Descriptive Analysis of First-Line Non-Small Cell Lung Cancer Treatment with Pembrolizumab in Tumors Expressing PD-L1 ≥ 50% in Patients Treated in Quebec’s University Teaching Hospitals (DALP-First Study)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Collection
2.3. Ethical Considerations
2.4. Study Measures
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Patients at Diagnosis of NSCLC
3.2. Efficacy
3.2.1. Treatment
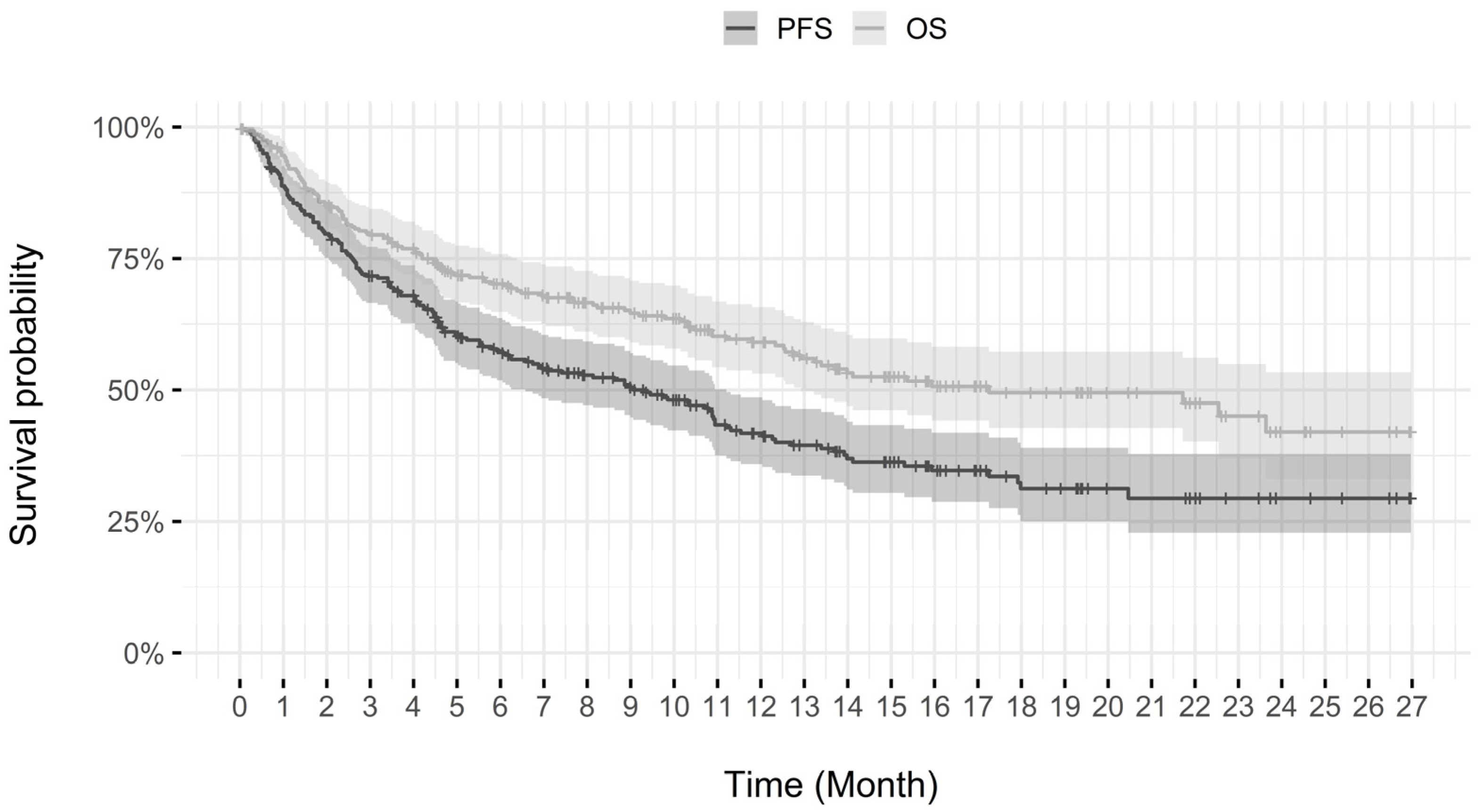
3.2.2. Progression-Free Survival and Overall Survival
3.3. Adverse Events
3.4. Subgroup Analysis
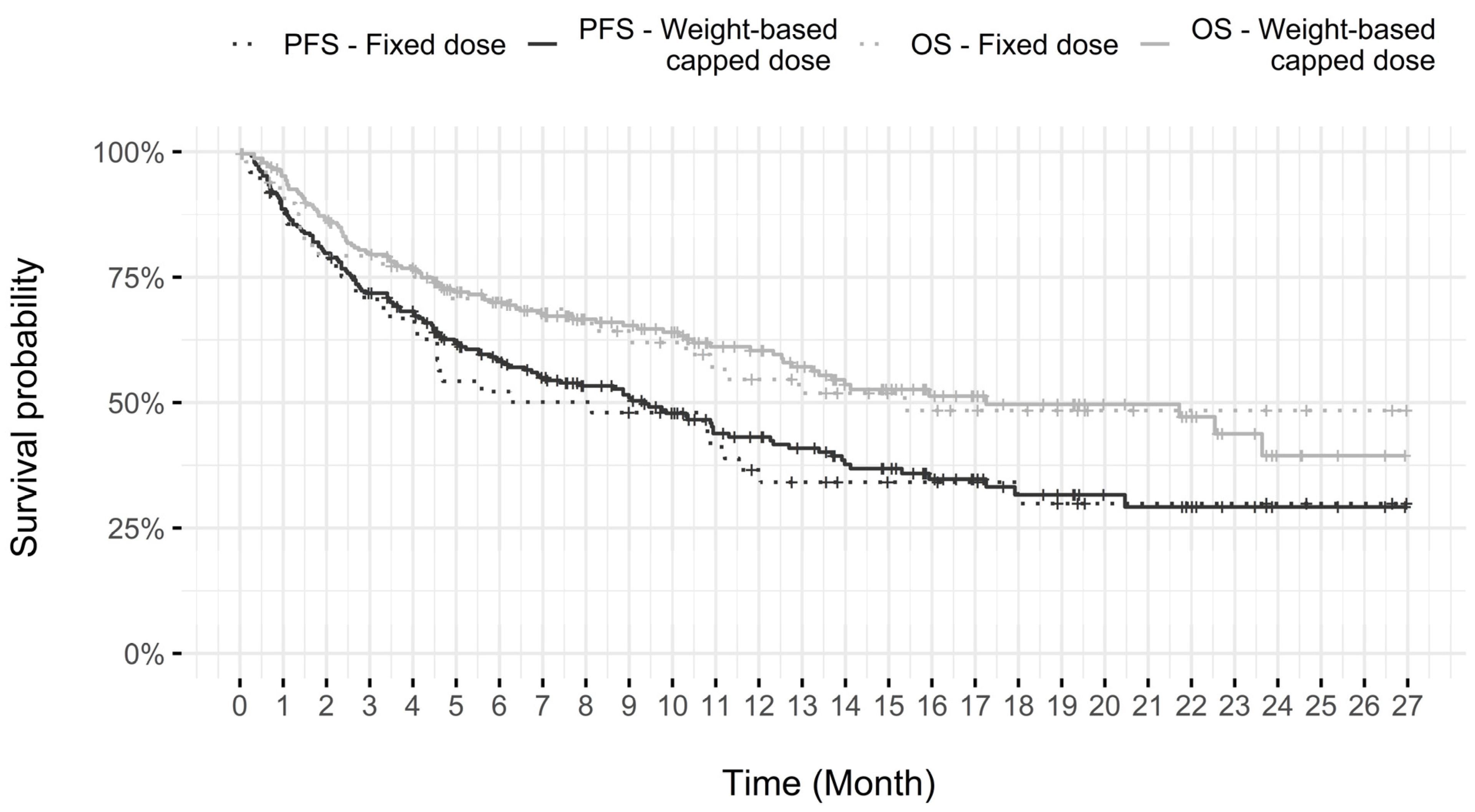
3.4.1. Dosing Modality
