The Effects of Lack of Awareness in Age-Related Quality of Life, Coping with Stress, and Depression among Patients with Malignant Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Patient Selection Process and Surveying Methods
2.3. Data Collection and Variables
2.4. Statistical Analysis
3. Results
3.1. Demographical Data
3.2. Unstandardized Survey
3.3. Standardized Surveys
3.4. Correlation Analysis
4. Discussion
4.1. Literature Findings Associated with This Study
4.2. Study Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atkins, M.B.; Curiel-Lewandrowski, C.; Fisher, D.E.; Swetter, S.M.; Tsao, H.; Aguirre-Ghiso, J.A.; Soengas, M.S.; Weeraratna, A.T.; Flaherty, K.T.; Herlyn, M.; et al. Melanoma research foundation. the state of melanoma: Emergent challenges and opportunities. Clin. Cancer Res. 2021, 27, 2678–2697. [Google Scholar] [CrossRef] [PubMed]
- Aabed, H.; Bloanca, V.; Crainiceanu, Z.; Bratosin, F.; Citu, C.; Diaconu, M.M.; Ciorica, O.; Bratu, T. The impact of SARS-CoV-2 pandemic on patients with malignant melanoma at a Romanian academic center: A four-year retrospective analysis. Int. J. Environ. Res. Public Health 2022, 19, 8499. [Google Scholar] [CrossRef]
- Del Fiore, P.; Russo, I.; Ferrazzi, B.; Monico, A.D.; Cavallin, F.; Filoni, A.; Tropea, S.; Russano, F.; Di Prata, C.; Buja, A.; et al. Melanoma in adolescents and young adults: Evaluation of the characteristics, treatment strategies, and prognostic factors in a monocentric retrospective study. Front. Oncol. 2021, 11, 725523, Erratum in Front. Oncol. 2021, 11, 793169. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Qadir, M.I.; Ghafoor, S. Malignant melanoma: Skin cancer-diagnosis, prevention, and treatment. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 291–297. [Google Scholar] [CrossRef]
- Moreira, A.; Heinzerling, L.; Bhardwaj, N.; Friedlander, P. Current melanoma treatments: Where do we stand? Cancers 2021, 13, 221. [Google Scholar] [CrossRef]
- Sood, S.; Jayachandiran, R.; Pandey, S. Current advancements and novel strategies in the treatment of metastatic melanoma. Integr. Cancer Ther. 2021, 20, 1534735421990078. [Google Scholar] [CrossRef]
