Low-Frequency PPM1D Gene Mutations Affect Treatment Response to CD19-Targeted CAR T-Cell Therapy in Large B-Cell Lymphoma
Abstract
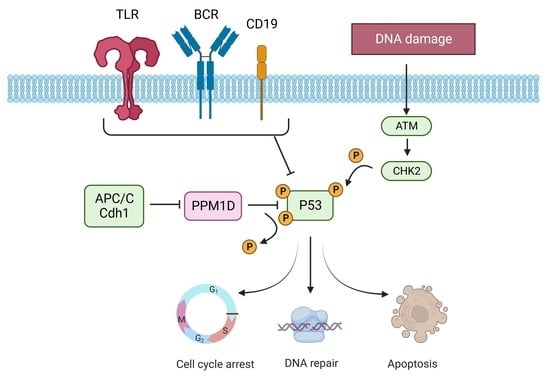
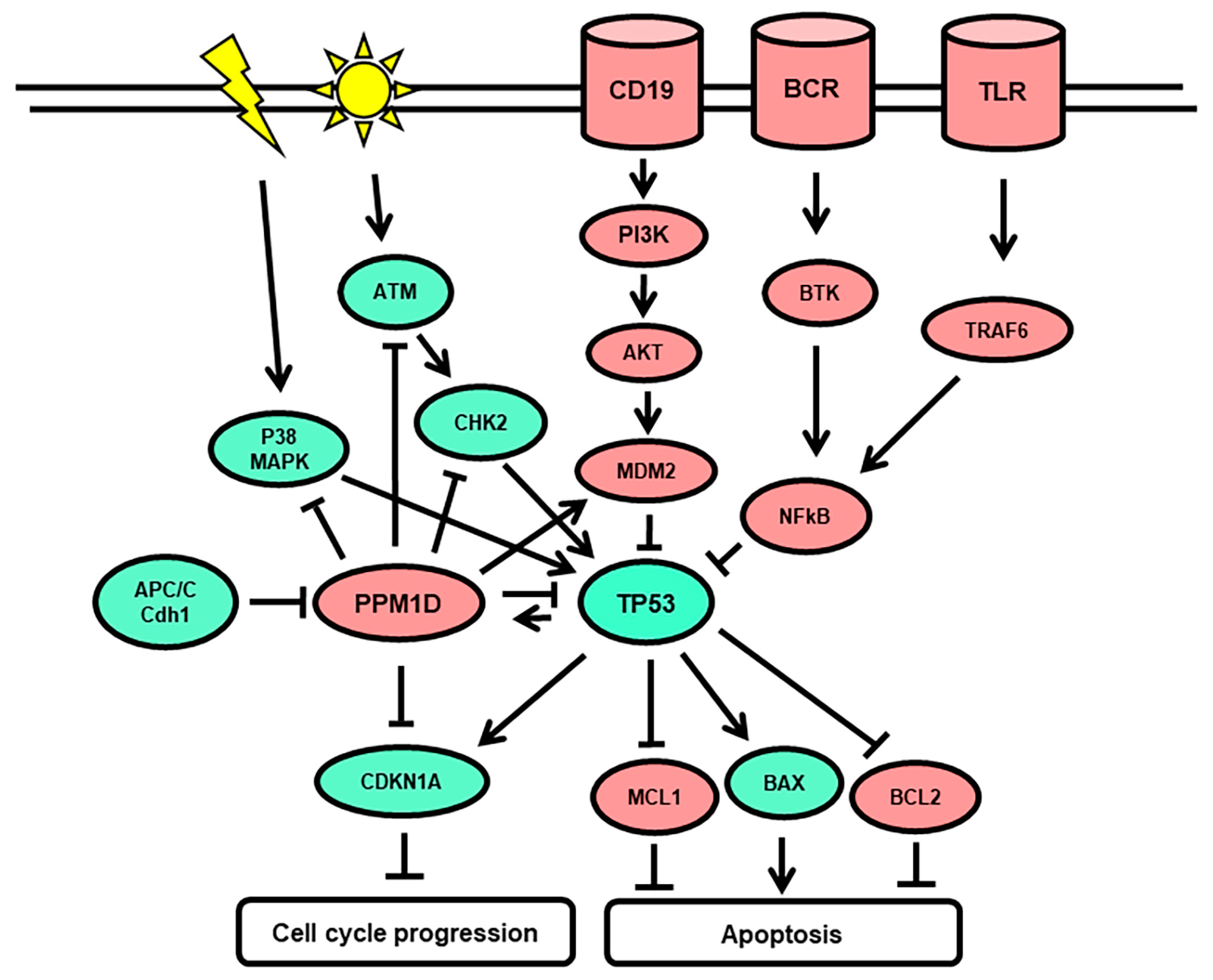
:1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. NGS Amplicon Sequencing
2.3. Clinical Data Analysis
3. Results
3.1. Prevalence of PPM1D Mutations in r/r DLBCL
3.2. Baseline Clinical Characteristics of the DLBCL Patient Cohort
3.3. Disease Features and CAR T-Cell Treatment
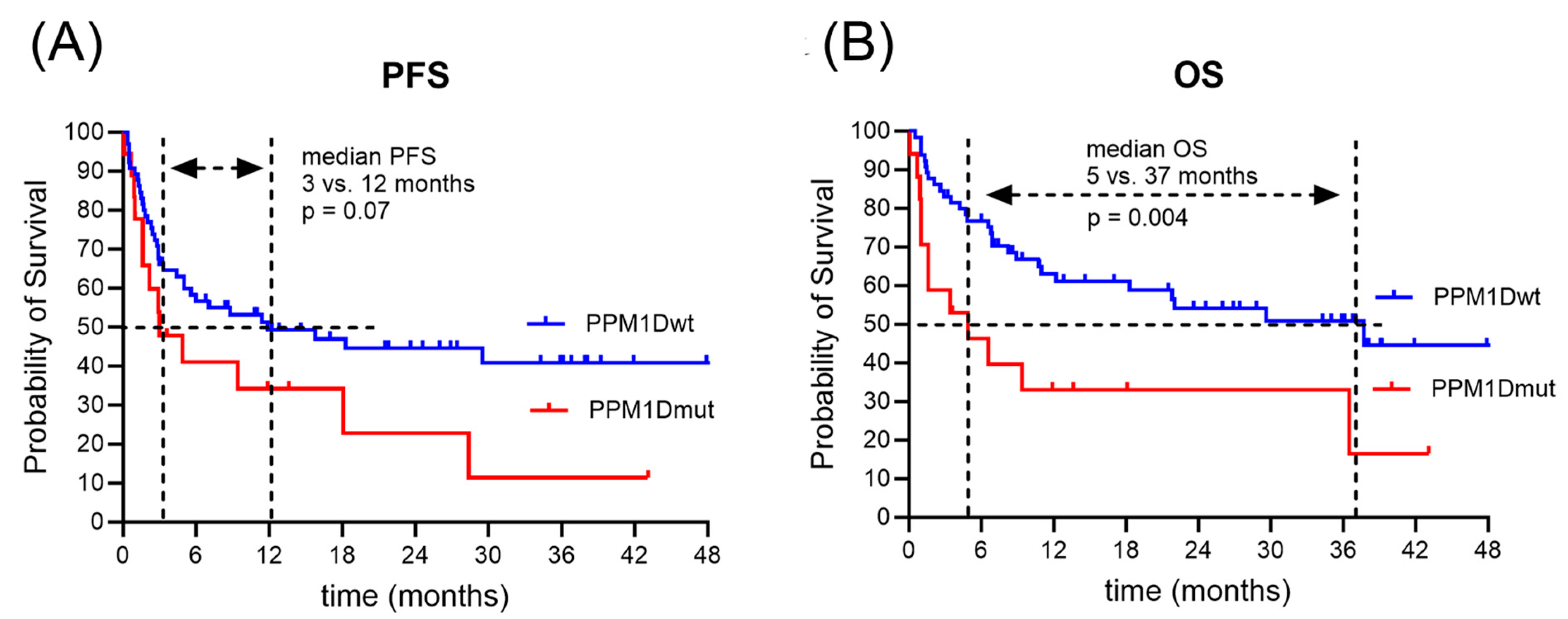
3.4. Clinical Outcome after CAR T-Cell Therapy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morton, L.M.; Wang, S.S.; Devesa, S.S.; Hartge, P.; Weisenburger, D.D.; Linet, M.S. Lymphoma Incidence Patterns by WHO Subtype in the United States, 1992–2001. Blood 2006, 107, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Congiu, A.G.; Culligan, D.J.; Gironella, M.; Yoon, D.H.; Ogura, M.; Rosta, A.; Zhu, J.; Launonen, A.; Nielsen, T.; et al. No Added Benefit of Eight Versus Six Cycles of CHOP When Combined with Rituximab in Previously Untreated Diffuse Large B-Cell Lymphoma Patients: Results from the International Phase III GOYA Study. Blood 2018, 132, 783. [Google Scholar] [CrossRef]
- Susanibar-Adaniya, S.; Barta, S.K. 2021 Update on Diffuse Large B Cell Lymphoma: A Review of Current Data and Potential Applications on Risk Stratification and Management. Am. J. Hematol. 2021, 96, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Philip, T.; Guglielmi, C.; Hagenbeek, A.; Somers, R.; Van der Lelie, H.; Bron, D.; Sonneveld, P.; Gisselbrecht, C.; Cahn, J.Y.; Harousseau, J.L. Autologous Bone Marrow Transplantation as Compared with Salvage Chemotherapy in Relapses of