Incorporating VR-RENDER Fusion Software in Robot-Assisted Partial Prostatectomy: The First Case Report
Abstract
1. Introduction
2. Case
2.1. Initial Assessments
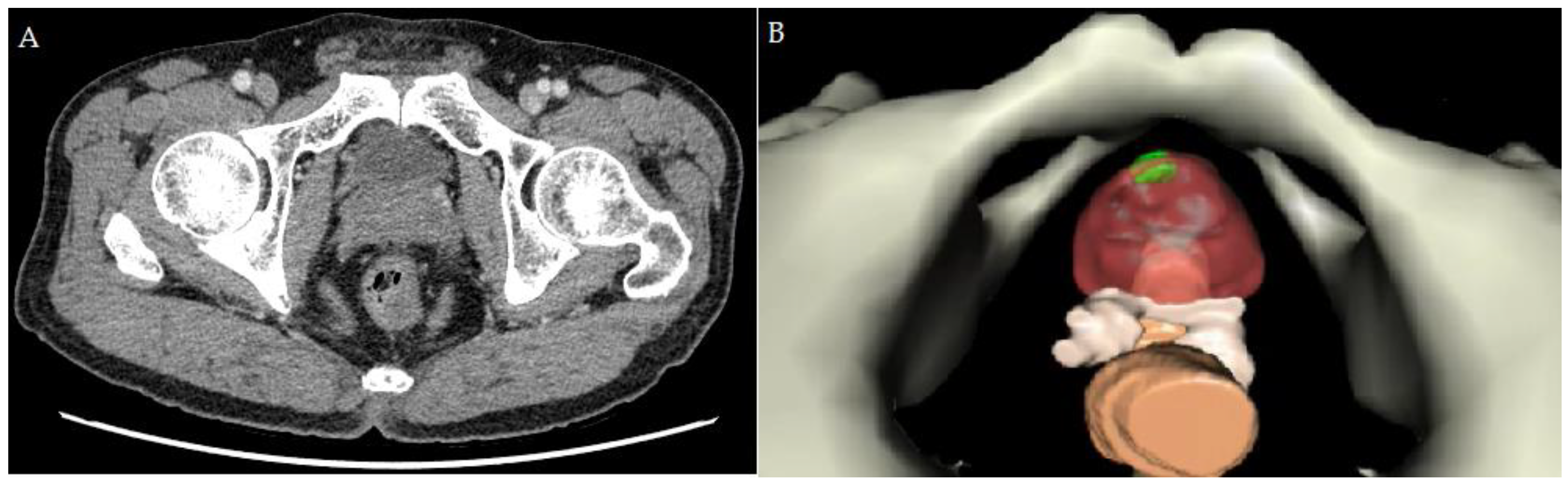
2.2. Creating the Personal 3D Model before RAPP
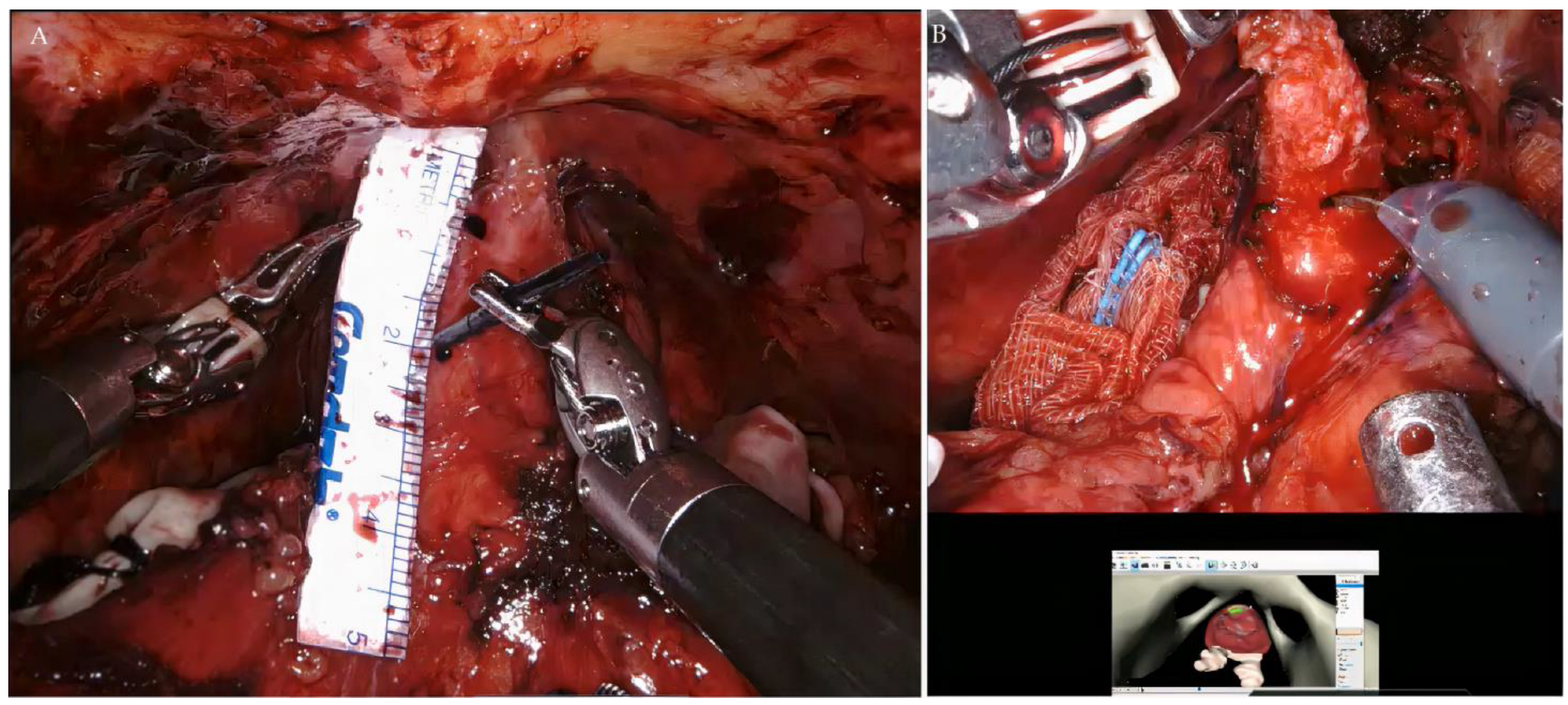
2.3. RAPP
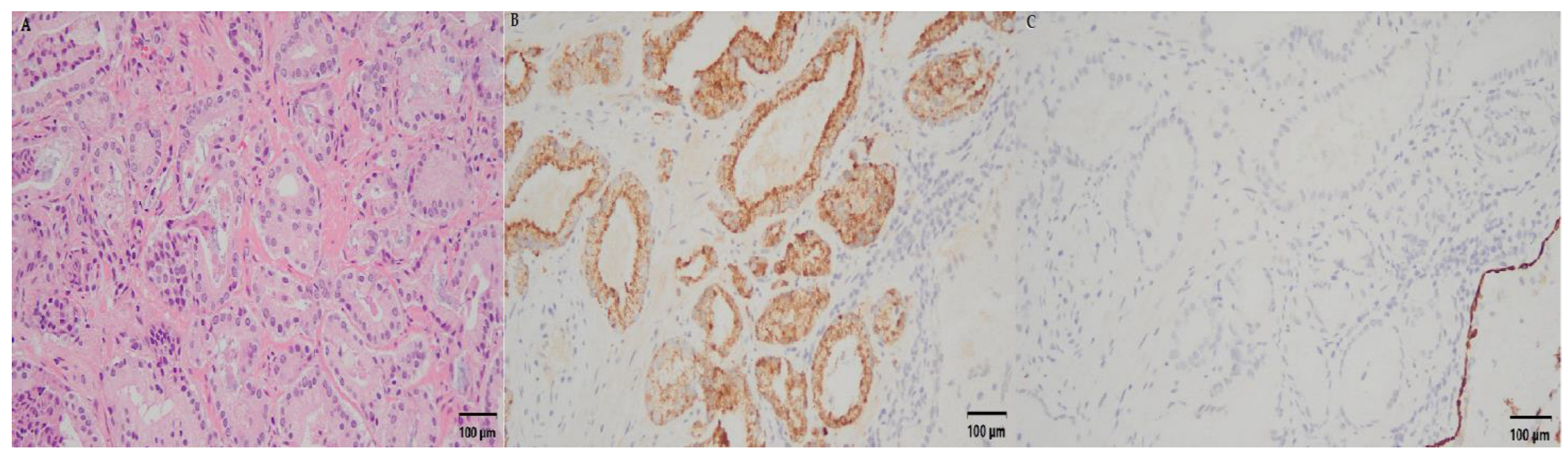
2.4. Oncological Outcome and Functional Outcome
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carroll, P.R.; Parsons, J.K.; Andriole, G.; Bahnson, R.R.; Castle, E.P.; Catalona, W.J.; Dahl, D.M.; Davis, J.W.; Epstein, J.I.; Etzioni, R.B.; et al. Prostate Cancer Early Detection, Version 2.2016. J. Natl. Compr. Cancer Netw. 2016, 14, 509–519. [Google Scholar] [CrossRef]
- Schaeffer, E.; Srinivas, S.; Antonarakis, E.S.; Armstrong, A.J.; Bekelman, J.E.; Cheng, H.; D’Amico, A.V.; Davis, B.J.; Desai, N.; Dorff, T.; et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.; Benfante, N.; Alvim, R.; Sjoberg, D.D.; Vickers, A.; Reuter, V.E.; Fine, S.W.; Vargas, H.A.; Wiseman, M.; Mamoor, M.; et al. Long-Term Outcomes of Active Surveillance for Prostate Cancer: The Memorial Sloan Kettering Cancer Center Experience. J. Urol. 2020, 203, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Tosoian, J.J.; Mamawala, M.; Epstein, J.I.; Landis, P.; Macura, K.J.; Simopoulos, D.N.; Carter, H.B.; Gorin, M.A. Active Surveillance of Grade Group 1 Prostate Cancer: Long-term Outcomes from a Large Prospective Cohort. Eur. Urol. 2020, 77, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Folkvaljon, Y.; Bratt, O.; Robinson, D.; Stattin, P. Defining Intermediate Risk Prostate Cancer Suitable for Active Surveillance. J. Urol. 2019, 201, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Willemse, P.-P.M.; Davis, N.F.; Grivas, N.; Zattoni, F.; Lardas, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Dell’Oglio, P.; Donaldson, J.F.; et al. Systematic Review of Active Surveillance for Clinically Localised Prostate Cancer to Develop Recommendations Regarding Inclusion of Intermediate-risk Disease, Biopsy Characteristics at Inclusion and Monitoring, and Surveillance Repeat Biopsy Strategy. Eur. Urol. 2022, 81, 337–346. [Google Scholar] [CrossRef]