- Sacchetto, L.; Rosso, S.; Comber, H.; Bouchardy, C.; Broganelli, P.; Galceran, J.; Hackl, M.; Katalinic, A.; Louwman, M.; Robsahm, T.E.; et al. Skin melanoma deaths within 1 or 3 years from diagnosis in Europe. Int. J. Cancer 2021, 148, 2898–2905. [Google Scholar] [CrossRef]
- Quinlan, C.; Gill, R.; Murphy, M. Increasing melanoma awareness among health and social care professionals in secondary care in an era of reduced skin cancer referrals due to COVID-19. Clin. Exp. Dermatol. 2020, 45, 920–921. [Google Scholar] [CrossRef]
- Goddard, L.; Yorozuya, L.; Hirokane, J. Art of prevention: The importance of melanoma surveillance. Int. J. Womens Dermatol. 2020, 6, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Steeb, T.; Wessely, A.; Mastnik, S.; Brinker, T.J.; French, L.E.; Niesert, A.C.; Berking, C.; Heppt, M.V. Patient attitudes and their awareness towards skin cancer-related apps: Cross-sectional survey. JMIR mHealth uHealth 2019, 7, e13844. [Google Scholar] [CrossRef] [PubMed]
- Damude, S.; Hoekstra-Weebers, J.E.; van Leeuwen, B.L.; Hoekstra, H.J. Melanoma patients’ disease-specific knowledge, information preference, and appreciation of educational YouTube videos for self-inspection. Eur. J. Surg. Oncol. 2017, 43, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Vojvodic, A.; Vlaskovic-Jovicevic, T.; Vojvodic, P.; Vojvodic, J.; Goldust, M.; Peric-Hajzler, Z.; Matovic, D.; Sijan, G.; Stepic, N.; Nguyen, V.T.; et al. Psychological impact of melanoma, how to detect, support and help. Open Access. Maced. J. Med. Sci. 2019, 7, 3043–3045. [Google Scholar] [CrossRef]
- Wiens, L.; Schäffeler, N.; Eigentler, T.; Garbe, C.; Forschner, A. Psychological distress of metastatic melanoma patients during treatment with immune checkpoint inhibitors: Results of a prospective study. Cancers 2021, 3, 2642. [Google Scholar] [CrossRef]
- McGannon, K.R.; Berry, T.R.; Rodgers, W.M.; Spence, J.C. Breast cancer representations in Canadian news media: A critical discourse analysis of meanings and the implications for identity. Qual. Res. Psychol. 2016, 13, 188–207. [Google Scholar] [CrossRef]
- Trusson, D.; Pilnick, A.; Roy, S. A new normal?: Women’s experiences of biographical disruption and liminality following treatment for early stage breast cancer. Soc. Sci. Med. 2016, 151, 121–129. [Google Scholar] [CrossRef]
