Correlation of Anticancer Drug Prices with Outcomes of Overall Survival and Progression-Free Survival in Clinical Trials in Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Therapeutic Drugs
2.2. Data Collection
2.3. Data Analysis
- Improvement OS (%) = (median OS (days) in experimental treatment arm)/(median OS (days) in control treatment arm);
- Improvement PFS (%) = (median PFS (days)in experimental treatment arm)/(median PFS (days) in control treatment arm);
- ΔOS (days) = (median OS [days] in experimental treatment arm) − (median OS (days) in control treatment arm);
- ΔPFS (days) = (median PFS [days] in experimental treatment arm) − (median PFS (days) in control treatment arm);
- The incremental drug costs of all anticancer drugs until disease progression or death in the experimental treatment arm were defined as the Cost Index (CI), as follows:
- CIpfs (Yen) = drug price/ΔPFS;
- CIos (Yen)= drug price/ΔOS.
2.4. Statistical Methods
3. Results
3.1. Background of Approved Drugs
3.2. Rates of Increase in OS and PFS Extension and Incremental Drug Costs
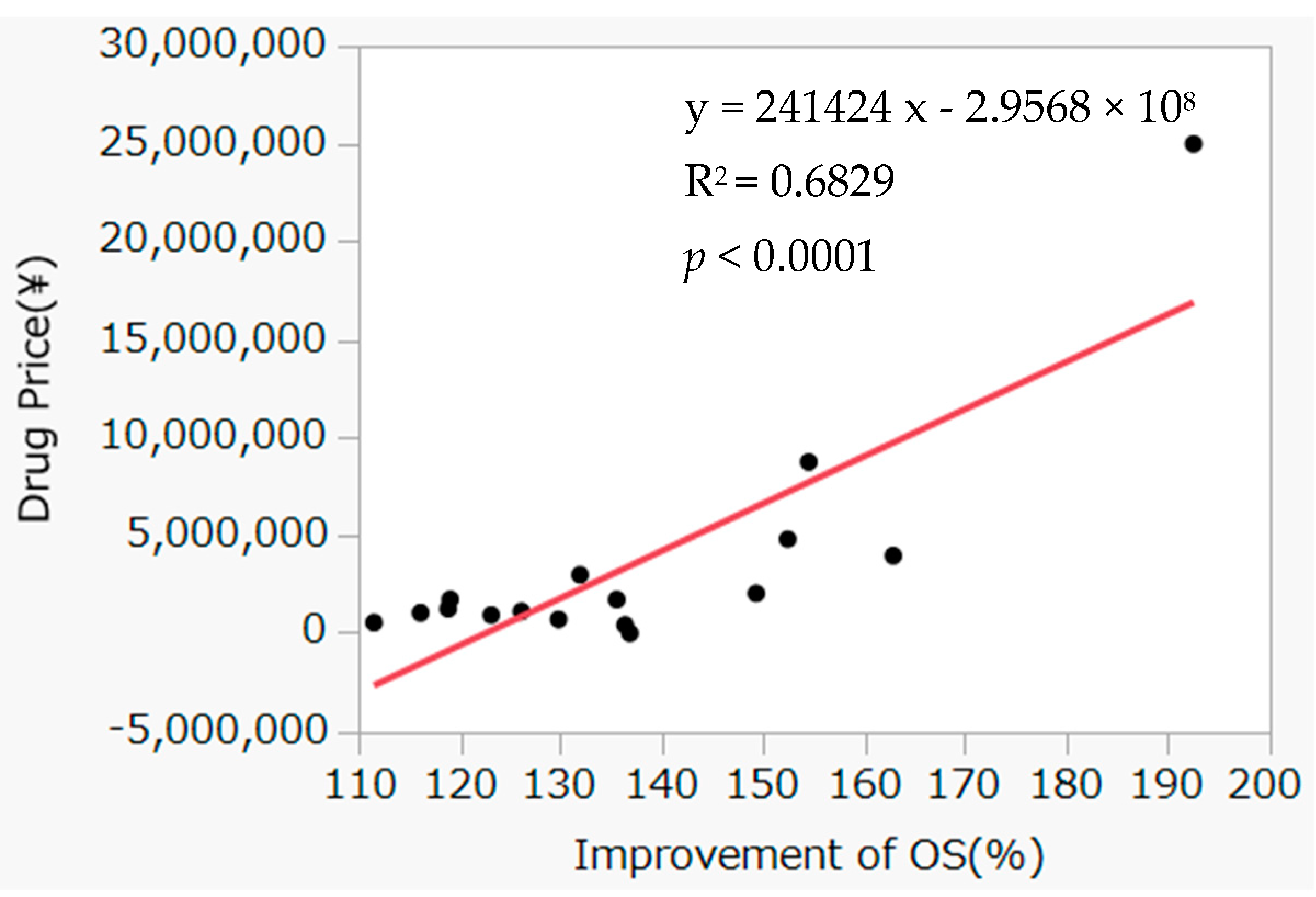
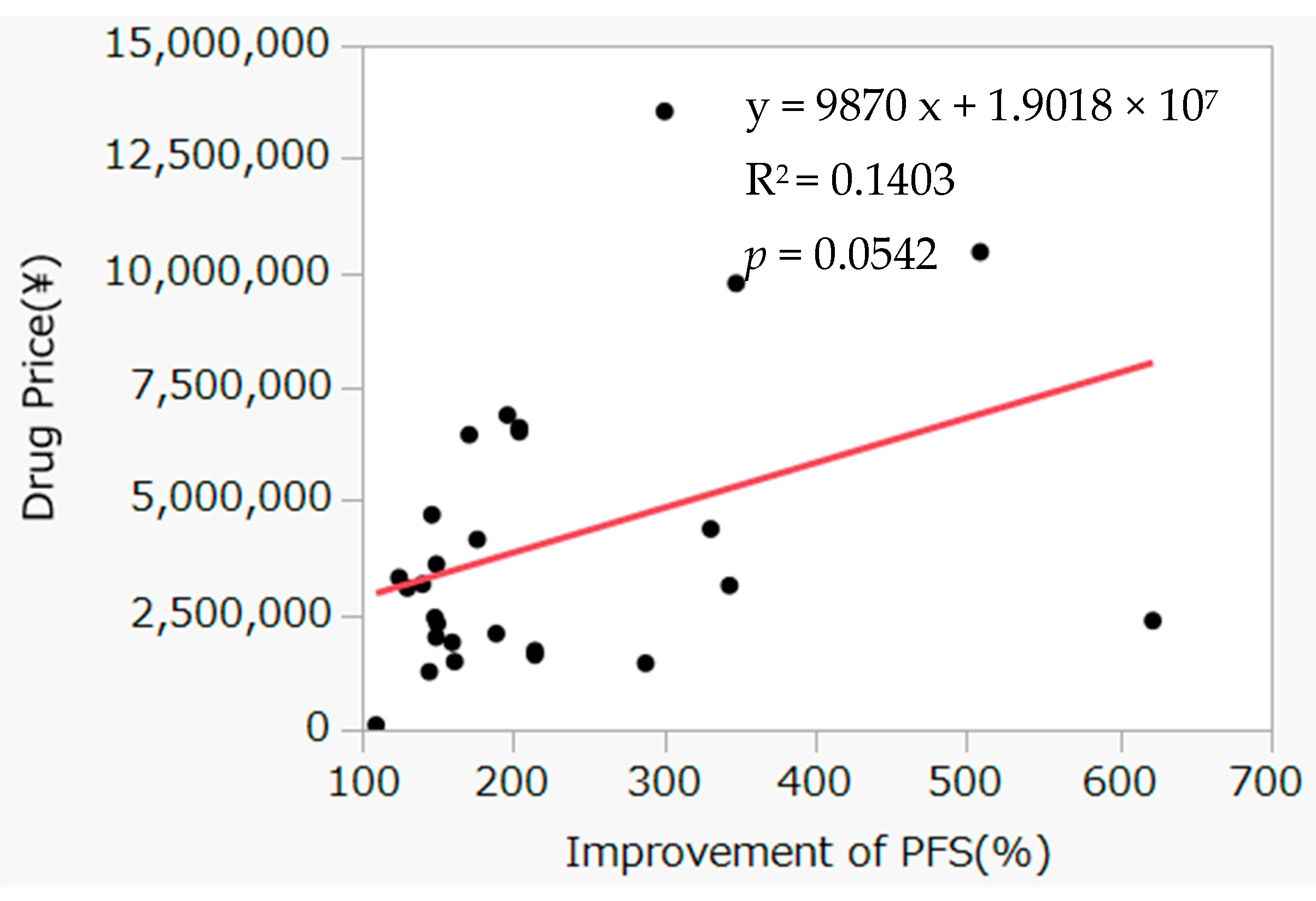
3.3. Correlation between Improvement Rate of OS and Incremental Drug Costs
4. Discussion
Future Issues and Definitions Based on the Present Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reich, M.R.; Shibuya, K. The future of Japan’s health system—Sustaining good health with equity at low cost. N. Engl. J. Med. 2015, 373, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, K.; Hashimoto, H.; Ikegami, N.; Nishi, A.; Tanimoto, T.; Miyata, H.; Takemi, K.; Reich, M.R. Future of Japan’s system of good health at