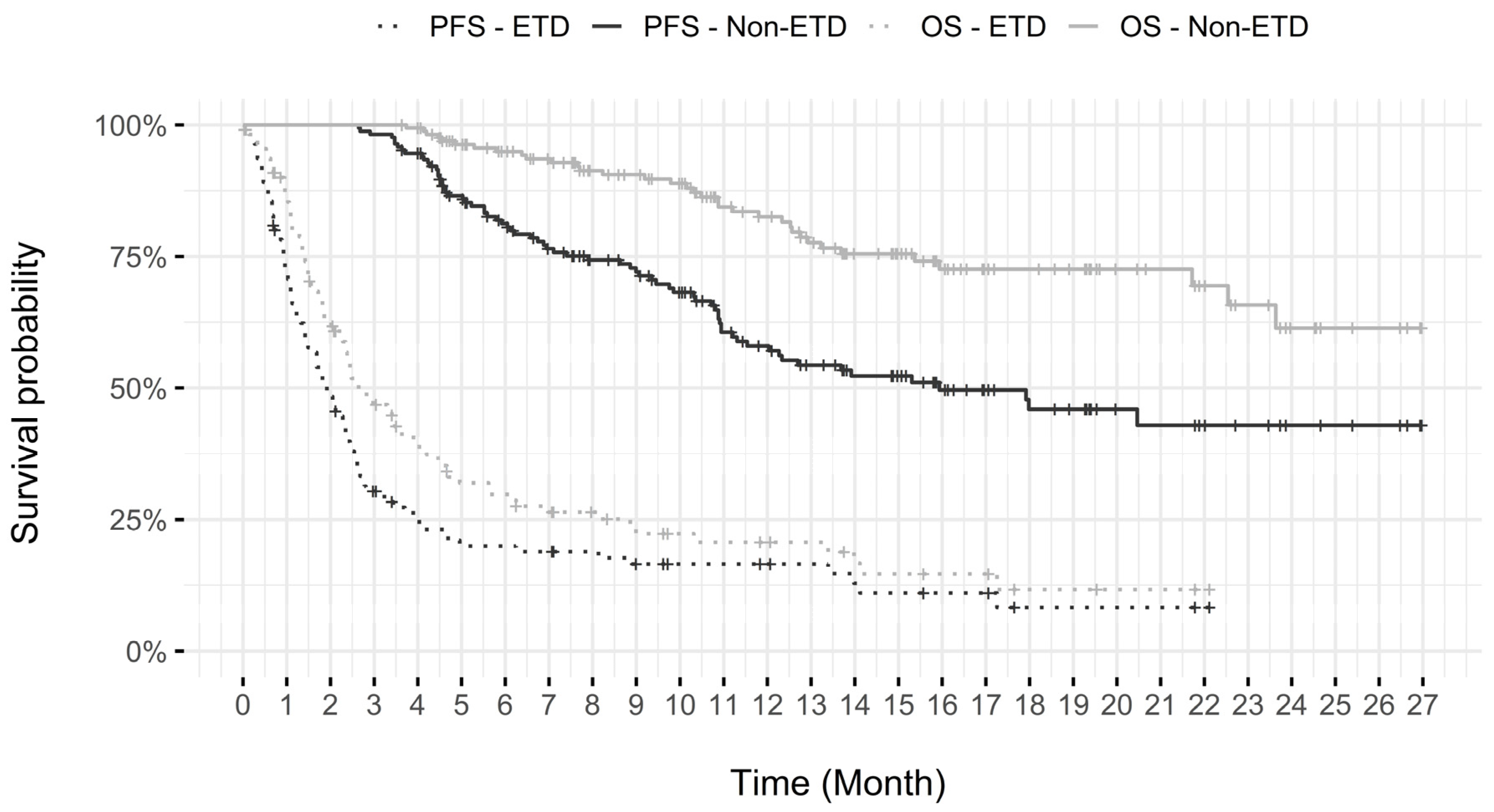
3.4.2. Early Treatment Discontinuation (ETD)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization—International Agency for Research on Cancer. Lung Cancer Statistics: Source Globocan 2020, on the Cancer Today Website. Available online: https://gco.iarc.fr/today/home (accessed on 27 October 2022).