- Sebri, V.; Durosini, I.; Mazzoni, D.; Pravettoni, G. The body after cancer: A qualitative study on breast cancer survivors’ body representation. Int. J. Environ. Res. Public Health 2022, 19, 12515. [Google Scholar] [CrossRef]
- Male, D.A.; Fergus, K.D.; Cullen, K. Sexual identity after breast cancer: Sexuality, body image, and relationship repercussions. Curr. Opin. Support Palliat. Care 2016, 10, 66–74. [Google Scholar] [CrossRef]
- Jabłoński, M.J.; Mirucka, B.; Streb, J.; Słowik, A.J.; Jach, R. Exploring the relationship between the body self and the sense of coherence in women after surgical treatment for breast cancer. Psychooncology 2019, 28, 54–60. [Google Scholar] [CrossRef]
- Sebri, V.; Mazzoni, D.; Triberti, S.; Pravettoni, G. The impact of unsupportive social support on the injured self in breast cancer patients. Front. Psychol. 2021, 12, 722211. [Google Scholar] [CrossRef] [PubMed]
- Steindel, S.J. International classification of diseases, 10th edition, clinical modification and procedure coding system: Descriptive overview of the next generation HIPAA code sets. J. Am. Med. Inform. Assoc. 2010, 17, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, P.; Yibulayin, F.; Feng, L.; Zhang, H.; Wushou, A. Spindle cell melanoma: Incidence and survival, 1973–2017. Oncol. Lett. 2018, 16, 5091–5099. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.; Bannister, P.; Rogers, I.; Sundin, J.; Al-Ayadhy, B.; James, P.W.; McNally, R.J.Q. Changing epidemiology and age-specific incidence of cutaneous malignant melanoma in England: An analysis of the national cancer registration data by age, gender and anatomical site, 1981–2018. Lancet Reg. Health Eur. 2021, 2, 100024. [Google Scholar] [CrossRef] [PubMed]
- Ontario Ministry of Health and Long-Term Care. Population Estimates; IntelliHealth: ON, Canada, 2019. [Google Scholar]
- Martins-Klein, B.; Bamonti, P.M.; Owsiany, M.; Naik, A.; Moye, J. Age differences in cancer-related stress, spontaneous emotion regulation, and emotional distress. Aging Ment. Health 2021, 25, 250–259. [Google Scholar] [CrossRef]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1. [Google Scholar] [CrossRef]
- Carver, C.S.; Scheier, M.F.; Weintraub, J.K. Assessing coping strategies: A theoretically based approach. J. Pers. Soc. Psychol. 1989, 56, 267–283. [Google Scholar] [CrossRef]