- Chandrasekar, T.; Bowler, N.; Schneider, A.; Goldberg, H.; Mark, J.R.; Trabulsi, E.J.; Lallas, C.D.; Gomella, L.G. Outcomes of Active Surveillance for Men with Intermediate Risk Prostate Cancer: A Population-Based Analysis. Urology 2021, 155, 101–109. [Google Scholar] [CrossRef]
- Lantz, A.; Bock, D.; Akre, O.; Angenete, E.; Bjartell, A.; Carlsson, S.; Modig, K.K.; Nyberg, M.; Kollberg, K.S.; Steineck, G.; et al. Functional and Oncological Outcomes After Open Versus Robot-assisted Laparoscopic Radical Prostatectomy for Localised Prostate Cancer: 8-Year Follow-up. Eur. Urol. 2021, 80, 650–660. [Google Scholar] [CrossRef]
- Carbonara, U.; Srinath, M.; Crocerossa, F.; Ferro, M.; Cantiello, F.; Lucarelli, G.; Porpiglia, F.; Battaglia, M.; Ditonno, P.; Autorino, R. Robot-assisted radical prostatectomy versus standard laparoscopic radical prostatectomy: An evidence-based analysis of comparative outcomes. World J. Urol. 2021, 39, 3721–3732. [Google Scholar] [CrossRef]
- Wolff, I.; Burchardt, M.; Gilfrich, C.; Peter, J.; Baunacke, M.; Thomas, C.; Huber, J.; Gillitzer, R.; Sikic, D.; Fiebig, C.; et al. Patients Regret Their Choice of Therapy Significantly Less Frequently after Robot-Assisted Radical Prostatectomy as Opposed to Open Radical Prostatectomy: Patient-Reported Results of the Multicenter Cross-Sectional IMPROVE Study. Cancers 2022, 14, 5356. [Google Scholar] [CrossRef]
- Bravi, C.A.; Tin, A.; Benfante, N.; Salonia, A.; Briganti, A.; Montorsi, F.; Mulhall, J.P.; Eastham, J.A.; Vickers, A.J. Comparison of Two Methods for Assessing Erectile Function Before Radical Prostatectomy. Eur. Urol. Oncol. 2021, 4, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Hopstaken, J.S.; Bomers, J.G.; Sedelaar, M.J.; Valerio, M.; Fütterer, J.J.; Rovers, M.M. An Updated Systematic Review on Focal Therapy in Localized Prostate Cancer: What Has Changed over the Past 5 Years? Eur. Urol. 2022, 81, 5–33. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Jeong, W.; Palma-Zamora, I.; Abdollah, F.; Butaney, M.; Corsi, N.; Wurst, H.; Arora, S.; Kachroo, N.; Hassan, O.; et al. Description of Surgical Technique and Oncologic and Functional Outcomes of the Precision Prostatectomy Procedure (IDEAL Stage 1–2b Study). Eur. Urol. 2022, 81, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Jeong, W.; Keeley, J.; Abdollah, F.; Hassan, O.; Gupta, N.; Menon, M. Subtotal surgical therapy for localized prostate cancer: A single-center precision prostatectomy experience in 25 patients, and SEER-registry data analysis. Transl. Androl. Urol. 2021, 10, 3155–3166. [Google Scholar] [CrossRef]
- Villers, A.; Puech, P.; Flamand, V.; Haber, G.-P.; Desai, M.M.; Crouzet, S.; Leroy, X.; Chopra, S.; Lemaitre, L.; Ouzzane, A.; et al. Partial Prostatectomy for Anterior Cancer: Short-term Oncologic and Functional Outcomes. Eur. Urol. 2017, 72, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Diana, M.; Noll, E.; Diemunsch, P.; Dallemagne, B.; Benahmed, M.A.; Agnus, V.; Soler, L.; Barry, B.; Namer, I.J.; Demartines, N.; et al. Enhanced-reality video fluorescence: A real-time assessment of intestinal viability. Ann. Surg. 2014, 259, 700–707. [Google Scholar] [CrossRef]
- Ou, Y.-C.; Yang, C.-R.; Wang, J.; Yang, C.-K.; Cheng, C.-L.; Patel, V.R.; Tewari, A.K. The learning curve for reducing complications of robotic-assisted laparoscopic radical prostatectomy by a single surgeon. BJU Int. 2011, 108, 420–425. [Google Scholar] [CrossRef]
- Ou, Y.-C.; Hung, S.-W.; Wang, J.; Yang, C.-K.; Cheng, C.-L.; Tewari, A.K. Retro-apical transection of the urethra during robot-assisted laparoscopic radical prostatectomy in an Asian population. BJU Int. 2012, 110, E57–E63. [Google Scholar] [CrossRef]
- Ou, Y.; Yang, C.; Wang, J.; Hung, S.; Cheng, C.; Tewari, A.; Patel, V. The trifecta outcome in 300 consecutive cases of robotic-assisted laparoscopic radical prostatectomy according to D’Amico risk criteria. Eur. J. Surg. Oncol. (EJSO) 2013, 39, 107–113. [Google Scholar] [CrossRef]
- Ou, Y.-C.; Yang, C.-K.; Chang, K.-S.; Wang, J.; Hung, S.-W.; Tung, M.-C.; Tewari, A.K.; Patel, V.R. Prevention and Management of Complications During Robotic-assisted Laparoscopic Radical Prostatectomy Following Comprehensive Planning: A Large Series Involving a Single Surgeon. Anticancer Res. 2016, 36, 1991–1998. [Google Scholar]
