 35 (48.6) |
| N/A: hematologic or diagnostic or screening | N (%) | 64 (22.9) | 29 (23.6) | 14 (27.5) | 15 (20.8) |
| Diagnosis | |||||
| Cancer stage 0, I, II | N (%) | 88 (34.8) * | 54 (46.6) | 24 (50.0) | 30 (44.1) |
| Cancer stage III or IV | N (%) | 103 (40.7) | 35 (30.2) | 12 (25.0) | 23 (33.8) |
| Non-cancer hematological diagnosis | N (%) | 62 (24.5) | 27 (23.3) | 12 (25.0) | 15 (22.1) |
| N/A: diagnostic or cancer screening | N | 2 (-) | 2 (-) | 2 (-) | 0 (-) |
| Missing/unknown | N | 24 (-) | 5 (-) | 1 (-) | 4 (-) |
| Method Patient Identified | |||||
| Clinician referral | N (%) | 39 (14.0) *** | 63 (51.2) | 28 (54.9) | 37 (51.4) |
| MA assessment | N (%) | 240 (86.0) | 60 (48.8) | 23 (45.1) | 35 (48.6) |
| Psychological Distress 4 (1–10, high = more) | |||||
| ≤6 | N (%) | -- | -- | 28 (54.9) * | 51 (70.8) |
| ≥7 | N (%) | -- | -- | 23 (45.1) | 21 (29.2) |
| Tobacco-Related Characteristics | |||||
| Smoking Status (MA Assessment) | |||||
| Smoked a cigarette today | N (%) | 195 (73.9) ** | 103 (83.7) | 45 (88.2) | 58 (80.6) |
| Smoked 1–30 days ago | N (%) | 69 (26.1) | 20 (16.3) | 6 (11.8) | 14 (19.4) |
| Used other nicotine/tobacco (not cigarettes) | 15 (-) | 0 (-) | 0 (-) | 0 (-) | |
| Cigarettes per day (number) | Mean (SD) | -- | -- | 11.7 (10.2) * | 9.1 (6.1) |
| Median | -- | -- | 10.0 | 7.0 | |
| Cigarettes per day (categorical) | |||||
| ≤5 | N (%) | -- | -- | 13 (25.5) | 25 (35.2) |
| 6 to 10 | N (%) | -- | -- | 19 (37.3) | 24 (33.8) |
| ≥11 | N (%) | -- | -- | 19 (37.3) | 22 (31.0) |
| Missing | 0 (-) | 1 (-) | |||
| Pack-years | Mean (SD) | -- | -- | 29.8 (19.0) | 37.3 (31.0) |
| Median | -- | -- | 23.0 | 32.4 | |
| Time to first cigarette after waking | |||||
| <30 min | (N, %) | -- | -- | 34 (66.7) | 46 (65.7) |
| 31–60 min | (N, %) | -- | -- | 8 (15.7) | 12 (17.1) |
| 61+ min | (N, %) | -- | -- | 9 (17.6) | 12 (17.1) |
| Missing/refused | N | -- | -- | 0 (-) | 2 (-) |
| Readiness to Quit | |||||
| Not ready to quit (≥6 months) | (N, %) | -- | -- | 21 (41.2) | 24 (34.3) |
| Ready to quit <30 days | (N, %) | -- | -- | 29 (56.9) | 45 (64.3) |
| Already quit (<30 days) | (N, %) | -- | -- | 1 (2.0) | 1 (1.4) |
| Missing/refused | N | -- | -- | 0 (-) | 2 (-) |
| Lives With Person Who Smokes | |||||
| No (or lives alone) | (N, %) | -- | -- | 34 (66.7) | 51 (70.8) |
| Yes | (N, %) | -- | -- | 17 (33.3) | 21 (29.2) |
| Treatment Engagement | |||||
| Sessions Completed (Number) | Mean (SD) | -- | -- | n/a | 2.04 (1.1) |
| Median | n/a | 2.0 | |||
| Sessions Completed (Categorical) | |||||
| 0/1 counseling sessions | N (%) | -- | -- | n/a | 30 (41.7) |
| 2+ sessions | N (%) | -- | -- | n/a | 42 (58.3) |
| STAR Prescription or NRT | |||||
| Yes | N (%) | -- | -- | n/a | 43 (59.7) |
3.2.7. Predictors of Enrollment at MGUH and MWHC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallaway, M.S.; Glover-Kudon, R.; Momin, B.; Puckett, M.; Lunsford, N.B.; Ragan, K.R.; Rohan, E.A.; Babb, S. Smoking cessation attitudes and practices among cancer survivors–United States, 2015. J. Cancer Surviv. 2019, 13, 66–74. [Google Scholar] [CrossRef]
- Jose, T.; Schroeder, D.R.; Warner, D.O. Changes in Cigarette Smoking Behavior in Cancer Survivors During Diagnosis and Treatment. Nicotine Tob. Res. 2022, 24, 1581–1588. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General; Printed with Corrections, January 2014; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2014.