low cost with equity: Beyond universal coverage. Lancet 2011, 378, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Ikegami, N.; Shibuya, K.; Izumida, N.; Noguchi, H.; Yasunaga, H.; Miyata, H.; Acuin, J.M.; Reich, M.R. Cost containment and quality of care in Japan: Is there a trade-off? Lancet 2011, 378, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Center. Cancer Statistics in Japan 2022. Available online: https://ganjoho.jp/public/qa_links/report/statistics/2022_en.html (accessed on 27 September 2022).
- Gregson, N.; Sparrowhawk, K.; Mauskopf, J.; Paul, J. Pricing medicines: Theory and practice, challenges and opportunities. Nat. Rev. Drug Discov. 2005, 4, 121–130. [Google Scholar] [CrossRef]
- Maeda, H.; Kurokawa, T. Acceptance of surrogate endpoints in clinical trials supporting approval of drugs for cancer treatment by the Japanese regulatory agency. Ann. Oncol. 2015, 26, 211–216. [Google Scholar] [CrossRef]
- Chen, E.Y.; Joshi, S.K.; Tran, A.; Prasad, V. Estimation of study time reduction using surrogate end points rather than overall survival in oncology clinical trials. JAMA Intern. Med. 2019, 179, 642–647. [Google Scholar] [CrossRef]
- He, Q.; Li, Q.; Lv, F.; Kaitin, K.I.; Shao, L. A survey of survival outcomes for targeted cancer drugs approved by the US Food and Drug Administration. Ther. Innov. Regul. Sci. 2021, 55, 676–684. [Google Scholar] [CrossRef]
- Kok, P.S.; Cho, D.; Yoon, W.H.; Ritchie, G.; Marschner, I.; Lord, S.; Friedlander, M.; Simes, J.; Lee, C.K. Validation of progression-free survival rate at 6 months and objective response for estimating overall survival in immune checkpoint inhibitor trials: A systematic review and meta-analysis. JAMA Netw. Open 2020, 3, e2011809. [Google Scholar] [CrossRef]