- Timircan, M.; Bratosin, F.; Vidican, I.; Suciu, O.; Turaiche, M.; Bota, A.V.; Mitrescu, S.; Marincu, I. Coping strategies and health-related quality of life in pregnant women with SARS-CoV-2 infection. Medicina 2021, 57, 1113. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Keller, S.D. A 12-item short-form health survey. Med. Care 1996, 34, 220–223. [Google Scholar] [CrossRef]
- Dehelean, L.; Papava, I.; Musat, M.I.; Bondrescu, M.; Bratosin, F.; Bucatos, B.O.; Bortun, A.-M.C.; Mager, D.V.; Romosan, R.S.; Romosan, A.-M.; et al. Coping strategies and stress related disorders in patients with COVID-19. Brain Sci. 2021, 11, 1287. [Google Scholar] [CrossRef]
- Finlay, A.Y.; Khan, G.K. Dermatology life quality index (DLQI)—A simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; Fox, R.S.; Mills, S.D.; Roesch, S.C.; Sadler, G.R.; Klonoff, E.A.; Malcarne, V.L. Reliability and validity of the perceived stress scale-10 in Hispanic Americans with English or Spanish language preference. J Health Psychol. 2019, 24, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Naszay, M.; Stockinger, A.; Jungwirth, D.; Haluza, D. Digital age and the public eHealth perspective: Prevailing health app use among Austrian internet users. Inform. Health Soc. Care 2018, 43, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Kessel, K.A.; Vogel, M.M.; Kessel, C.; Bier, H.; Biedermann, T.; Friess, H.; Herschbach, P.; von Eisenhart-Rothe, R.; Meyer, B.; Kiechle, M.; et al. Mobile health in oncology: A patient survey about app-assisted cancer care. JMIR mHealth uHealth 2017, 5, e81. [Google Scholar] [CrossRef]
- Brütting, J.; Bergmann, M.; Garzarolli, M.; Rauschenberg, R.; Weber, C.; Berking, C.; Tilgen, W.; Schadendorf, D.; Meier, F.; NVKH supporting group. Information-seeking and use of information resources among melanoma patients of German skin cancer centers. J. Dtsch. Dermatol. Ges. 2018, 16, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, A.; Kochs, C.; Blum, A.; Capellaro, M.; Czeschik, C.; Dettenborn, T.; Dill, D.; Dippel, E.; Eigentler, T.; Feyer, P.; et al. Malignant melanoma S3-guideline “diagnosis, therapy and follow-up of melanoma”. J. Ger. Soc. Dermatol. 2013, 11 (Suppl. 6), 116–126. [Google Scholar] [CrossRef]