- Fondation Québécoise du Cancer. Faits et Statistiques sur le Cancer, on the Fondation Québécoise du Cancer Website. Available online: https://fqc.qc.ca/fr/information/le-cancer/statistiques (accessed on 15 November 2022).
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Spira, A.; Ettinger, D.S. Multidisciplinary management of lung cancer. N. Engl. J. Med. 2004, 350, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.H.; Harrington, D.; Belani, C.P.; Langer, C.; Sandler, A.; Krook, J.; Zhu, J.; Johnson, D.H.; Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N. Engl. J. Med. 2002, 346, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eight Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50 (KEYNOTE-024). J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Guévremont, C.; Letarte, N.; Bérard, G.; Marcotte, N.; Jamal, R.; Blais, N.; El Raichani, L.; Programme de Gestion Thérapeutique des Médicaments (PGTM) (2020). Analyse Descriptive de l’Utilisation des Anti-PD-1 (Pembrolizumab et Nivolumab) pour des Patients Atteints de Cancer Dans les CHU du Québec. PGTM Website. Available online: http://pgtm.org/documentation/FSW/AD%20Anti%20PD%201_final%20corr.pdf (accessed on 25 October 2022).
- Goldstein, D.A.; Gordon, N.; Davidescu, M.; Leshno, M.; Steuer, C.E.; Patel, N.; Stemmer, S.M.; Zer, A. A Pharmaco-economic Analysis of Personalized Dosing vs Fixed Dosing of Pembrolizumab in First line PD-L1-Positive Non-Small Cell Lung Cancer. J. Natl. Cancer Inst. 2017, 109, 63. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; de Léséleuc, L.; Butcher, R. Dosing and Timing of Immuno-Oncology Drugs. Ottawa: CADTH; 2019 Month. (CADTH Technology Review: Optimal Use 360 Report; no. 25). Available online: https://www.cadth.ca/sites/default/files/ou-tr/ho0008-dosing-timing-immuno-oncology-drugs.pdf (accessed on 27 February 2023).
- Bérard, G.; Guévremont, C.; Marcotte, N.; Legeleux, L.; Letarte, N.; Vanhuyse; Programme de Gestion Thérapeutique des Médicaments (PGTM) (2018). Pembrolizumab (KeytrudaMC)—Quelle Stratégie Posologique Devrait-on Privilégier: Dose en Fonction du Poids, Dose Fixe ou Dose en Fonction du Poids Avec Dose Maximale? PGTM Website. Available online: http://pgtm.org/documentation/FSW/Pembrolizumab_Strat%C3%A9gie%20posologique.pdf (accessed on 25 October 2022).
- Institut National D’excellence en Santé et en Services Sociaux (INESSS). Choix de la Posologie du Nivolumab et du Pembrolizumab—Rapport en Soutien à l’Outil d’Aide à la Décision. Rapport Rédigé par Catherine Awad. Québec, Qc: INESSS. 2020. Available online: https://numerique.banq.qc.ca/patrimoine/details/52327/4153429 (accessed on 25 October 2022).