- Delgado, A.; Guddati, A.K. Clinical endpoints in oncology—A primer. Am. J. Cancer Res. 2021, 11, 1121–1131. [Google Scholar]
- Satoh, E.; Sasaki, Y.; Ohkuma, R.; Takahashi, T.; Kubota, Y.; Ishida, H.; Hamada, K.; Kiuchi, Y.; Tsunoda, T. Lack of correlation between the costs of anticancer drugs and clinical benefits in Japan. Cancer Sci. 2018, 109, 3896–3901. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Y.; Li, H.; Chen, Y.; Guo, Y.; Han, S.; Shi, L.; Guan, X. Prices and clinical benefit of national price-negotiated anticancer medicines in China. Pharmacoecon 2022, 40, 715–724. [Google Scholar] [CrossRef]
- Trotta, F.; Mayer, F.; Barone-Adesi, F.; Esposito, I.; Punreddy, R.; Da Cas, R.; Traversa, G.; Perrone, F.; Martini, N.; Gyawali, B.; et al. Anticancer drug prices and clinical outcomes: A cross-sectional study in Italy. BMJ Open 2019, 9, e033728. [Google Scholar] [CrossRef]
- Saluja, R.; Arciero, V.S.; Cheng, S.; McDonald, E.; Wong, W.W.L.; Cheung, M.C.; Chan, K.K.W. Examining trends in cost and clinical benefit of novel anticancer drugs over time. J. Oncol. Pract. 2018, 14, e280–e294. [Google Scholar] [CrossRef]
- Maeda, H.; Okabe, A.; Sakakura, K.; Ng, D.B.; Akazawa, M. Relationships between developmental strategies for additional indications and price revisions for anticancer drugs in Japan. BMC Health Serv. Res. 2021, 21, 1329. [Google Scholar] [CrossRef]
- Pharmaceuticals and Medical Devices Agency. Prescribed Drug Information Search. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuSearch/ (accessed on 27 September 2022). (In Japanese).