- Mehnert, A.; Brähler, E.; Faller, H.; Härter, M.; Keller, M.; Schulz, H.; Wegscheider, K.; Weis, J.; Boehncke, A.; Hund, B.; et al. Four-week prevalence of mental disorders in patients with cancer across major tumor entities. J. Clin. Oncol. 2014, 32, 3540–3546. [Google Scholar] [CrossRef]
- Martos-Méndez, M.J. Self-efficacy and adherence to treatment: The mediating effects of social support. J. Behav. Health Soc. Issues 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Ko, C.Y.; Maggard, M.; Livingston, E.H. Evaluating health utility in patients with melanoma, breast cancer, colon cancer, and lung cancer: A nationwide, population-based assessment. J. Surg. Res. 2003, 114, 1–5. [Google Scholar] [CrossRef]
- Boyle, D.A. Psychological adjustment to the melanoma experience. Semin. Oncol. Nurs. 2003, 19, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Cornish, D.; Holterhues, C.; van de Poll-Franse, L.V.; Coebergh, J.W.; Nijsten, T. A systematic review of health-related quality of life in cutaneous melanoma. Ann. Oncol. 2009, 20 (Suppl. 6), VI51–VI58. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.-O.; Prodan, M.; Reddyreddy, A.R.; Seclaman, E.; Crainiceanu, Z.; Bloanca, V.; Bratosin, F.; Dumitru, C.; Pilut, C.N.; Alambaram, S.; et al. The epidemiology of malignant melanoma during the first two years of the COVID-19 pandemic: A systematic review. Int. J. Environ. Res. Public Health 2023, 20, 305. [Google Scholar] [CrossRef] [PubMed]
- Both, H.; Essink-Bot, M.L.; Busschbach, J.; Nijsten, T. Critical review of generic and dermatology-specific health-related quality of life instruments. J. Investig. Dermatol. 2007, 127, 2726–2739. [Google Scholar] [CrossRef]
- Wiener, D.C.; Argote-Greene, L.M.; Ramesh, H.; Audisio, R.A.; Jaklitsch, M.T. Choices in the management of asymptomatic lung nodules in the elderly. Surg. Oncol. 2004, 13, 239–248. [Google Scholar] [CrossRef]
- Macdonald, J.B.; Dueck, A.C.; Gray, R.J.; Wasif, N.; Swanson, D.L.; Sekulic, A.; Pockaj, B.A. Malignant melanoma in the elderly: Different regional disease and poorer prognosis. J. Cancer 2011, 2, 538–543. [Google Scholar] [CrossRef]
- Bandura, A. Health promotion from the perspective of social cognitive theory. Psychol. Health 1998, 13, 623–649. [Google Scholar] [CrossRef]
| Variables * | Group A (n = 162) | Group B (n = 76) | Significance |
|---|---|---|---|
| Demographics | |||
