Abstract
Background: Metastatic renal cell carcinoma (mRCC) is an aggressive cancer characterised by an increased recurrence rate and an inadequate response to treatment. This study aimed to investigate the importance of the neutrophil-to-lymphocyte ratio (NLR) as a prognostic marker for long-term survival in patients with mRCC. Methods: We retrospectively analysed data from 74 patients with mRCC treated at our medical centre with tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs). We evaluated the predictive value of NLR for overall survival (OS) in these patients. Results: The median OS was 5.1 months in the higher NLR group (≥3) and 13.3 months in the lower NLR group (<3) (p < 0.0001). There was no significant difference in the OS between the TKI and ICI therapies in the low NLR group (12.9 vs. 13.6 months, p = 0.411) or in the high NLR group (4.7 vs. 5.5 months, p = 0.32). Both univariate and multivariate analyses revealed that a higher NLR was an independent prognostic factor of long-term survival in patients with mRCC treated with first-line therapy. Conclusions: This retrospective study showed that adding NLR to other Memorial Sloan Kettering Cancer Center (MSKCC) and International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) variables might improve the prognostic and predictive power of these models.
1. Introduction
The most common histological type of renal cell carcinoma (RCC) is clear cell carcinoma (ccRCC), which accounts for approximately 85% of all cases. It is the sixth most common type of cancer in men and the ninth most common type in women [1,2].
Renal cell carcinoma is a well-vascularized and immunogenic tumour characterized by a massive infiltration of various immune cells. Consequently, the current therapeutic approaches include anti-angiogenic agents, cancer immunotherapy, or both [3,4].
An elevated neutrophil-to-lymphocyte ratio (NLR) can reflect both the presence of neutrophilia and lymphopenia and may suggest impaired cell-mediated immunity in patients with cancer. Therefore, NLR is considered a robust prognostic biomarker in certain tumours, including digestive or genitourinary cancers [5,6,7].
Hence, can we incorporate the NLR, which is easily calculated using complete blood cell counts and widely measured in daily clinical practice?
Even reporting the clinical experience of a small number of patients may aid in the identification of potential additional biomarkers for predicting survival and enhancing patient management.
2. Materials and Methods
We retrospectively analysed 74 eligible patients with metastatic renal clear cell carcinoma treated at our department of medical oncology at the Elias Emergency University Clinic Hospital, Bucharest, Romania, from the 1 January 2020 to the 31 October 2022.
The selection criteria were as follows: a histologic diagnosis of metastatic or locally advanced unresectable RCC, clear cell histology and aged over 18 years. Informed consent was obtained from all subjects involved in the study. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Elias Emergency University Clinic Hospital (no. 7170/12 January 2023)
All the patients were deemed eligible for first-line therapy with tyrosine kinase inhibitors or immunotherapy, depending on the risk assessed with the IMDC and MSKCC prognostic models.
The study population was stratified into NLR low (<3) or NLR high (>3) according to a cut-off point value established at three. We performed the receiver-operating characteristic (ROC) curve analysis by calculating the area under the curve (AUC) to determine the specific cut-off values of NLR. Univariate and multivariate analyses were used to evaluate factors influencing the response to first-line therapy. The parameters analysed were age at diagnosis, gender, tumoral stage, histology, metastatic sites, various serum variables, and the neutrophil-to-lymphocyte ratio (NRL). The data were analysed using the Kaplan–Meier method and log-rank tests. Statistical significance was established when p < 0.05.
3. Results
3.1. Patient Characteristics
All the patients had a clear cell histology. The clinicopathological features of the 74 patients are listed in Table 1.

Table 1.
The clinical–pathological characteristics.
3.2. The Relationship between Clinicopathological Parameters and Survival
According to the univariate analysis, poor cancer-specific survival had significant relationships with a Karnofsky score of <80% (HR 11.60, 95%; p < 0.001), late treatment initiation (over 12 months) (HR 1.04 95% CI p = 0.009), haemoglobin < LLN (HR 5.52 CI 95% p = 0.002), LHD over 1.5 times ULN (HR 3.31, 95% CI p = 0.002), an NLR of ≥3 (HR 10.31, 95% CI p < 0.001) and high IMDC and MSKCC scores (HR 6.11, 95% CI, p < 0.001) (Supplementary Table S1, Table 2).