- U.S. Department of Health and Human Services. Smoking Cessation. A Report of the Surgeon General; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2020.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am. J. Prev. Med. 2008, 35, 158–176. [Google Scholar] [CrossRef]
- Shields, P.G.; Herbst, R.S.; Arenberg, D.; Benowitz, N.L.; Bierut, L.; Luckart, J.B.; Cinciripini, P.; Collins, B.; David, S.; Davis, J.; et al. Smoking Cessation, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 1430–1468. [Google Scholar] [CrossRef]
- Croyle, R.; Morgan, G.; Fiore, M. Addressing a Core Gap in Cancer Care: The NCI Cancer MoonshotSM Initiative to Help Oncology Patients Stop Smoking. N. Engl. J. Med. 2019, 380, 512–515. [Google Scholar] [CrossRef]
- Taylor, K.L.; Fallon, S.; Subramaniam, D.; Davis, K.; To, C.; Lobo, T.; Tercyak, K.P.; Friberg, J.; Tynan, M.; Russell, E.; et al. Implementation of the Smoking Treatment and Recovery (STAR) program: Healthy cancer survivorship through integrated tobacco control. J. Cancer Surviv. 2020, 14, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, K.P.; Hauser, L.; Clark, C.; Odumosu, O.; Dahl, N.; Peregoy, J.; Sheffield, C.W.; Klesges, R.C.; Anderson, R.T. An Evaluation of the Process and Quality Improvement Measures of the University of Virginia Cancer Center Tobacco Treatment Program. Int. J. Environ. Res. Public Health 2020, 17, 4707. [Google Scholar] [CrossRef]
- Meyer, C.; Mitra, S.; Ruebush, E.; Sisler, L.; Wang, K.; Goldstein, A.O. A Lean Quality Improvement Initiative to Enhance Tobacco Use Treatment in a Cancer Hospital. Int. J. Environ. Res. Public Health 2020, 17, 2165. [Google Scholar] [CrossRef]
- D’Angelo, H.; Ramsey, A.T.; Rolland, B.; Chen, L.-S.; Bernstein, S.L.; Fucito, L.M.; Webb Hooper, M.; Adsit, R.; Pauk, D.; Rosenblum, M.S.; et al. Pragmatic Application of the RE-AIM Framework to Evaluate the Implementation of Tobacco Cessation Programs Within NCI-Designated Cancer Centers. Front. Public Health 2020, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Tong, E.K.; Wolf, T.; Cooke, D.T.; Fairman, N.; Chen, M.S. The Emergence of a Sustainable Tobacco Treatment Program across the Cancer Care Continuum: A Systems Approach for Implementation at the University of California Davis Comprehensive Cancer Center. Int. J. Environ. Res. Public Health 2020, 17, 3241. [Google Scholar] [CrossRef]
- D’Angelo, H.; Rolland, B.; Adsit, R.; Baker, T.B.; Rosenblum, M.; Pauk, D.; Morgan, G.D.; Fiore, M.C. Tobacco Treatment Program Implementation at NCI Cancer Centers: Progress of the NCI Cancer Moonshot-Funded Cancer Center Cessation Initiative. Cancer Prev. Res. 2019, 12, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Alton, D.; Eng, L.; Lu, L.; Song, Y.; Su, J.; Farzanfar, D.; Mohan, R.; Krys, O.; Mattina, K.; Harper, C.; et al. Perceptions of Continued Smoking and Smoking Cessation Among Patients With Cancer. J. Oncol. Pract. 2018, 14, e269–e279. [Google Scholar] [CrossRef] [PubMed]
- Arifin, A.J.; McCracken, L.C.; Nesbitt, S.; Warner, A.; Dinniwell, R.E.; Palma, D.A.; Louie, A.V. Does free nicotine replacement improve smoking cessation rates in cancer patients? Curr. Oncol. 2020, 27, 14–18. [Google Scholar] [CrossRef]
- Powell, B.J.; Waltz, T.J.; Chinman, M.J.; Damschroder, L.J.; Smith, J.L.; Matthieu, M.M.; Proctor, E.K.; Kirchner, J.E. A refined compilation of implementation strategies: Results from the Expert Recommendations for Implementing Change (ERIC) project. Implement. Sci. 2015, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Burris, J.L.; Borger, T.N.; Baker, T.B.; Bernstein, S.L.; Ostroff, J.S.; Rigotti, N.A.; Joseph, A.M. Proposing a Model of Proactive Outreach to Advance Clinical Research and Care Delivery for Patients Who Use Tobacco. J. Gen. Intern Med. 2022, 37, 2548–2552. [Google Scholar] [CrossRef]