| Gender (women) | 74 (45.7%) | 36 (47.4%) | 0.807 |
| Age, mean (mean ± SD) ** | 43.0 ± 13.9 | 67.2 ± 7.1 | <0.001 |
| Area of residence (urban) | 91 (56.2%) | 53 (69.7%) | 0.045 |
| Relationship status (married) | 138 (85.2%) | 71 (93.4%) | 0.070 |
| Substance use | |||
| Frequent alcohol consumption | 20 (12.3%) | 17 (22.4%) | 0.046 |
| Smoking | 53 (32.7%) | 29 (38.2%) | 0.410 |
| Comorbidities | <0.001 | ||
| 0–1 | 116 (71.6%) | 18 (23.7%) | |
| 2 | 21 (13.0%) | 31 (40.8%) | |
| ≥3 | 25 (15.4%) | 27 (35.5%) | |
| History of major depression | 19 (11.7%) | 6 (7.9%) | 0.368 |
| Depressed mood or anhedonia | 71 (43.8%) | 49 (28.9%) | 0.028 |
| Cancer staging | 0.150 | ||
| 0 (In situ) | 5 (3.1%) | 4 (5.3%) | |
| I | 13 (8.0%) | 5 (6.6%) | |
| II | 41 (25.3%) | 29 (38.2%) | |
| III | 103 (63.6%) | 38 (50.0%) |
| Questions (Answer—Yes) | Group A (n = 162) | Group B (n = 76) | (V) Significance |
|---|---|---|---|
| Do you know someone with malignant melanoma? | 8 (4.9%) | 2 (2.6%) | (0.054) 0.408 |
| Did you know about malignant melanoma before diagnosis? | 64 (39.5%) | 22 (28.9%) | (0.102) 0.113 |
| Do you think melanoma is contagious? | 19 (11.7%) | 5 (6.6%) | (0.079) 0.219 |
| Do you believe melanoma can be cured? | 79 (48.8%) | 31 (40.8%) | (0.181) 0.249 |
| Is malignant melanoma caused by sun exposure? | 85 (52.2%) | 26 (34.2%) | (0.170) 0.008 |
| Do you use a smartphone, tablet, or personal computer? | 147 (90.7%) | 52 (68.4%) | (0.281) < 0.001 |
| Do you prefer television as source of information? | 53 (32.7%) | 69 (90.8%) | (0.541) < 0.001 |
| Do you prefer the internet as source of information? | 120 (74.1%) | 37 (48.7%) | (0.249) < 0.001 |
| Do you use sunscreen? | 42 (25.9%) | 11 (14.5%) | (0.128) 0.047 |
| Did you ever visit a doctor to evaluate your skin moles? | 38 (23.5%) | 7 (9.2%) | (0.167) 0.009 |
| Do you believe your melanoma could have been prevented if someone had told you how to protect? | 108 (66.7%) | 61 (80.3%) | (0.139) 0.031 |
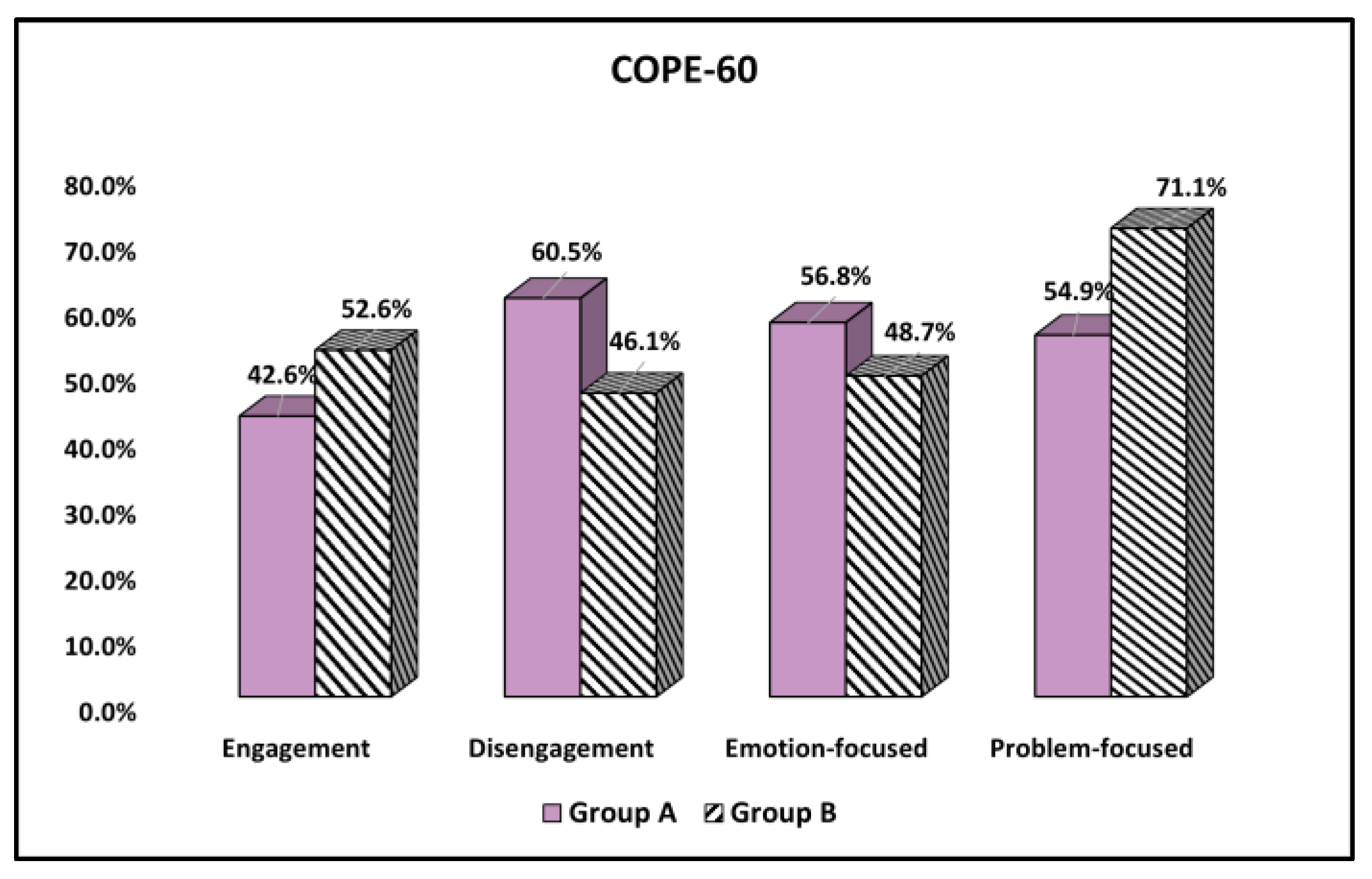
| Questions (Yes) | Likelihood | Group A (n = 162) | Group B (n = 76) | (V) Significance |
|---|---|---|---|---|
| Engagement | (0.093) 0.147 | |||
| Low (1–2) | 93 (57.4%) | 36 (47.4%) | ||
| High (3–4) | 69 (42.6%) | 40 (52.6%) | ||
| Disengagement | (0.135) 0.036 | |||