Table 2.
Univariate analysis.
On multivariate analysis, only a Karnofsky score of <80% (HR 16.008, 95% CI p = 0.009), a time from diagnosis to the start of systemic treatment of >12 months (HR 10.819, 95% CI p = 0.0011) and an NLR of ≥ 3 (HR 4.650, 95% CI p = 0.006) were significantly and independently associated with inferior overall survival (Table 3, Figure 1).

Table 3.
Multivariate analysis.
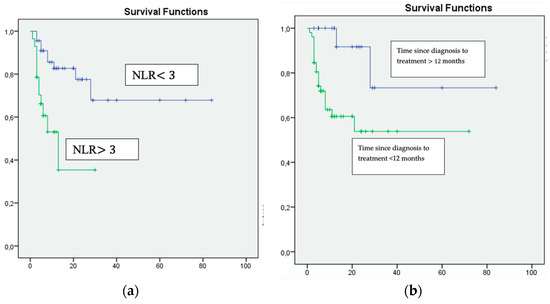
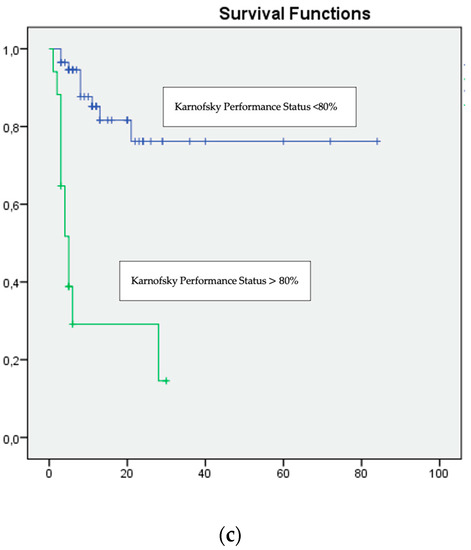
Figure 1.
Multivariate analysis: (a) NLR ≥ 3, (b) Time since diagnosis to treatment <12 months and (c) Karnofsky Performance Status <80%, were associated with unfavourable survival.
Systemic therapy was administered to all the patients after the diagnosis of mRCC. TKIs were used most frequently (n = 50, 67.5%). Immunotherapy with Nivolumab and Ipilimumab was used in 24 (32.4 %) patients.
The median follow-up was conducted for 15.3 (range, 4.3–22.6) months. The overall survival for all patients was a median of 12.7 months. In the entire cohort, the median overall survival for patients with an NLR of ≤3 was 13.3 months vs. 5.1 months for those with an NLR of >3.
There was no significant difference in the OS between the TKI and ICI therapies in the low NLR group (12.9 months vs. 13.6 months, p = 0.411) or in the high NLR group (4.7 months vs. 5.5 months, p = 0.32).
4. Discussion
Renal cell carcinoma represents 2.4% of all cancer diagnoses, and its incidence has increased globally over the last two decades [8].
Surgery can be a curative procedure for a minority of patients who present with early-stage disease. However, for advanced and metastatic stages, systemic therapy is essential. Renal cell carcinoma is a highly immunogenic and chemotherapy-resistant tumour [9]. Currently, anti-angiogenic drugs and immune checkpoint inhibitors have been established as the new standard of care for patients with mRCC [10,11]. Immuno-oncology-based doublet combinations have a highly significant effect on patients with an intermediate or poor prognosis [12]. Therefore, combination therapy is only the best choice for some patients. However, monotherapy with TKI may be an appropriate treatment option for favourable-risk patients to prevent the potential toxicities associated with ICI therapy [13].
Although most factors that affect the prognosis are related to the tumour pathology and the patient’s clinical and biological characteristics, the potential outcome for each patient remains uncertain.