- Gali, K.; Pike, B.; Kendra, M.S.; Tran, C.; Fielding-Singh, P.; Jimenez, K.; Mirkin, R.; Prochaska, J.J. Integration of Tobacco Treatment Services into Cancer Care at Stanford. Int. J. Environ. Res. Public Health 2020, 17, 2101. [Google Scholar] [CrossRef]
- Richter, K.P.; Ellerbeck, E.F. It’s time to change the default for tobacco treatment. Addiction 2015, 110, 381–386. [Google Scholar] [CrossRef]
- Amato, K.A.; Reid, M.E.; Bansal-Travers, M.; Ochs-Balcom, H.M.; Cummings, K.M.; Mahoney, M.; Marshall, J.; Hyland, A. Patient Cessation Activity after Automatic Referral to a Dedicated Cessation Support Service. J. Smok. Cessat. 2018, 13, 78–86. [Google Scholar] [CrossRef]
- Nomura, A.; Tanigawa, T.; Muto, T.; Oga, T.; Fukushima, Y.; Kiyosue, A.; Miyazaki, M.; Hida, E.; Satake, K. Clinical Efficacy of Telemedicine Compared to Face-to-Face Clinic Visits for Smoking Cessation: Multicenter Open-Label Randomized Controlled Noninferiority Trial. J. Med. Internet Res. 2019, 21, e13520. [Google Scholar] [CrossRef]
- Carlson, L.E.; Lounsberry, J.J.; Maciejewski, O.; Wright, K.; Collacutt, V.; Taenzer, P. Telehealth-delivered group smoking cessation for rural and urban participants: Feasibility and cessation rates. Addict. Behav. 2012, 37, 108–114. [Google Scholar] [CrossRef]
- Snoswell, C.L.; Comans, T.A. Does the Choice Between a Telehealth and an In-Person Appointment Change Patient Attendance? Telemed. E-Health 2021, 27, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Drerup, B.; Espenschied, J.; Wiedemer, J.; Hamilton, L. Reduced No-Show Rates and Sustained Patient Satisfaction of Telehealth During the COVID-19 Pandemic. Telemed. E-Health 2021, 27, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.P.; Park, C.; Silverman, C.L.; Eckhoff, D.O.; Guest, J.C.; Díaz, D.A. Accessibility and utilisation of telehealth services among older adults during COVID-19 pandemic in the United States. Health Soc. Care Community 2022, 30, e2657–e2669. [Google Scholar] [CrossRef]
- Cantor, J.H.; McBain, R.K.; Pera, M.F.; Bravata, D.M.; Whaley, C.M. Who Is (and Is Not) Receiving Telemedicine Care During the COVID-19 Pandemic. Am. J. Prev. Med. 2021, 61, 434–438. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Center Cessation Initiative Telehealth Working Group. Telehealth Delivery of Tobacco Cessation Treatment in Cancer Care: An Ongoing Innovation Accelerated by the COVID-19 Pandemic. J. Natl. Compr. Cancer Netw. 2021, 19 (Suppl. S1), S21–S24. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.J.; Dy, S.M.; Rosen, M.A. Team-training in healthcare: A narrative synthesis of the literature. BMJ Qual. Saf. 2014, 23, 359–372. [Google Scholar] [CrossRef]
- Bunnell, C.A.; Gross, A.H.; Weingart, S.N.; Kalfin, M.J.; Partridge, A.; Lane, S.; Burstein, H.J.; Fine, B.; Hilton, N.A.; Sullivan, C.; et al. High performance teamwork training and systems redesign in outpatient oncology. BMJ Qual. Saf. 2013, 22, 405–413. [Google Scholar] [CrossRef]
- Edmondson, A. Psychological Safety and Learning Behavior in Work Teams. Adm. Sci. Q. 1999, 44, 350–383. [Google Scholar] [CrossRef]
- Ivers, N.; Jamtvedt, G.; Flottorp, S.; Young, J.M.; Odgaard-Jensen, J.; French, S.D.; O’Brien, M.A.; Johansen, M.; Grimshaw, J.; Oxman, A.D. Audit and feedback: Effects on professional practice and healthcare outcomes. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Jamtvedt, G.; Flottorp, S.; Ivers, N. Audit and Feedback as a Quality Strategy; Health Policy Series, No. 53; European Observatory on Health Systems and Policies: Copenhangen, Denmark, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549284/ (accessed on 4 December 2022).