- Welch, H.G.; Albertsen, P.C. Reconsidering Prostate Cancer Mortality—The Future of PSA Screening. N. Engl. J. Med. 2020, 382, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.A.; von Landenberg, N.; Cole, A.P.; Gild, P.; Choueiri, T.K.; Lipsitz, S.R.; Trinh, Q.-D.; Kibel, A.S. Contemporary national trends in prostate cancer risk profile at diagnosis. Prostate Cancer Prostatic Dis. 2020, 23, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Ngoo, K.S.; Honda, M.; Kimura, Y.; Yumioka, T.; Iwamoto, H.; Morizane, S.; Hikita, K.; Takenaka, A. Longitudinal study on the impact of urinary continence and sexual function on health-related quality of life among Japanese men after robot-assisted radical prostatectomy. Int. J. Med. Robot. Comput. Assist. Surg. 2019, 15, e2018. [Google Scholar] [CrossRef]
- Hanna, B.; Ranasinghe, W.; Lawrentschuk, N. Risk stratification and avoiding overtreatment in localized prostate cancer. Curr. Opin. Urol. 2019, 29, 612–619. [Google Scholar] [CrossRef]
- Borges, R.C.; Tourinho-Barbosa, R.R.; Glina, S.; Macek, P.; Mombet, A.; Sanchez-Salas, R.; Cathelineau, X. Impact of Focal Versus Whole Gland Ablation for Prostate Cancer on Sexual Function and Urinary Continence. J. Urol. 2021, 205, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Huber, P.M.; Afzal, N.; Arya, M.; Boxler, S.; Dudderidge, T.; Emberton, M.; Guillaumier, S.; Hindley, R.G.; Hosking-Jervis, F.; Leemann, L.; et al. Focal HIFU therapy for anterior compared to posterior prostate cancer lesions. World J. Urol. 2021, 39, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Ouzzane, A.; Puech, P.; Lemaitre, L.; Leroy, X.; Nevoux, P.; Betrouni, N.; Haber, G.-P.; Villers, A. Combined Multiparametric MRI and Targeted Biopsies Improve Anterior Prostate Cancer Detection, Staging, and Grading. Urology 2011, 78, 1356–1362. [Google Scholar] [CrossRef]
- Huber, P.M.; Afzal, N.; Arya, M.; Boxler, S.; Dudderidge, T.; Emberton, M.; Guillaumier, S.; Hindley, R.G.; Hosking-Jervis, F.; Leemann, L.; et al. Prostate Specific Antigen Criteria to Diagnose Failure of Cancer Control following Focal Therapy of Nonmetastatic Prostate Cancer Using High Intensity Focused Ultrasound. J. Urol. 2020, 203, 734–742. [Google Scholar] [CrossRef]