IMDC and MSKCC criteria are already broadly adopted to estimate RCC patient prognosis. However, can we improve these scores?
Studying the role of cellular inflammatory markers in the interaction between immune response and cancer is challenging. The neutrophil-to-lymphocyte ratio (NLR) reflects a dynamic balance between innate and adaptive immune activity. Therefore, a high NLR suggests chronic inflammation and immune distress [14,15].
Today, NLR is widely reported as a reliable and readily available prognostic marker in various solid cancers, but with no widely accepted cut-off point. Normal NLR values are between one and two. Elevated values, defined as an NLR of ≥3, are regarded as pathological [16,17].
Recent published studies of patients with solid metastatic tumours have shown (using multivariate Cox regressions and time-dependent sensitivity analysis) that the optimal NLR cut-off value varies from 2.5 to 5 [18,19,20]. Therefore, our cut-off point of three was based on a previous analysis with similar findings [21]. For example, an extensive systematic review and meta-analysis investigated the association between NLR, disease-free progression and overall survival in 18 studies with 2735 patients selected. The results indicated that an elevated pre-treatment NLR of ≥3 was significantly associated with poorer OS and DFS (HR = 2.31, 95% p < 0.001) [22].
According to our study, a high baseline NLR (≥3) was correlated with a worse OS (13.3 months vs. 5.1 months, p = 0.001) with no considerable differences between patients treated with TKIs or ICIs (4.7 months vs. 5.5 months, p = 0.32).
Our findings agree with those of A. K. A Lalani from Dana–Farber Cancer Institute, who reported a significantly longer survival for patients treated with PD-1/PD-L1 immunotherapy and a low NLR at baseline. In addition, a maintained low NLR after six weeks of treatment further improved outcomes [23]. Similarly, a large systematic review by Chen X also demonstrated that a high NLR at baseline or pre-therapy was significantly associated with a worse overall survival (HR, 2.23; 95% CI, 1.84–2.70; p < 0.001) in patients with mRCC treated with ICIs [24].
Several studies evaluating the NLR as a personalized outcome prediction tool in patients treated with TKIs have clearly established an increased NLR value as a negative prognostic factor [25,26,27]. For instance, A.J. Templeton confirmed in a retrospective analysis of 5549 subjects with mRCC that a higher NLR at baseline was associated with an adverse OS and PFS. In this context, an increase in NLR after six weeks of therapy reassured that the therapy was associated with a good clinical response and better survival [28].
These data support our study’s conclusion about the significative predictive value of NLR in patients with RCC receiving both immunotherapy and TKIs.
Despite the rapidly growing body of literature on NLR, the mechanism underlying the association of this marker of inflammation remains poorly understood. Our results encourage the routine monitoring of NLR to predict recurrence, progress and survival outcomes in patients with RCC.
This study has several limitations. First, it is a retrospective analysis of a small number of patients who received different first-line regimens, including TKI or ICIs, according to the approved therapies available in Romania. Moreover, the data were collected during the COVID-19 pandemic, which caused treatment modifications and immensely disrupted the therapies’ acceptability and availability.
5. Conclusions
A survival prognosis is essential but very challenging. This study confirms that a high pre-treatment neutrophil-to-lymphocyte ratio of ≥3 predicts an unfavourable outcome in patients with advanced RCC treated with first-line ICIs or TKIs. In addition, in our univariate and multivariate models for OS, a poor performance status and ≥one-year interval between diagnosis and treatment initiation were also associated with inferior outcomes. Therefore, NLR may be considered an additional variable that improves the prognostic prediction of the IMDC and MSKCC models. However, a more extensive prospective study is needed to validate these results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol30020187/s1, Table S1: Statistical analysis of potential prognostic markers.