- Colquhoun, H.; Michie, S.; Sales, A.; Ivers, N.; Grimshaw, J.M.; Carroll, K.; Chalifoux, M.; Eva, K.; Brehaut, J. Reporting and design elements of audit and feedback interventions: A secondary review. BMJ Qual. Saf. 2017, 26, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ostroff, J.S.; Bolutayo Gaffney, K.L.; O’Brien, M.; de Leon-Sanchez, S.T.; Whitlock, C.W.; Kotsen, C.S.; Carter-Harris, L.; Banerjee, S.C.; Schofield, E. Training oncology care providers in the assessment and treatment of tobacco use and dependence. Cancer 2021, 127, 3010–3018. [Google Scholar] [CrossRef]
- Sheffer, C.E.; Payne, T.; Ostroff, J.S.; Jolicoeur, D.; Steinberg, M.; Czabafy, S.; Foulds, J.; Bars, M.; Wassum, K.; Perry, B.; et al. Increasing the Quality and Availability of Evidence-based Treatment for Tobacco Dependence through Unified Certification of Tobacco Treatment Specialists. J. Smok. Cessat. 2016, 11, 229–235. [Google Scholar] [CrossRef]
- Ahluwalia, J.S.; Gibson, C.A.; Kenney, R.E.; Wallace, D.D.; Resnicow, K. Smoking Status as a Vital Sign. J. Gen. Intern Med. 1999, 14, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Land, S.R.; Toll, B.A.; Moinpour, C.M.; Mitchell, S.A.; Ostroff, J.S.; Hatsukami, D.K.; Duffy, S.A.; Gritz, E.R.; Rigotti, N.A.; Brandon, T.H.; et al. Research Priorities, Measures, and Recommendations for Assessment of Tobacco Use in Clinical Cancer Research. Clin. Cancer Res. 2016, 22, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Rickert, W.; Robinson, J. Measuring the Heaviness of Smoking: Using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br. J. Addict. 1989, 84, 791–800. [Google Scholar] [CrossRef]
- Biener, L.; Abrams, D.B. The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991, 10, 360–365. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology–Distress Management Version 1; NCCN: Washington, PA, USA, 2016. [Google Scholar]
- Hohl, S.D.; Matulewicz, R.S.; Salloum, R.G.; Ostroff, J.S.; Baker, T.B.; Schnoll, R.; Warren, G.; Bernstein, S.L.; Minion, M.; Lenhoff, K.; et al. Integrating Tobacco Treatment into Oncology Care: Reach and Effectiveness of Evidence-Based Tobacco Treatment across National Cancer Institute–Designated Cancer Centers. J. Clin. Oncol. 2022, 22, JCO-22. [Google Scholar] [CrossRef]
- Cancer Survivors and Smoking. National Cancer Institute: Cancer Trends Progress Report. 2022. Available online: https://progressreport.cancer.gov/after/smoking (accessed on 23 February 2023).
- Cornelius, M.E. Tobacco Product Use Among Adults—United States, 2020. MMWR Morb. Mortal Wkly. Rep. 2022, 71, 397–405. [Google Scholar] [CrossRef]
- Landrine, H.; Corral, I.; Campbell, K.M. Racial disparities in healthcare provider advice to quit smoking. Prev. Med. Rep. 2018, 10, 172–175. [Google Scholar] [CrossRef]
- Rojewski, A.M.; Bailey, S.R.; Bernstein, S.L.; Cooperman, N.A.; Gritz, E.R.; Karam-Hage, M.A.; Piper, M.E.; Rigotti, N.A.; Warren, G.W. Considering Systemic Barriers to Treating Tobacco Use in Clinical Settings in the United States. Nicotine Tob. Res. 2018, 21, 1453–1461. [Google Scholar] [CrossRef]
- Just ASK Quality Improvement Project & Clinical Study. American College of Surgeons. Available online: https://www.facs.org/quality-programs/cancer-programs/pdsa-just-ask/ (accessed on 4 December 2022).
- Salloum, R.G.; D’Angelo, H.; Theis, R.P.; Rolland, B.; Hohl, S.; Pauk, D.; LeLaurin, J.H.; Asvat, Y.; Chen, L.-S.; Day, A.T.; et al. Mixed-methods economic evaluation of the implementation of tobacco treatment programs in National Cancer Institute-designated cancer centers. Implement. Sci. Commun. 2021, 2, 41. [Google Scholar] [CrossRef] [PubMed]
| Strategy | Teams Involved | Team Actions | Facilitators | Barriers | Outcomes |
|---|---|---|---|---|---|