- Amrane, K.; Geier, M.; Corre, R.; Léna, H.; Léveiller, G.; Gadby, F.; Lamy, R.; Bizec, J.L.; Goarant, E.; Robinet, G.; et al. First-line pembrolizumab for non-small cell lung cancer patients with PD-L1 ≥50% in a multicenter real-life cohort: The PEMBREIZH study. Cancer Med. 2020, 9, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Pasello, G.; Lorenzi, M.; Calvetti, L.; Oliani, C.; Pavan, A.; Favaretto, A.; Palazzolo, G.; Giovanis, P.; Zustovich, F.; Bonetti, A.; et al. Multicenter Real-World Study on Effectiveness and Early Discontinuation Predictors in Patients with Non-small Cell Lung Cancer Receiving Nivolumab. Oncologist 2022, 27, e484–e493. [Google Scholar] [CrossRef] [PubMed]
- Freshwater, T.; Kondic, A.; Ahamadi, M.; Li, C.H.; de Greef, R.; de Alwis, D.; Stone, J.A. Evaluation of dosing strategy for pembrolizumab for oncology indications. J. Immunother. Cancer 2017, 5, 43. [Google Scholar] [CrossRef] [PubMed]
| Gender | Number of Patients | % | |
|---|---|---|---|
| Men | 129 | 46.2 | |
| Women | 150 | 53.8 | |
| Age | |||
| Mean ± standard deviation | 68.1 ± 8.5 | ||
| Median | 68 | ||
| Range (min–max) | 34–94 | ||
| Interquartile range | 62–74 | ||
| Patients ≥ 70 years | 123 | 44.1% | |
| Weight (kg) | |||
| Mean ± standard deviation | 68.3 ± 16.6 | ||
| Median | 67 | ||
| Range (min–max) | 32–127 | ||
| Interquartile range | 56–80 | ||
| ECOG PS score | Number of patients | % | |
| 0–1 | 211 | 75.7 | |
| >1–≤2 | 37 | 13.3 | |
| >2 | 14 | 5.0 | |
| Unknown/NFF | 17 | 6.1 | |
| Autoimmune disease | |||
| Yes | 25 | 9.0 | |
| No | 254 | 91.0 | |
| Lung cancer staging | |||
| Metastatic | 244 | 87.5 | |
| Advanced (stage III) | 35 | 12.5 | |
| PD-L1 Score | |||
| Positive ≥ 50% | 276 | 98.9 | |
| Positive, but < 50% | 2 | 0.7 | |
| Negative | 0 | - | |
| Unknown/NFF | 1 | 0.4 | |
| Brain metastases | |||
| Absence | 197 | 70.6 | |
| Presence | 63 | 22.6 | |
| Treated and stable | 52 | 18.6 | |
| Untreated and/or unstable | 10 | 3.6 | |
| Unknown if treated or not | 1 | 0.4 | |
| Unknown/NFF | 19 | 6.8 | |
| Variables * | HR | 95% Confidence Interval | p-Value | ||
|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||
| Dose | Fixed vs. weight-based capped dose (n = 49 vs. n = 230) | 0.97 | 0.61 | 1.53 | 0.88 |
| Age | Less than 70 vs. 70 or more (n = 156 vs. n = 123) | 1.02 | 0.71 | 1.47 | 0.91 |
| Sex | Men vs. women (n = 129 vs. n = 150) | 1.02 | 0.71 | 1.47 | 0.92 |
| Weight | 50 kg or more vs. less than 50 kg (n = 246 vs. n = 33) | 2.61 | 1.65 | 4.13 | <0.0001 |
| ECOG PS > 2 (n = 262) | 2 or less vs. more than 2 (n = 248 vs. n = 14) | 4.11 | 2.13 | 7.93 | <0.0001 |
| ECOG PS > 1 (n =262) | 1 or less vs. more than 1 (n = 211 vs. n = 51) | 1.34 | 0.77 | 2.34 | 0.07 |
| Brain metastases (n = 259) | None or treated vs. untreated/unstable (n = 249 vs. n = 10) | 2.18 | 1.01 | 4.7 | 0.047 |
| Autoimmune disease | No vs. yes (n = 254 vs. n = 25) | 1.34 | 0.77 | 2.34 | 0.31 |
| Number of Cycles Received | Fixed Dose (n = 49) | Weight-Based Capped Dose (n = 230) |
|---|---|---|
| Mean ± standard deviation | 9.3 ± 9.7 | 9.0 ± 8.2 |
| Median | 5 | 6 |
| Range (min–max) | 1–36 | 1–34 |
| Interquartile range | 2–14 | 2.25–13 |
| Non-ETD Population (More than 4 Cycles of Pembrolizumab) (n = 166) | ETD Population (4 Cycles of Pembrolizumab or Less) (n = 113) | ||||