Author Contributions
Conceptualization, A.I.P.; Methodology, C.B. and C.N.; Formal analysis, A.I.P.; Investigation, A.I.P.; Writing—original draft, A.I.P.; Writing—review and editing, C.O.S.; Supervision, C.F.P., I.M.S., H.-T.C., R.C.V., A.-M.P., M.O., C.I., L.I.B., I.F.B. and O.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Elias Emergency University Clinic Hospital (no. 7170/12 January 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data supporting this study are included within the article and supporting materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, I.; Pircher, A.; Pichler, R. Targeting the Tumor Microenvironment in Renal Cell Cancer Biology and Therapy. Front. Oncol. 2019, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Bamias, A.; Escudier, B.; Sternberg, C.N.; Zagouri, F.; Dellis, A.; Djavan, B.; Tzannis, K.; Kontovinis, L.; Stravodimos, K.; Papatsoris, A.; et al. Current Clinical Practice Guidelines for the Treatment of Renal Cell Carcinoma: A Systematic Review and Critical Evaluation. Oncologist 2017, 22, 667–679. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, R.; Ren, F.; Guo, R.; Zhang, P. Prognostic and clinicopathological significance of neutrophil-to-lymphocyte ratio in patients with oral cancer. Biosci. Rep. 2018, 38, BSR20181550. [Google Scholar] [CrossRef]
- Faria, S.S.; Fernandes, P.C., Jr.; Silva, M.J.; Lima, V.C.; Fontes, W.; Eterovic, A.K.; Forget, P. The neutrophil-to-lymphocyte ratio: A narrative review. Ecancermedicalscience 2016, 10, 702. [Google Scholar] [CrossRef]
- Mjaess, G.; Chebel, R.; Karam, A.; Moussa, I.; Pretot, D.; Abi Tayeh, G.; Sarkis, J.; Semaan, A.; Peltier, A.; Aoun, F.; et al. Prognostic role of neutrophil-to-lymphocyte ratio (NLR) in urological tumors: An umbrella review of evidence from systematic reviews and meta-analyses. Acta Oncol. 2021, 60, 704–713. [Google Scholar]
- World Health Organization (WHO). Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 10 February 2023).
- Díaz-Montero, C.M.; Rini, B.I.; Finke, J.H. The immunology of renal cell carcinoma. Nat. Rev. Nephrol. 2020, 16, 721–735. [Google Scholar] [CrossRef]
- Jonasch, E.; Atkins, M.B.; Chowdhury, S.; Mainwaring, P. Combination of Anti-Angiogenics and Checkpoint Inhibitors for Renal Cell Carcinoma: Is the Whole Greater Than the Sum of Its Parts? Cancers 2022, 14, 644. [Google Scholar] [CrossRef]
- Song, Y.; Fu, Y.; Xie, Q.; Zhu, B.; Wang, J.; Zhang, B. Anti-angiogenic Agents in Combination With Immune Checkpoint Inhibitors: A Promising Strategy for Cancer Treatment. Front. Immunol. 2020, 11, 1956. [Google Scholar] [CrossRef]
- Rossi, E.; Bersanelli, M.; Gelibter, A.J.; Borsellino, N.; Caserta, C.; Doni, L.; Maruzzo, M.; Mosca, A.; Pisano, C.; Verzoni, E.; et al. Combination Therapy in Renal Cell Carcinoma: The Best Choice for Every Patient? Curr. Oncol. Rep. 2021, 23, 147. [Google Scholar] [CrossRef] [PubMed]
- Fontes-Sousa, M.; Magalhães, H.; Oliveira, A.; Carneiro, F.; Dos Reis, F.P.; Madeira, P.S.; Meireles, S. Reviewing Treatment Options for Advanced Renal Cell Carcinoma: Is There Still a Place for Tyrosine Kinase Inhibitor (TKI) Monotherapy? Adv. Ther. 2022, 39, 1107–1125. [Google Scholar] [CrossRef] [PubMed]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef] [PubMed]
- Isaac, V.; Wu, C.Y.; Huang, C.T.; Baune, B.T.; Tseng, C.L.; McLachlan, C.S. Elevated neutrophil to lymphocyte ratio predicts mortality in medical inpatients with multiple chronic conditions. Medicine 2016, 95, e3832, Erratum in Medicine 2016, 95, e0916. [Google Scholar] [CrossRef]