| Opt-out approach to identify and reach all new patients who have smoked tobacco in the past 30 days | EHR IT team (programmer, provider informaticist) and clinical administration team (Dir. of Oncology Services) | EHR modifications of MA workflow and development of weekly reporting tools. | Clinical administration team helps to obtain modifications prioritized by the EHR team. | Low priority of work requests submitted to EHR programmers results in delays in improvements. | Number (%) of all hem/onc patients assessed for tobacco use (monthly and overall). (See Figure 1, Figure 2, Figure 3 and Figure 4.) |
| Clinical team: medical assistants (MAs) and Dir. of Nursing Services | MAs document tobacco status at every visit and provide a STAR flyer to those who have used tobacco or nicotine in <30 days. | Monthly audit and feedback with lead MAs on percentage of patients assessed for tobacco use. | MAs’ time constraints, need for regular training, and staff turnover. | Identification of more current smokers compared to referral methods (see Figure 1 and Figure 2). | |
| STAR team: TTS, NP, outreach staff (interns), data manager, clinical psychologist supervisor for TTS | Outreach and intake completion of new patients (smoked in < 30 days) via phone, email, and/or EHR portal. | Availability of staff time; shared Google voice line allows staff to return calls from office or home; monthly EHR messages sent to providers re status of their patients. | Limited integration of STAR procedures with EHR (e.g., the contact attempts made to STAR patients are not tracked in EHR). | Tracking data on % reached and % enrolled (see Figure 1 and Figure 2). | |
| Centralized approach to providing tobacco treatment at 2 hospitals | STAR team | TTS phone-based sessions: TTS provided evidence-based behavioral counseling and assessed potential need for medication and NP visit. | Use of Google calendar for immediate scheduling of counseling during the intake call; phone-based calls reduce staff time needed. | Patient cancellations/no shows/rescheduling requires staff time. | Tracking data on number of counseling sessions completed; phone sessions require less time for TTSs and patients than telehealth or in-person visits. |
| Administrative, STAR, clinical, and pharmacy teams | In-person visits: NPs and TTSs collaborate on medication and behavioral strategies. Pharmacy stores and distributes NRT donated by CVS. | Admin team identified NPs with available time for STAR and needed the clinical space; NP and TTS review prior session(s) in advance of the upcoming session. | Clinical space for the visits; limited clinic availability reduces options for patients. | TTSs provide treatment to patients at two different hospitals, providing greater efficiency. | |
| STAR, clinical, IT, and pharmacy teams | Telehealth visits: The IT team set up telehealth procedures after pandemic onset. NPs and TTSs provide same counseling and medication options as for in-person visits. | When available, technical assistance is provided to connect patient and NPs for the sessions; otherwise, regular phone calls were used when telehealth calls failed or were not possible. | State licenses necessary for MD and VA; some patients have connection issues with telehealth platform. | Patients receive counseling at home. Medications are delivered to patients’ homes. | |
| Audit/feedback/training | STAR and clinical teams | STAR data manager and staff audit MA tobacco assessments each month. | Feedback provided to lead MA who communicates with MA team; STAR team meets with clinical team every 6 months to review procedures. | High MA turnover, busy clinics. | Data provide % of patients who are assessed for tobacco use each month. Feedback and training result in higher % of patients assessed; (see Figure 3 and Figure 4). |
| STAR, oncology admin, and clinical teams | Attendance at STAR meetings every other month. | Regular attendance by all team members; remote meetings assist with attendance. | Lack of attendance due to competing priorities and staff shortages. | Gain input from multiple teams and address issues more quickly than via email exchanges. | |