| Low (1–2) | 64 (39.5%) | 41 (53.9%) | ||
| High (3–4) | 98 (60.5%) | 35 (46.1%) | ||
| Emotion-focused | (0.075) 0.241 | |||
| Low (1–2) | 70 (43.2%) | 39 (51.3%) | ||
| High (3–4) | 92 (56.8%) | 37 (48.7%) | ||
| Problem-focused | (0.153) 0.018 | |||
| Low (1–2) | 73 (45.1%) | 22 (28.9%) | ||
| High (3–4) | 89 (54.9%) | 54 (71.1%) |
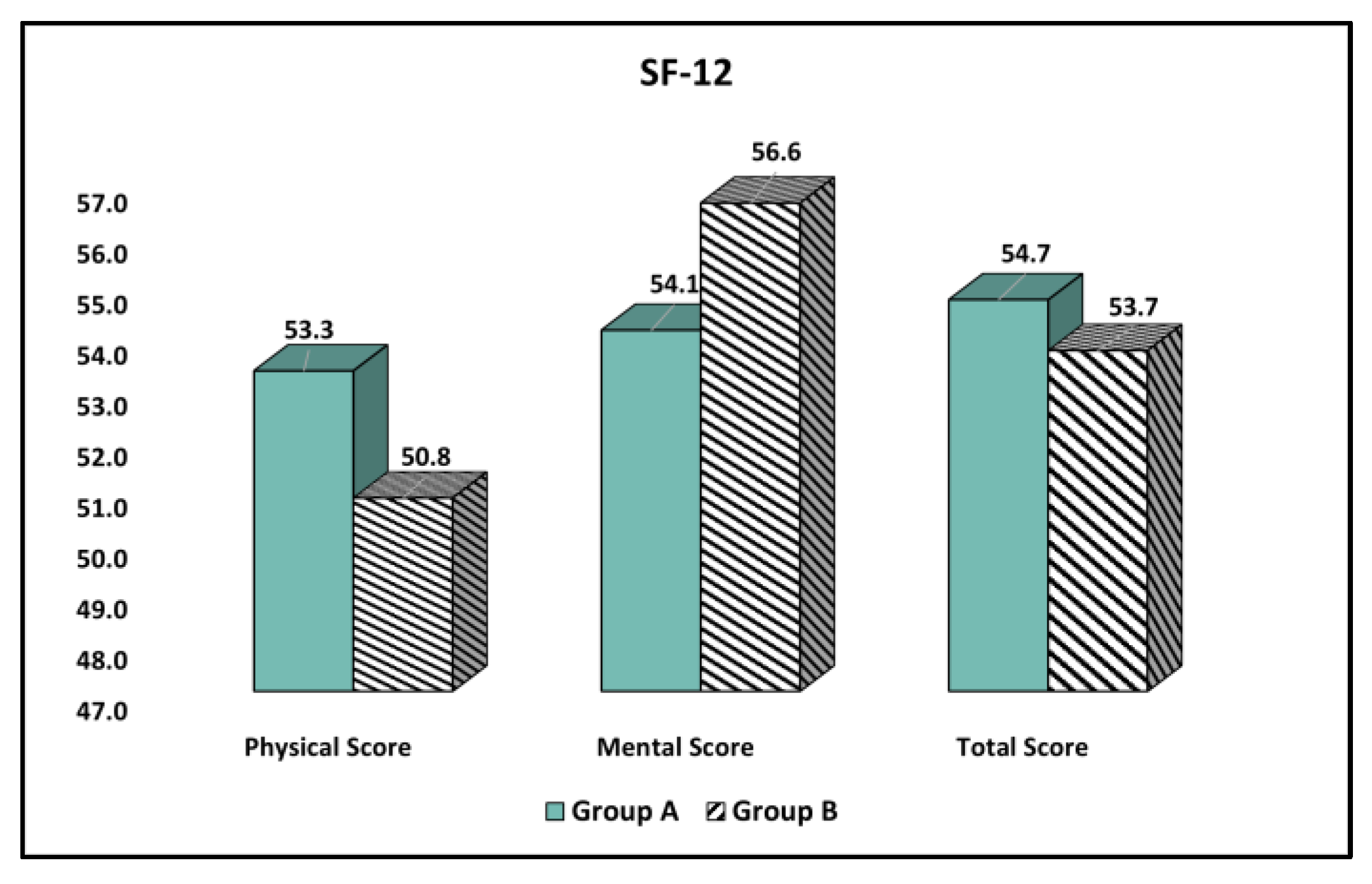
| Physical and Mental Health (Mean ± SD) | Group A (n = 162) | Group B (n = 76) | t Statistic, df, SE | Significance (95% CI), p-Value |
|---|---|---|---|---|
| Physical Score | 55.3 ± 7.6 | 50.8 ± 8.0 | t = 4.18, df = 236, SE = 1.07 | (2.83, 6.61) <0.001 |
| Mental Score | 54.1 ± 8.5 | 56.6 ± 6.9 | t = 2.24, df = 236, SE = 1.11 | (−4.69, −0.32) 0.026 |
| Total Score | 54.7 ± 8.1 | 53.7 ± 7.5 | t = 0.90, df = 236, SE = 1.10 | (−1.16, 3.16) 0.364 |
| Answers (a Lot & Very Much) | Group A (n = 162) | Group B (n = 76) | (V) Significance |
|---|---|---|---|
| Item 1 (sore, itchy, painful) | 22 (13.6%) | 9 (11.8%) | (0.024) 0.710 |
| Item 2 (embarrassment) | 26 (16.0%) | 5 (6.6%) | (0.131) 0.043 |
| Item 3 (shopping/home) | 3 (1.9%) | 5 (6.6%) | (0.122) 0.059 |
| Item 4 (clothes) | 19 (11.7%) | 5 (6.6%) | (0.064) 0.218 |
| Item 5 (social activities) | 38 (23.5%) | 11 (14.5%) | (0.103) 0.110 |
| Item 6 (sport) | 12 (7.4%) | 9 (11.8%) | (0.072) 0.260 |
| Item 7 (working/studying) | 25 (15.4%) | 8 (10.5%) | (0.066) 0.307 |
| Item 8 (interpersonal problems) | 34 (21.0%) | 17 (22.4%) | (0.0159) 0.808 |
| Item 9 (sexual difficulties) | 41 (25.3%) | 35 (46.1%) | (0.207) 0.002 |
| Item 10 (treatment difficulties) | 48 (29.6%) | 51 (67.1%) | (0.354) < 0.001 |
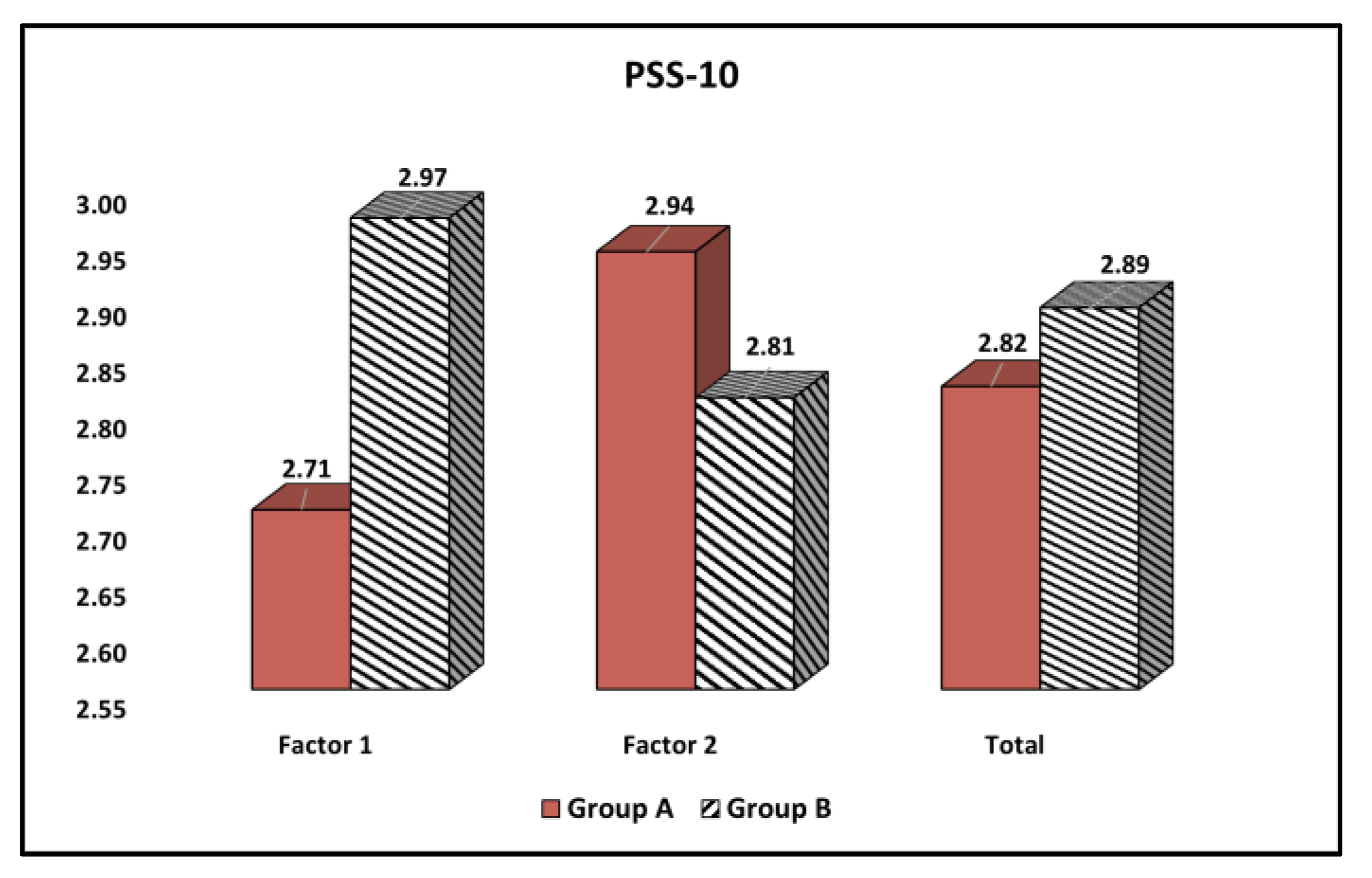
| Factors (Mean ± SD) | Group A (n = 162) | Group B (n = 76) | t Statistic, df, SE | Significance (95% CI), p-Value |
|---|---|---|---|---|
| Factor 1 (Perceived Helplessness) | 2.71 ± 0.83 | 2.97 ± 0.92 | t = 2.17, df = 236, SE = 0.13 | (−0.49,−0.02) 0.036 |
| Factor 2 (Perceived Self-Efficacy) | 2.94 ± 0.89 | 2.81 ± 0.84 | t = 1.06, df = 236, SE = 0.12 | (−0.10,0.36) 0.286 |
| Total | 2.82 ± 0.86 | 2.89 ± 0.88 | t = 0.58, df = 236, SE = 1.12 | (−0.30,0.16) 0.561 |
| Factors | Group A (Depressive Symptoms and Anhedonia) | Group B (Depressive Symptoms and Anhedonia) |
|---|---|---|
| Area of residence (rural) | 0.081 | 0.164 |