- Otunctemur, A.; Dursun, M.; Besiroglu, H.; Ozer, K.; Horsanali, O.; Ozbek, E. Clinical Significance of Preoperative Neutrophil-to-Lymphocyte Ratio in Renal Cell Carcinoma. Int. Braz. J. Urol. 2016, 42, 678–684. [Google Scholar] [CrossRef]
- Allenet, C.; Klein, C.; Rouget, B.; Margue, G.; Capon, G.; Alezra, E.; Blanc, P.; Estrade, V.; Bladou, F.; Robert, G.; et al. Can Pre-Operative Neutrophil-to-Lymphocyte Ratio (NLR) Help Predict Non-Metastatic Renal Carcinoma Recurrence after Nephrectomy? (UroCCR-61 Study). Cancers 2022, 14, 5692. [Google Scholar] [CrossRef]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy 2021, 122, 474–488. [Google Scholar] [CrossRef]
- Setakornnukul, J.; Chanvimalueng, W.; Patumanond, J.; Thephamongkhol, K. Cutoff point of neutrophil-to-lymphocyte ratio for predicting survival in nasopharyngeal carcinoma. Medicine 2021, 100, e27095. [Google Scholar] [CrossRef]
- Forget, P.; Khalifa, C.; Defour, J.P.; Latinne, D.; van Pel, M.C.; de Kock, M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes 2017, 10, 12. [Google Scholar] [CrossRef]
- Hu, K.; Lou, L.; Ye, J.; Zhang, S. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: A meta-analysis. BMJ Open 2015, 5, e006404. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, B.; Jia, W.; Zhang, Z.; Chen, Q.; Wang, D. Prognostic value of pretreatment neutrophil-to-lymphocyte ratio in renal cell carcinoma: A systematic review and meta-analysis. BMC Urol. 2020, 20, 90. [Google Scholar] [CrossRef]
- Lalani, A.A.; Xie, W.; Martini, D.J.; Steinharter, J.A.; Norton, C.K.; Krajewski, K.M.; Duquette, A.; Bossé, D.; Bellmunt, J.; van Allen, E.M.; et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J. Immunother. Cancer 2018, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Meng, F.; Jiang, R. Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker for Patients With Metastatic Renal Cell Carcinoma Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 746976. [Google Scholar] [CrossRef] [PubMed]
- Na, N.; Yao, J.; Cheng, C.; Huang, Z.; Hong, L.; Li, H.; Qiu, J. Meta-analysis of the efficacy of the pretreatment neutrophil-to-lymphocyte ratio as a predictor of prognosis in renal carcinoma patients receiving tyrosine kinase inhibitors. Oncotarget 2016, 7, 44039–44046. [Google Scholar] [CrossRef]
- Shang, B.; Guo, L.; Shen, R.; Cao, C.; Xie, R.; Jiang, W.; Wen, L.; Bi, X.; Shi, H.; Zheng, S.; et al. Prognostic Significance of NLR About NETosis and Lymphocytes Perturbations in Localized Renal Cell Carcinoma With Tumor Thrombus. Front. Oncol. 2021, 11, 771545. [Google Scholar] [CrossRef]
- Nishiyama, N.; Hirobe, M.; Kikushima, T.; Matsuki, M.; Takahashi, A.; Yanase, M.; Ichimatsu, K.; Egawa, M.; Hayashi, N.; Negishi, T.; et al. The neutrophil-lymphocyte ratio has a role in predicting the effectiveness of nivolumab in Japanese patients with metastatic renal cell carcinoma: A multi-institutional retrospective study. BMC Urol. 2020, 20, 110. [Google Scholar] [CrossRef]
- Templeton, A.J.; Knox, J.J.; Lin, X.; Simantov, R.; Xie, W.; Lawrence, N.; Broom, R.; Fay, A.P.; Rini, B.; Donskov, F.; et al. Change in Neutrophil-to-lymphocyte Ratio in Response to Targeted Therapy for Metastatic Renal Cell Carcinoma as a Prognosticator and Biomarker of Efficacy. Eur. Urol. 2016, 70, 358–364. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).