| STAR team | Monthly notifications sent to providers via EHR on their patients’ enrollment and smoking status. | Buy-in and awareness of providers improves when updated on their patients’ progress; providers appreciate receiving the updates on their patients. | Limited time for providers to review messages and encourage patients regarding quitting and remaining smoke-free. | Providers are kept informed and can reinforce cessation at visits; messages remind providers to refer other patients. | |
| STAR team | TTS meets monthly with clinical psychologist supervisor. | Clinical psychologist supervises TTSs on motivational interviewing and behavioral cessation strategies. | Limited time to discuss all patients. | TTS uses feedback to improve counseling for patients. | |
| Clinical admin and STAR teams | Attend standing faculty meeting for annual STAR updates and review of data on outreach, treatment engagement, and abstinence. | Updates provided during regularly scheduled faculty meeting. Provider permission to contact patients without a referral is confirmed. | Providers’ time and interest; time available on meeting agenda is limited. | Providers are reminded of the rationale for tobacco treatment, institutional program support, and to refer their patients. |
| Not Enrolled (n = 359) | Enrolled (n = 178) | Enrolled/Not Engaged 1 (n = 51) | Engaged (n = 127) | 6-Month Not Quit (n = 106) | 6-Month Quit (n = 40) | ||
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age | M (SD) | 59.8 (10.6) | 59.8 (12.6) | 59.3 (10.2) | 59.9 (10.8) | 59.2 (10.9) | 60.8 (11.2) |
| ≤60 | N (%) | 158 (44.0) | 84 (47.2) | 27 (52.9) | 57 (44.9) | 54 (50.9) * | 14 (35.0) |
| ≥61 | N (%) | 201 (56.0) | 94 (52.8) | 24 (47.2) | 70 (55.1) | 52 (49.1) | 26 (65.0) |
| Sex | |||||||
| Female | N (%) | 165 (46.0) *** | 105 (59.0) | 29 (56.9) | 76 (59.8) | 66 (62.3) | 22 (55.0) |
| Race | |||||||
| African American | N (%) | 148 (42.7) *** | 113 (64.9) | 35 (70.0) | 78 (62.9) | 64 (62.1) | 27 (67.5) |
| White | N (%) | 166 (47.8) | 46 (26.4) | 14 (28.0) | 32 (25.8) | 31 (30.1) | 8 (20.0) |
| Other (e.g., Asian, NHPI 2) | N (%) | 33 (9.5) | 15 (8.6) | 1 (2.0) | 14 (11.3) | 8 (7.8) | 5 (12.5) |
| Missing | 12 (-) | 4 (-) | 1 (-) | 3 (-) | 3 (-) | 0 (-) | |
| Ethnicity | |||||||
| Hispanic or Latino | N (%) | 15 (4.4) | 9 (5.3) | 1 (2.0) | 8 (6.6) | 4 (4.0) | 3 (7.5) |
| Non-Hispanic | N (%) | 328 (95.6) | 162 (94.7) | 49 (98.0) | 113 (93.4) | 97 (96.0) | 37 (92.5) |
| Missing/unknown | N (%) | 16 (-) | 7 (-) | 1 (-) | 6 (-) | 5 (-) | 0 (-) |
| Insurance Type | |||||||
| Private | N (%) | 170 (47.6) ** | 64 (36.2) | 20 (39.2) | 44 (34.9) | 39 (36.8) | 16 (40.0) |
| Medicare or military | N (%) | 145 (40.6) | 86 (48.6) | 22 (43.1) | 64 (50.8) | 50 (47.2) | 19 (47.5) |
| Medicaid or other govt | N (%) | 42 (11.8) | 27 (15.3) | 9 (17.6) | 18 (14.3) | 17 (16.0) | 5 (12.5) |
| No insurance/unknown | N (%) | 1 (-) | 1 (-) | 0 (-) | 1 (-) | 0 (-) | 0 (-) |
| Clinical Variables | |||||||
| Primary Diagnosis | |||||||
| Breast cancer | N (%) | 43 (12.0) | 24 (13.5) | 4 (7.8) | 20 (15.7) | 15 (14.2) | 6 (15.0) |
| Head/neck cancer | N (%) | 14 (3.9) | 4 (2.2) | 0 (0.0) | 4 (3.1) | 2 (1.9) | 1 (2.5) |
| Gastrointestinal cancer | N (%) | 73 (20.3) | 27 (15.2) | 10 (19.6) | 17 (13.4) | 14 (13.2) | 2 (5.0) |
| Genitourinary cancer | N (%) | 52 (14.5) | 11 (6.2) | 4 (7.8) | 7 (5.5) | 3 (2.8) | 5 (12.5) |
| Leukemia/lymphoma | N (%) | 25 (7.0) | 15 (8.4) | 3 (5.9) | 12 (9.4) | 12 (11.3) | 3 (7.5) |
| Lung cancer | N (%) | 42 (11.7) | 38 (21.3) | 12 (23.5) | 26 (20.5) | 22 (20.8) | 10 (25.0) |
| Other (brain, gyn, skin) | N (%) | 51 (14.2) | 23 (12.9) | 9 (17.6) | 15 (11.8) | 12 (11.3) | 6 (15.0) |
| Heme Dx (e.g., anemia, DVT) | N (%) | 44 (12.3) | 21 (11.8) | 6 (11.8) | 15 (11.8) | 14 (13.2) | 6 (15.0) |
| Lung cancer screening | N (%) | 6 (1.7) | 13 (7.3) | 3 (5.9) | 10 (7.9) | 11 (10.4) | 0 (0.0) |
