Real-World Molecular Biomarker Testing Patterns and Results for Advanced Gastroesophageal Cancers in the United States
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Demographics and Baseline Characteristics
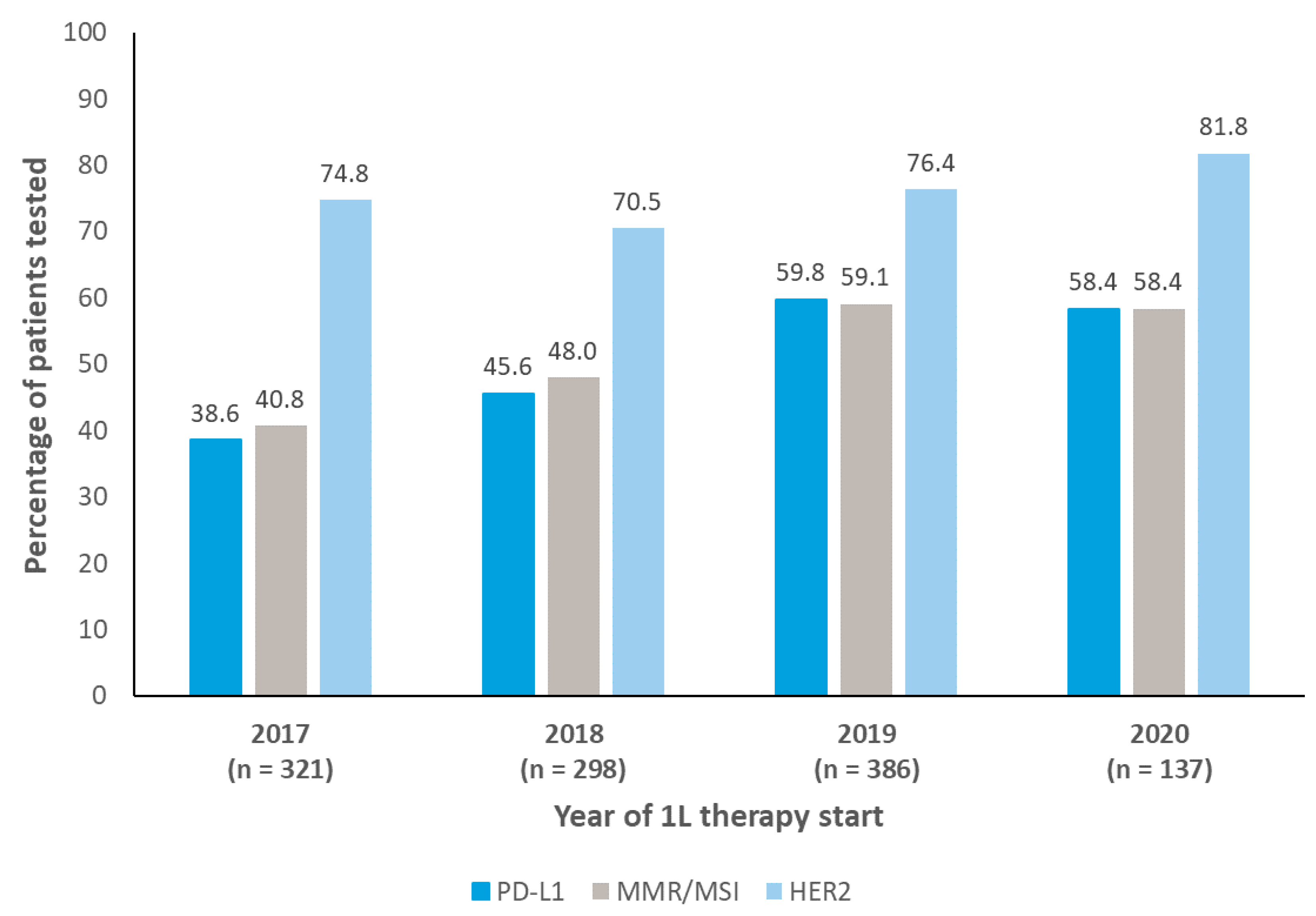
PD-L1, MMR/MSI, and HER2 Testing
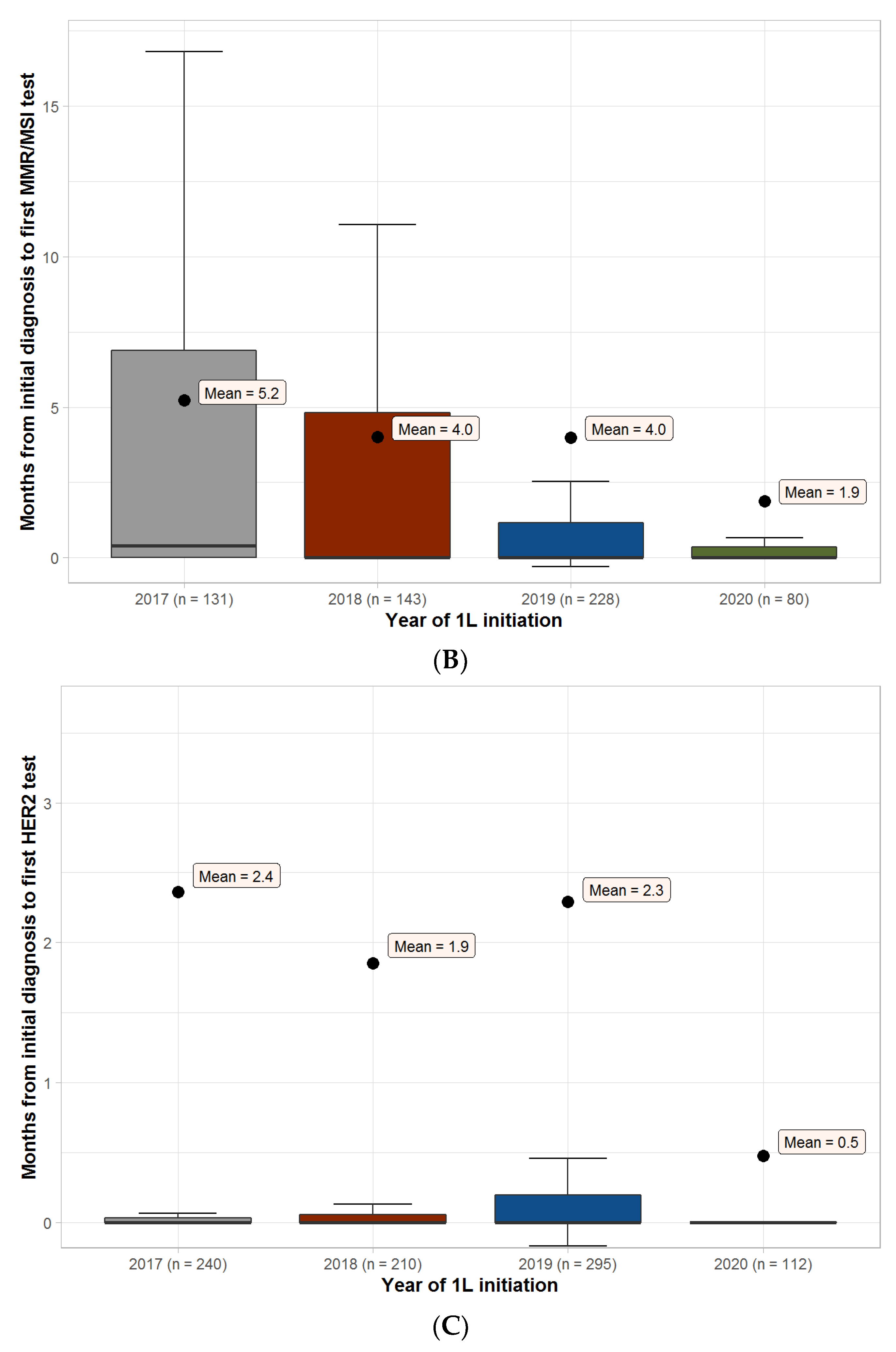
3.2. Overall Biomarker Testing Details
Biomarker Testing Combinations
3.3. Biomarker Testing Rates by Primary Tumor Site and Histology
3.4. Treatment Sequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Estimated Number of New Cases, Worldwide, both Sexes, all Ages (excl. NMSC). International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&include_nmsc_other=1 (accessed on 29 August 2022).
- National Cancer Institute. Esophageal Cancer—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/esoph.html (accessed on 29 August 2022).