- Ministry of Health, Labour and Welfare. Central Council of Social Insurance Medical Services. Available online: https://www.mhlw.go.jp/stf/shingi/shingi-chuo_128153.html (accessed on 27 September 2022). (In Japanese).
- Ministry of Health, Labour and Welfare. Medical Care Insurance. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/iryouhoken/index.html (accessed on 27 September 2022). (In Japanese).
- Yakuji Nippo Ltd. NHI Drug Price Standards version October 2019; Yakuji Nippo Ltd.: Tokyo, Japan, 2019. (In Japanese) [Google Scholar]
- Jiho Inc. NHI Drug Price Standards Quick Reference Tables; Jiho Inc.: Tokyo, Japan, 2020. (In Japanese) [Google Scholar]
- Maeda, H.; Kurokawa, T. Recent trends for drug lag in clinical development of oncology drugs in Japan: Does the oncology drug lag still exist in Japan? Int. J. Clin. Oncol. 2015, 20, 1072–1080. [Google Scholar] [CrossRef]
- Maeda, H.; Fukuda, Y.; Uchida, M. Assessment of drugs approved by public knowledge-based applications (Kouchi-shinsei) during the last two decades in Japan. Clin. Pharmacol. Ther. 2021, 110, 1127–1135. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef]
- Takura, T. An evaluation of clinical economics and cases of cost-effectiveness. Intern. Med. 2018, 57, 1191–1200. [Google Scholar] [CrossRef]
- Shiroiwa, T.; Fukuda, T.; Ikeda, S.; Takura, T. New decision-making processes for the pricing of health technologies in Japan: The FY 2016/2017 pilot phase for the introduction of economic evaluations. Health Policy 2017, 121, 836–841. [Google Scholar] [CrossRef]
- Kido, K.; Matsumaru, N.; Tsukamoto, K. Health technology assessment in Japan: A pharmaceutical industry perspective. Ther. Innov. Regul. Sci. 2019, 53, 472–480. [Google Scholar] [CrossRef]
- Fukuda, A.; Igarashi, A. Universal health coverage and cancer drugs—A cost-effectiveness perspective (in Japanese). Gan Kagaku Ryoho 2016, 43, 1311–1315. [Google Scholar]
- Ministry of Health, Labour and Welfare. The Revision of the Guideline for Clinical Evaluation Methods of Anticancer Drugs in Japan. Available online: http://home.att.ne.jp/red/akihiro/anticancer/MHLW_gl_notice.pdf (accessed on 27 September 2022). (In Japanese).
- Kang, S.Y.; DiStefano, M.J.; Socal, M.P.; Anderson, G.F. Using external reference pricing in Medicare Part D to reduce drug price differentials with other countries. Health Aff. (Millwood) 2019, 38, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, G.M.; Kluetz, P.G.; Schneider, J.; Goldberg, K.B.; McKee, A.E.; Pazdur, R. Oncology drug approvals: Evaluating endpoints and evidence in an era of breakthrough therapies. Oncologist 2017, 22, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Cherla, A.; Naci, H.; Kesselheim, A.S.; Gyawali, B.; Mossialos, E. Assessment of coverage in England of cancer drugs qualifying for US Food and Drug Administration accelerated approval. JAMA Intern. Med. 2021, 181, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.; Fu, A.C.; Fisher, T.; Meletiche, D.; Pawar, V. Oncology drugs and added benefit: Insights from 3 European health technology assessment agencies on the role of efficacy endpoints. J. Med. Econ. 2022, 25, 1–6. [Google Scholar] [CrossRef]
- Kilickap, S.; Demirci, U.; Karadurmus, N.; Dogan, M.; Akinci, B.; Sendur, M.A.N. Endpoints in oncology clinical trials. J. BUON 2018, 23, 1–6. [Google Scholar]
- Ministry of Health, Labour and Welfare. Overview of Fundamental Reform of the NHI Drug Price System in Japan. Available online: https://www.mhlw.go.jp/file/06-Seisakujouhou-12400000-Hokenkyoku/0000114381_2.pdf (accessed on 27 September 2022). (In Japanese).