| Diagnostic, other, non-LCS | N (%) | 9 (2.5) | 2 (1.2) | 0 (0.0) | 2 (1.6) | 1 (0.9) | 1 (2.5) |
| Tobacco-Related Cancer 3 | |||||||
| Yes | N (%) | 156 (43.5) | 75 (42.1) | 21 (41.2) | 54 (42.5) | 39 (36.8) | 17 (42.5) |
| No | N (%) | 144 (40.1) | 68 (38.2) | 22 (43.1) | 46 (36.2) | 42 (39.6) | 16 (40.0) |
| N/A: heme or diagnostic | N (%) | 59 (16.4) | 35 (19.7) | 8 (15.7) | 27 (21.3) | 25 (23.6) | 7 (17.5) |
| Cancer Stage | |||||||
| Cancer stage 0, I, II | N (%) | 94 (29.4) * | 61 (39.9) | 14 (29.2) * | 47 (44.8) | 36 (41.4) | 17 (45.9) |
| Cancer stage III or IV | N (%) | 180 (56.3) | 70 (45.8) | 28 (58.3) | 42 (40.0) | 36 (41.4) | 14 (37.8) |
| N/A: non-cancer hem dx | N (%) | 46 (14.4) | 22 (14.4) | 6 (12.5) | 16 (15.2) | 15 (17.2) | 6 (16.2) |
| N/A: diagnostic, ca screen | N | 13 (-) | 13 (-) | 3 (-) | 10 (-) | 11 (-) | 1 (-) |
| Missing/unknown | N | 26 (-) | 26 (-) | 0 (-) | 12 (-) | 8 (-) | 2 (-) |
| Method Patient Identified | |||||||
| Clinician referral | N (%) | 24 (6.7) *** | 60 (33.7) | 12 (23.5) * | 48 (37.8) | 39 (36.8) | 15 (37.5) |
| MA assessment | N (%) | 335 (93.3) | 118 (66.3) | 39 (76.5) | 79 (62.2) | 67 (63.2) | 25 (62.5) |
| Psych. Distress 4 (1 = low to 10 = high) | -- | -- | |||||
| ≤6 | M (SD) | -- | -- | 26 (60.5) ** | 49 (41.5) | 17 (47.2) | 42 (44.7) |
| ≥7 | M (SD) | -- | -- | 17 (39.5) | 69 (58.5) | 19 (52.8) | 52 (55.3) |
| Missing | N | -- | -- | 8 (-) | 9 (-) | 4 (-) | 12 (-) |
| Baseline Tobacco Variables | |||||||
| MA Smoking Assessment | |||||||
| Smoked a cigarette today | N (%) | 233 (69.3) * | 137 (77.4) | 38 (74.5) | 99 (78.6) | 86 (81.9) | 24 (60.0) *** |
| Smoked 1–30 days ago | N (%) | 103 (30.7) | 40 (22.6) | 13 (25.5) | 27 (21.4) | 19 (18.1) | 16 (40.0) |
| Used other nicotine/tobacco | 23 (-) | 1 (-) | 0 (-) | 1 (-) | 1 (-) | 0 (-) | |
| Cigarettes Per Day (N) | M (SD) | -- | -- | 9.1 (7.8) | 10.6 (9.5) | 11.4 (9.2) ** | 7.6 (8.3) |
| Median | -- | -- | 6.5 | 9.0 | 10.0 | 6.0 | |
| Cigarettes Per Day (categ.) | |||||||
| ≤5 | N (%) | -- | -- | 20 (40.0) | 47 (37.3) | 31 (29.8) * | 19 (47.5) |
| 6 to 10 | N (%) | -- | -- | 18 (36.0) | 41 (32.5) | 38 (36.5) | 14 (35.0) |
| ≥11 | N (%) | -- | -- | 12 (24.0) | 38 (22.4) | 35 (33.7) | 7 (17.5) |
| Pack-Years | M (SD) | -- | -- | 31.9 (25.0) | 36.6 (28.0) | 34.1 (26.1) | 34.7 (31.5) |
| Median | -- | -- | 22.5 | 29.5 | 27.0 | 24.25 | |
| First Cigarette After Waking | -- | -- | |||||
| <30 min | (N, %) | -- | -- | 23 (45.1) * | 73 (63.5) | 58 (59.2) | 18 (47.4) |
| 31–60 min | (N, %) | -- | -- | 12 (23.5) | 18 (15.7) | 18 (18.4) | 5 (13.2) |
| 61+ min | (N, %) | -- | -- | 16 (31.4) | 24 (20.9) | 22 (22.4) | 15 (39.5) |
| Missing/refused | N | -- | -- | 0 (-) | 12 (-) | 8 (-) | 2 (-) |
| Readiness to Quit | -- | -- | |||||
| Not ready to quit (≥6 mos) | (N, %) | -- | -- | 18 (35.3) | 38 (34.2) | 39 (41.1) *** | 7 (18.4) |
| Ready to quit ≤30 days | (N, %) | -- | -- | 24 (47.1) | 64 (57.7) | 51 (53.7) | 19 (50.0) |
| Already quit (<30 days) | (N, %) | -- | -- | 9 (17.6) | 9 (8.1) | 5 (5.3) | 12 (31.6) |
| Missing/refused | N | -- | -- | 0 (-) | 16 (-) | 11 (-) | 2 (-) |
| Lives w. Person Smoking | -- | -- | |||||
| No (or lives alone) | (N, %) | -- | -- | 29 (56.9) ** | 90 (74.4) | 68 (66.7) ** | 33 (84.6) |
| Yes | (N, %) | -- | -- | 22 (43.1) | 31 (25.6) | 34 (33.3) | 6 (15.4) |
| Missing | N | -- | -- | 0 (-) | 6 (-) | 4 (-) | 1 (-) |
| STAR Engagement | |||||||
| Sessions Complete | M (SD) | -- | -- | n/a | 2.52 (1.6) | 1.62 (1.7) ** | 2.35 (1.8) |
| Median | -- | -- | n/a | 2.0 | 1.0 | 3.0 | |
| Sessions Complete (categ.) | -- | -- | |||||
| 0/1 sessions | N (%) | -- | -- | n/a | 43 (33.9) | 61 (57.5) * | 16 (40.0) |
| 2+ sessions | N (%) | -- | -- | n/a | 84 (66.1) | 45 (42.5) | 24 (60.0) |
| STAR Prescription or NRT | -- | -- | |||||
| Yes | N (%) | -- | -- | n/a | 60 (47.2) | 38 (35.8) | 13 (32.5) |
| MGUH (n = 125) | ||
|---|---|---|
| OR (95% CI) | p Value | |
| Diagnosis | ||