- National Cancer Institute. Stomach Cancer—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/stomach.html (accessed on 29 August 2022).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.Z.; Almhanna, K. Beyond Chemotherapy, PD-1, and HER-2: Novel Targets for Gastric and Esophageal Cancer. Cancers 2021, 13, 4322. [Google Scholar] [CrossRef]
- Catenacci, D.V.; Chao, J.; Muro, K.; Al-Batran, S.E.; Klempner, S.J.; Wainberg, Z.A.; Shah, M.A.; Rha, S.Y.; Ohtsu, A.; Liepa, A.M.; et al. Toward a Treatment Sequencing Strategy: A Systematic Review of Treatment Regimens in Advanced Gastric Cancer/Gastroesophageal Junction Adenocarcinoma. Oncologist 2021, 26, e1704–e1729. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Corvera, C.; Das, P.; Denlinger, C.S.; Enzinger, P.C.; Fanta, P.; Farjah, F.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2019, 17, 855–883. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef]
- Kim, S.; Barzi, A.; Rajdev, L. Biomarker-driven targeted therapies for gastric/gastro-esophageal junction malignancies. Semin Oncol. 2018, 45, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Yashiro, M. Biomarkers of gastric cancer: Current topics and future perspective. World J. Gastroenterol. 2018, 24, 2818–2832. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. FDA Grants Accelerated Approval to Pembrolizumab for Advanced Gastric Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-advanced-gastric-cancer (accessed on 29 August 2022).
- Merck. Merck Provides Update on KEYTRUDA® (pembrolizumab) Indication in Third-Line Gastric Cancer in the US. Available online: https://www.merck.com/news/merck-provides-update-on-keytruda-pembrolizumab-indication-in-third-line-gastric-cancer-in-the-us/ (accessed on 29 August 2022).
- Chao, J.; Fuchs, C.S.; Shitara, K.; Tabernero, J.; Muro, K.; Van Cutsem, E.; Bang, Y.J.; De Vita, F.; Landers, G.; Yen, C.J.; et al. Assessment of Pembrolizumab Therapy for the Treatment of Microsatellite Instability-High Gastric or Gastroesophageal Junction Cancer Among Patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol. 2021, 7, 895–902. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. FDA Approves Pembrolizumab for Esophageal or GEJ Carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-esophageal-or-gej-carcinoma (accessed on 29 August 2022).
- U.S. Food & Drug Administration. FDA Approves Nivolumab in Combination with Chemotherapy for Metastatic Gastric Cancer and Esophageal Adenocarcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-combination-chemotherapy-metastatic-gastric-cancer-and-esophageal (accessed on 29 August 2022).
- Kato, K.; Shah, M.A.; Enzinger, P.; Bennouna, J.; Shen, L.; Adenis, A.; Sun, J.M.; Cho, B.C.; Ozguroglu, M.; Kojima, T.; et al. KEYNOTE-590: Phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. Future Oncol. 2019, 15, 1057–1066. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Vivaldi, C.; Catanese, S.; Massa, V.; Pecora, I.; Salani, F.; Santi, S.; Lencioni, M.; Vasile, E.; Falcone, A.; Fornaro, L. Immune Checkpoint Inhibitors in Esophageal Cancers: Are we Finally Finding the Right Path in the Mist? Int. J. Mol. Sci. 2020, 21, 1658. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.L.; Moon, S.; Adamson, B.J.; Baxi, S.S. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. Medrxiv 2020. [Google Scholar] [CrossRef]
- Birnbaum, B.; Nussbaum, N.; Seidl-Rathkopf, K.; Agrawal, M.; Estevez, M.; Estola, E.; Haimson, J.; He, L.; Larson, P.; Richardson, P. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv 2020, arXiv:2001.09765. [Google Scholar]
- Hess, L.M.; Li, X.; Wu, Y.; Goodloe, R.J.; Cui, Z.L. Defining treatment regimens and lines of therapy using real-world data in oncology. Future Oncol. 2021, 17, 1865–1877. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.1.0; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org (accessed on 29 August 2022).