Chemotherapy-Sensitive Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 1995, 333, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- von Matt, S.; Bacher, U.; Banz, Y.; Taleghani, B.M.; Novak, U.; Pabst, T. Outcome of Patients with Diffuse Large B-Cell Lymphoma Relapsing after Autologous Transplant before Availability of CAR-T Cell Treatment. Mediterr. J. Hematol. Infect. Dis. 2023, 15, e2023025. [Google Scholar] [CrossRef] [PubMed]
- Mathys, A.; Bacher, U.; Banz, Y.; Legros, M.; Mansouri Taleghani, B.; Novak, U.; Pabst, T. Outcome of Patients with Mantle Cell Lymphoma after Autologous Stem Cell Transplantation in the Pre-CAR T-Cell Era. Hematol. Oncol. 2022, 40, 292–296. [Google Scholar] [CrossRef] [PubMed]
- González-Barca, E.; Boumendil, A.; Blaise, D.; Trněný, M.; Masszi, T.; Finel, H.; Michieli, M.G.; Bittenbring, J.T.; Gritti, G.; Snowden, J.A.; et al. Outcome in Patients with Diffuse Large B-Cell Lymphoma Who Relapse after Autologous Stem Cell Transplantation and Receive Active Therapy. A Retrospective Analysis of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transpl. 2020, 55, 393–399. [Google Scholar] [CrossRef]
- Narkhede, M.; Mehta, A.; Ansell, S.M.; Goyal, G. CAR T-Cell Therapy in Mature Lymphoid Malignancies: Clinical Opportunities and Challenges. Ann. Transl. Med. 2021, 9, 1036. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Sawalha, Y. Relapsed/Refractory Diffuse Large B-Cell Lymphoma: A Look at the Approved and Emerging Therapies. J. Pers. Med. 2021, 11, 1345. [Google Scholar] [CrossRef]
- Wittibschlager, V.; Bacher, U.; Seipel, K.; Porret, N.; Wiedemann, G.; Haslebacher, C.; Hoffmann, M.; Daskalakis, M.; Akhoundova, D.; Pabst, T. CAR T-Cell Persistence Correlates with Improved Outcome in Patients with B-Cell Lymphoma. Int. J. Mol. Sci. 2023, 24, 5688. [Google Scholar] [CrossRef]