| Frequent alcohol consumption | 0.094 | 0.106 |
| Number of comorbidities | 0.078 | (ES = 0.084) 0.291 * |
| COPE-60—Disengagement coping methods | (ES = 0.112) 0.336 * | 0.184 |
| COPE-60—Problem-focused coping methods | 0.137 | 0.120 |
| SF-12 (physical score) | 0.191 | (ES = 0.123) −0.352 * |
| SF-12 (mental score) | (ES = 0.198) −0.446 * | (ES = 0.074) −0.273 * |
| DLQI—Item 2 (embarrassment) | 0.168 | 0.039 |
| DLQI—Item 9 (sexual difficulties) | (ES = 0.060) 0.245 * | (ES = 0.116) 0.341 * |
| DLQI—Item 10 (treatment difficulties) | 0.130 | (ES = 0.069) 0.264 * |
| PSS-10—Perceived Helplessness | 0.059 | (ES = 0.051) 0.228 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, A.-O.; Boeriu, E.; Decean, L.; Bloanca, V.; Bratosin, F.; Levai, M.C.; Vasamsetti, N.G.; Alambaram, S.; Oprisoni, A.L.; Miutescu, B.; et al. The Effects of Lack of Awareness in Age-Related Quality of Life, Coping with Stress, and Depression among Patients with Malignant Melanoma. Curr. Oncol. 2023, 30, 1516-1528. https://doi.org/10.3390/curroncol30020116
Toma A-O, Boeriu E, Decean L, Bloanca V, Bratosin F, Levai MC, Vasamsetti NG, Alambaram S, Oprisoni AL, Miutescu B, et al. The Effects of Lack of Awareness in Age-Related Quality of Life, Coping with Stress, and Depression among Patients with Malignant Melanoma. Current Oncology. 2023; 30(2):1516-1528. https://doi.org/10.3390/curroncol30020116
Chicago/Turabian StyleToma, Ana-Olivia, Estera Boeriu, Luminita Decean, Vlad Bloanca, Felix Bratosin, Mihaela Codrina Levai, Neeharika Gayatri Vasamsetti, Satish Alambaram, Andrada Licinia Oprisoni, Bogdan Miutescu, and et al. 2023. "The Effects of Lack of Awareness in Age-Related Quality of Life, Coping with Stress, and Depression among Patients with Malignant Melanoma" Current Oncology 30, no. 2: 1516-1528. https://doi.org/10.3390/curroncol30020116
APA StyleToma, A.-O., Boeriu, E., Decean, L., Bloanca, V., Bratosin, F., Levai, M. C., Vasamsetti, N. G., Alambaram, S., Oprisoni, A. L., Miutescu, B., Hemaswini, K., Juganaru, I., Bondar, A.-C., & Moise, M. L. (2023). The Effects of Lack of Awareness in Age-Related Quality of Life, Coping with Stress, and Depression among Patients with Malignant Melanoma. Current Oncology, 30(2), 1516-1528. https://doi.org/10.3390/curroncol30020116