- Egebjerg, K.; Garbyal, R.S.; Hasselby, J.P.; Baeksgaard, L.; Mau-Sørensen, M. Prevalence of HER2 overexpression and amplification in squamous cell carcinoma of the esophagus: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 161, 103339. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Choi, M.G.; Kim, K.; Kim, K.M.; Kim, S.T.; Park, S.H.; Cristescu, R.; Peter, S.; Lee, J. High PD-L1 expression in gastric cancer (GC) patients and correlation with molecular features. Pathol. Res. Pract. 2020, 216, 152881. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.J.; Fuchs, C.; Wyrwicz, L.; Lee, K.W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Kawazoe, A.; Yanez, P.; Li, N.; Lonardi, S.; Kolesnik, O.; Barajas, O.; Bai, Y.; Shen, L.; Tang, Y.; et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021, 600, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kawazoe, A.; Lordick, F.; Janjigian, Y.Y.; Shitara, K. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: An emerging paradigm. Nat. Rev. Clin. Oncol. 2021, 18, 473–487. [Google Scholar] [CrossRef]
- Mamdani, H.; Jalal, S.I. Where to Start and What to Do Next: The Sequencing of Treatments in Metastatic Esophagogastric Cancer. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, 170–185. [Google Scholar] [CrossRef] [PubMed]




| PD-L1 (N = 1142) | MMR/MSI (N = 1142) | |||
|---|---|---|---|---|
| Variable | PD-L1 Tested (N = 571) | PD-L1 Not Tested (N = 571) | MMR/MSI Tested (N = 582) | MMR/MSI Not Tested (N = 560) |
| Diagnosed initially with advanced disease (n (%)) | 489 (85.6) | 511 (89.5) | 498 (85.6) | 502 (89.6) |
| ECOG performance status at 1L start (n (%)) | ||||
| 0 | 178 (31.2) | 161 (28.2) | 184 (31.6) | 155 (27.7) |
| 1 | 202 (35.4) | 184 (32.2) | 211 (36.3) | 175 (31.3) |
| 2 | 55 (9.6) | 60 (10.5) | 52 (8.9) | 63 (11.3) |
| 3 | 16 (2.8) | 20 (3.5) | 15 (2.6) | 21 (3.8) |
| 4 | 0 | 2 (0.4) | 0 | 2 (0.4) |
| Not reported | 80 (14.0) | 104 (18.2) | 75 (12.9) | 109 (19.5) |
| Primary tumor site and histology (n (%)) a | ||||
| Esophageal | n = 258 | n = 283 | n = 248 | n = 293 |
| Adenocarcinoma | 187 (72.4) | 174 (61.4) | 194 (78.2) | 167 (56.9) |
| Adenosquamous | 2 (0.7) | 2 (0.7) | 2 (0.8) | 2 (0.6) |
| Squamous cell carcinoma | 67 (25.9) | 105 (37.1) | 49 (19.7) | 123 (41.9) |
| Other | 2 (0.7) | 2 (0.7) | 3 (1.2) | 1 (0.3) |
| Gastric | n = 187 | n = 159 | n = 203 | n = 143 |
| Adenocarcinoma | 180 (96.2) | 157 (98.7) | 199 (98.0) | 138 (96.5) |
| Adenosquamous | 1 (0.5) | 0 | 1 (0.4) | 0 |
| Other | 6 (3.2) | 2 (1.2) | 3 (1.4) | 5 (3.4) |
| Gastroesophageal junction | n = 126 | n = 129 | n = 131 | n = 124 |
| Adenocarcinoma | 122 (96.8) | 122 (94.5) | 126 (96.1) | 118 (95.1) |
| Adenosquamous | 0 | 1 (0.7) | 1 (0.7) | 0 |
| Squamous cell carcinoma | 1 (0.7) | 6 (4.6) | 1 (0.7) | 6 (4.8) |
| Other | 3 (0.2) | 0 | 3 (2.2) | 0 |
| Follow-up from 1L start, months (median (IQR)) | 10 (6, 15) | 9 (6, 15) | 10 (6, 15) | 9 (6, 14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehta, R.; Liepa, A.M.; Zheng, S.; Chatterjee, A. Real-World Molecular Biomarker Testing Patterns and Results for Advanced Gastroesophageal Cancers in the United States. Curr. Oncol. 2023, 30, 1869-1881. https://doi.org/10.3390/curroncol30020145
Mehta R, Liepa AM, Zheng S, Chatterjee A. Real-World Molecular Biomarker Testing Patterns and Results for Advanced Gastroesophageal Cancers in the United States. Current Oncology. 2023; 30(2):1869-1881. https://doi.org/10.3390/curroncol30020145
Chicago/Turabian StyleMehta, Rutika, Astra M. Liepa, Shen Zheng, and Anindya Chatterjee. 2023. "Real-World Molecular Biomarker Testing Patterns and Results for Advanced Gastroesophageal Cancers in the United States" Current Oncology 30, no. 2: 1869-1881. https://doi.org/10.3390/curroncol30020145
APA StyleMehta, R., Liepa, A. M., Zheng, S., & Chatterjee, A. (2023). Real-World Molecular Biomarker Testing Patterns and Results for Advanced Gastroesophageal Cancers in the United States. Current Oncology, 30(2), 1869-1881. https://doi.org/10.3390/curroncol30020145