- Chen, P.Y.; Chiang, P.H.; Liu, Y.Y.; Chuang, Y.C.; Cheng, Y.T. Primary whole-gland ablation for localized prostate cancer with high-intensity focused ultrasound: The important predictors of biochemical recurrence. Int. J. Urol. 2018, 25, 615–620. [Google Scholar] [CrossRef]
- D’Agostino, J.; Wall, J.; Soler, L.; Vix, M.; Duh, Q.-Y.; Marescaux, J. Virtual Neck Exploration for Parathyroid Adenomas. JAMA Surg. 2013, 148, 232–238. [Google Scholar] [CrossRef]
- D’Agostino, J.; Diana, M.; Vix, M.; Nicolau, S.; Soler, L.; Bourhala, K.; Hassler, S.; Wu, H.-S.; Marescaux, J. Three-Dimensional Metabolic and Radiologic Gathered Evaluation Using VR-RENDER Fusion: A Novel Tool to Enhance Accuracy in the Localization of Parathyroid Adenomas. World J. Surg. 2013, 37, 1618–1625. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-H.; Chen, L.-H.; Lin, Y.-S.; Hsu, C.-Y.; Tung, M.-C.; Huang, S.-W.; Wu, C.-H.; Ou, Y.-C. Incorporating VR-RENDER Fusion Software in Robot-Assisted Partial Prostatectomy: The First Case Report. Curr. Oncol. 2023, 30, 1699-1707. https://doi.org/10.3390/curroncol30020131
Yang C-H, Chen L-H, Lin Y-S, Hsu C-Y, Tung M-C, Huang S-W, Wu C-H, Ou Y-C. Incorporating VR-RENDER Fusion Software in Robot-Assisted Partial Prostatectomy: The First Case Report. Current Oncology. 2023; 30(2):1699-1707. https://doi.org/10.3390/curroncol30020131
Chicago/Turabian StyleYang, Che-Hsueh, Li-Hsun Chen, Yi-Sheng Lin, Chao-Yu Hsu, Min-Che Tung, Shih-Wei Huang, Chi-Hsiang Wu, and Yen-Chuan Ou. 2023. "Incorporating VR-RENDER Fusion Software in Robot-Assisted Partial Prostatectomy: The First Case Report" Current Oncology 30, no. 2: 1699-1707. https://doi.org/10.3390/curroncol30020131
APA StyleYang, C.-H., Chen, L.-H., Lin, Y.-S., Hsu, C.-Y., Tung, M.-C., Huang, S.-W., Wu, C.-H., & Ou, Y.-C. (2023). Incorporating VR-RENDER Fusion Software in Robot-Assisted Partial Prostatectomy: The First Case Report. Current Oncology, 30(2), 1699-1707. https://doi.org/10.3390/curroncol30020131