| Stage 0, I, II | 1 | |
| Stage III or IV | 0.46 (0.19–1.16) | 0.099 |
| Hematologic condition | 0.98 (0.25–3.92) | 0.976 |
| Method of patient identification | ||
| MA assessment | 1 | |
| Provider referral | 2.64 (0.86–8.06) | 0.088 |
| Time to first cigarette after waking | ||
| After 60 min | 1 | |
| 31 to 60 min | 1.53 (0.46–5.14) | 0.489 |
| Within 30 min | 3.92 (1.40–10.96) | 0.009 |
| Lives with smoker | ||
| No or lives alone | 1 | |
| Yes | 0.42 (0.17–1.04) | 0.061 |
| Distress score (0–10) | ||
| 6 or lower | 1 | |
| 7+ | 2.31 (0.97–5.48) | 0.059 |
| MGUH (n = 131) | ||
|---|---|---|
| OR (95% CI) | p Value | |
| Age groups | ||
| 60 and under | 1 | |
| 61+ | 1.27 (0.54–2.95) | 0.583 |
| Time to first cigarette after waking | ||
| Within 30 min | 1 | |
| 31 to 60 min | 0.92 (0.25–3.37) | 0.895 |
| After 60 min | 2.18 (0.85–5.61) | 0.105 |
| Last day smoked | ||
| Today | 1 | |
| One or more days ago | 2.48 (0.96–6.37) | 0.060 |
| Lives with smoker | ||
| No or lives alone | 1 | |
| Yes | 0.51 (0.18–1.47) | 0.215 |
| Number of counseling sessions | ||
| 0 to 1 | 1 | |
| 2+ | 2.80 (1.19–6.58) | 0.018 |
| Model 1: MGUH (n = 437) | Model 2: MWHC (n = 353) | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age | ||||
| ≤60 | — | 1 | ||
| ≥61 | — | 0.64 (0.38–1.06) | 0.084 | |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 1.38 (0.89–2.13) | 0.153 | 1.43 (0.82–2.51) | 0.208 |
| Race | ||||
| White or other | 1 | 1 | ||
| Black/African American | 2.59 (1.64–4.10) | <0.001 | 2.23 (0.87–5.69) | 0.093 |
| Diagnosis | ||||
| Stage 0, I, II cancer | 1 | 1 | ||
| Stage III or IV cancer | 0.80 (0.50–1.30) | 0.372 | 0.72 (0.40–1.30) | 0.280 |
| Hematologic condition | 0.89 (0.45–1.76) | 0.728 | 0.88 (0.45–1.70) | 0.697 |
| Insurance type | ||||
| Private insurance | 1 | – | ||
| Medicare or military plan | 1.51 (0.95–2.40) | 0.084 | – | |
| Medicaid or other govt-sponsored | 1.01 (0.50–2.01) | 0.984 | – | |
| MA tobacco assessment | ||||
| 1 or more days ago | 1 | 1 | ||
| Smoked today | 1.29 (0.79–2.09) | 0.315 | 1.44 (0.77–2.69) | 0.249 |
| Method of patient identification | ||||
| MA assessment | 1 | 1 | ||
| Provider referral | 6.21 (3.25–11.89) | <0.001 | 5.86 (3.39–10.13) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, K.L.; Webster, M.A.; Philips, J.G.; Whealan, J.M.; Lobo, T.; Davis, K.M.; Breece, C.J.; Wheeley, J.R.; Childs, J.E.; Le, A.Q.; et al. Integrating Tobacco Use Assessment and Treatment in the Oncology Setting: Quality Improvement Results from the Georgetown Lombardi Smoking Treatment and Recovery Program. Curr. Oncol. 2023, 30, 3755-3775. https://doi.org/10.3390/curroncol30040285
Taylor KL, Webster MA, Philips JG, Whealan JM, Lobo T, Davis KM, Breece CJ, Wheeley JR, Childs JE, Le AQ, et al. Integrating Tobacco Use Assessment and Treatment in the Oncology Setting: Quality Improvement Results from the Georgetown Lombardi Smoking Treatment and Recovery Program. Current Oncology. 2023; 30(4):3755-3775. https://doi.org/10.3390/curroncol30040285
Chicago/Turabian StyleTaylor, Kathryn L., Marguerite A. Webster, Joanna G. Philips, Julia M. Whealan, Tania Lobo, Kimberly M. Davis, Chavalia J. Breece, Jennifer R. Wheeley, Jack E. Childs, Ariel Q. Le, and et al. 2023. "Integrating Tobacco Use Assessment and Treatment in the Oncology Setting: Quality Improvement Results from the Georgetown Lombardi Smoking Treatment and Recovery Program" Current Oncology 30, no. 4: 3755-3775. https://doi.org/10.3390/curroncol30040285
APA StyleTaylor, K. L., Webster, M. A., Philips, J. G., Whealan, J. M., Lobo, T., Davis, K. M., Breece, C. J., Wheeley, J. R., Childs, J. E., Le, A. Q., Williams, R. M., Veytsman, I. G., & Kim, C. (2023). Integrating Tobacco Use Assessment and Treatment in the Oncology Setting: Quality Improvement Results from the Georgetown Lombardi Smoking Treatment and Recovery Program. Current Oncology, 30(4), 3755-3775. https://doi.org/10.3390/curroncol30040285