|---|---|---|---|---|---|
| Number of patients | % | Number of patients | % | ||
| Still receiving initial treatment at data cut-off | 75 | 45.2 | 1 | 0.9 | |
| Discontinued Treatment | 91 | 54.8 | 112 | 99.1 | |
| Reason of discontinuation | |||||
| Progressive disease | 58 | 34.9 | 42 | 37.2 | |
| IRAE or other side effect | 23 | 13.9 | 21 | 18.6 | |
| Death less than 3 weeks after last dose | 1 | 0.6 | 24 | 21.2 | |
| Patient decision | 1 | 0.6 | 12 | 10.6 | |
| Complete response to treatment | 1 | 0.6 | 0 | - | |
| Other | 5 | 3.0 | 9 | 8.0 | |
| Loss to follow-up | 2 | 1.2 | 4 | 3.5 | |
| PGTM | Pembreizh | KEYNOTE—024 | KEYNOTE—042 (Subgroup of Patients with PD-L1 ≥ 50%) | ||
|---|---|---|---|---|---|
| Number of patients | 279 | 108 | 154 | 299 | |
| Median age (years) | 68 | 67 | 64.5 | 63 | |
| ECOG PS 0–1 | 75.7% | 76.9% | 99.4% | 100% | |
| Brain metastases | 22.6% | 17.6% | 11.7% | 6% | |
| Unstable or untreated | 3.6% | NA | 0% | 0% | |
| Median follow-up (months) (range) | 7.5 (0.03 *–26.8) | 8.2 (0.9–20.9) | 11.2 | 12.8 | |
| PFS (months) (95% CI) | 9.4 (6.6–11.2) | 10.1 (8.8–NR) | 10.3 (6.7–NR) | 7.1 (5.9–9.0) | |
| PFS at 6 months | 57.4% | 62.7% | 62.1% | ≈53% | |
| OS (months) (95% CI) | 17.3 (12.9–NR) | 15.2 (13.9–NR) | 30.0 (18.3–NR) | 20.0 (15.4–24.9) | |
| OS at 6 months | 70.1% | 86.2% | 80.2% | ≈74% | |
| IRAE (All grade) | 34.4% ** | 46.3% | 73.4% | 63% | |
| IRAE (Grade 3–4) | 8.6% | 8% | 9.7% | 8% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bérard, G.; Guévremont, C.; Marcotte, N.; Schroeder, C.; Bouchard, N.; Rajan, R., on behalf of the Programme de Gestion Thérapeutique des Médicaments (PGTM). Descriptive Analysis of First-Line Non-Small Cell Lung Cancer Treatment with Pembrolizumab in Tumors Expressing PD-L1 ≥ 50% in Patients Treated in Quebec’s University Teaching Hospitals (DALP-First Study). Curr. Oncol. 2023, 30, 3251-3262. https://doi.org/10.3390/curroncol30030247
Bérard G, Guévremont C, Marcotte N, Schroeder C, Bouchard N, Rajan R on behalf of the Programme de Gestion Thérapeutique des Médicaments (PGTM). Descriptive Analysis of First-Line Non-Small Cell Lung Cancer Treatment with Pembrolizumab in Tumors Expressing PD-L1 ≥ 50% in Patients Treated in Quebec’s University Teaching Hospitals (DALP-First Study). Current Oncology. 2023; 30(3):3251-3262. https://doi.org/10.3390/curroncol30030247
Chicago/Turabian StyleBérard, Ghislain, Chantal Guévremont, Nathalie Marcotte, Coleen Schroeder, Nicole Bouchard, and Raghu Rajan on behalf of the Programme de Gestion Thérapeutique des Médicaments (PGTM). 2023. "Descriptive Analysis of First-Line Non-Small Cell Lung Cancer Treatment with Pembrolizumab in Tumors Expressing PD-L1 ≥ 50% in Patients Treated in Quebec’s University Teaching Hospitals (DALP-First Study)" Current Oncology 30, no. 3: 3251-3262. https://doi.org/10.3390/curroncol30030247
APA StyleBérard, G., Guévremont, C., Marcotte, N., Schroeder, C., Bouchard, N., & Rajan, R., on behalf of the Programme de Gestion Thérapeutique des Médicaments (PGTM). (2023). Descriptive Analysis of First-Line Non-Small Cell Lung Cancer Treatment with Pembrolizumab in Tumors Expressing PD-L1 ≥ 50% in Patients Treated in Quebec’s University Teaching Hospitals (DALP-First Study). Current Oncology, 30(3), 3251-3262. https://doi.org/10.3390/curroncol30030247