| Characteristic | Number | Percentage | |
|---|---|---|---|
| Compound type | Molecular-targeted drug | 43 | 53.8% |
| Antibody | 15 | 18.8% | |
| Cytotoxic drug | 11 | 13.8% | |
| Hormonal drug | 6 | 7.5% | |
| Immunotherapy drug | 5 | 6.3% | |
| Other | 0 | 0.0% | |
| Indication | Hematologic tumor | 30 | 37.5% |
| Solid tumor | 50 | 62.5% | |
| Dosage form | Oral | 43 | 53.8% |
| Injection | 37 | 46.3% | |
| Review period (days) | Average (±SD) | 73.1 (±44.4) | |
| Median (interquartile) | 21,694 (4855.0–93,396.8) | ||
| Maximum | 289 | ||
| Minimum | 21 | ||
| Primary endpoint support approval | OS | 18 | 22.5% |
| PFS | 28 | 35.0% | |
| Response rate | 13 | 16.3% | |
| Time to Progression | 3 | 3.8% | |
| Others | 18 | 22.5% | |
| NHI drug price | Average (±SD; Yen) | 88,416.2 (±148,974.7) | |
| Median (interquartile) (Yen) | 21,694 (4855.0–93,396.8) | ||
| <JPY 1000 | 2 | 2.5% | |
| <JPY 10,000 | 34 | 42.5% | |
| <JPY 100,000 | 25 | 31.3% | |
| <JPY 1,000,000 | 19 | 23.8% | |
| >JPY 1,000,000 | 0 | 0.0% | |
| Calculation system for NHI price standard | Cost accounting method | 24 | 30.0% |
| Similar efficacy comparison method (I) | 51 | 63.8% | |
| Similar efficacy comparison method (II) | 3 | 3.8% | |
| Inter-specification adjustment | 2 | 2.5% | |
| Corrective premium rate | With premium rate | 56 | 70.0% |
| None | 24 | 30.0% | |
| Type of pharmaceutical company | Japanese domestic company | 31 | 38.8% |
| Foreign company | 49 | 61.3% | |
| Overall Survival (n = 16) | Progression Free Survival (n = 28) | p Value | |
|---|---|---|---|
| Drug Price (Yen) | 208,877 | 1,372,417 | 0.0223 |
| Cost Index (Yen) | 205,431 | 64,540 | 0.2297 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okabe, A.; Hayashi, H.; Maeda, H. Correlation of Anticancer Drug Prices with Outcomes of Overall Survival and Progression-Free Survival in Clinical Trials in Japan. Curr. Oncol. 2023, 30, 1776-1783. https://doi.org/10.3390/curroncol30020137
Okabe A, Hayashi H, Maeda H. Correlation of Anticancer Drug Prices with Outcomes of Overall Survival and Progression-Free Survival in Clinical Trials in Japan. Current Oncology. 2023; 30(2):1776-1783. https://doi.org/10.3390/curroncol30020137
Chicago/Turabian StyleOkabe, Ayano, Haruto Hayashi, and Hideki Maeda. 2023. "Correlation of Anticancer Drug Prices with Outcomes of Overall Survival and Progression-Free Survival in Clinical Trials in Japan" Current Oncology 30, no. 2: 1776-1783. https://doi.org/10.3390/curroncol30020137
APA StyleOkabe, A., Hayashi, H., & Maeda, H. (2023). Correlation of Anticancer Drug Prices with Outcomes of Overall Survival and Progression-Free Survival in Clinical Trials in Japan. Current Oncology, 30(2), 1776-1783. https://doi.org/10.3390/curroncol30020137