- Seipel, K.; Abbühl, M.; Bacher, U.; Nilius, H.; Daskalakis, M.; Pabst, T. Clinical Impact of Single Nucleotide Polymorphism in CD-19 on Treatment Outcome in FMC63-CAR-T Cell Therapy. Cancers 2023, 15, 3058. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef]
- Locke, F.L.; Rossi, J.M.; Neelapu, S.S.; Jacobson, C.A.; Miklos, D.B.; Ghobadi, A.; Oluwole, O.O.; Reagan, P.M.; Lekakis, L.J.; Lin, Y.; et al. Tumor Burden, Inflammation, and Product Attributes Determine Outcomes of Axicabtagene Ciloleucel in Large B-Cell Lymphoma. Blood Adv. 2020, 4, 4898–4911. [Google Scholar] [CrossRef]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.-A.; Kersten, M.-J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 640–654. [Google Scholar] [CrossRef]
- Nydegger, A.; Novak, U.; Kronig, M.-N.; Legros, M.; Zeerleder, S.; Banz, Y.; Bacher, U.; Pabst, T. Transformed Lymphoma Is Associated with a Favorable Response to CAR-T-Cell Treatment in DLBCL Patients. Cancers 2021, 13, 6073. [Google Scholar] [CrossRef] [PubMed]
- Pabst, T.; Joncourt, R.; Shumilov, E.; Heini, A.; Wiedemann, G.; Legros, M.; Seipel, K.; Schild, C.; Jalowiec, K.; Mansouri Taleghani, B.; et al. Analysis of IL-6 Serum Levels and CAR T Cell-Specific Digital PCR in the Context of Cytokine Release Syndrome. Exp. Hematol. 2020, 88, 7–14.e3. [Google Scholar] [CrossRef] [PubMed]
- Lakomy, T.; Akhoundova, D.; Nilius, H.; Kronig, M.-N.; Novak, U.; Daskalakis, M.; Bacher, U.; Pabst, T. Early Use of Corticosteroids Following CAR T-Cell Therapy Correlates with Reduced Risk of High-Grade CRS without Negative Impact on Neurotoxicity or Treatment Outcome. Biomolecules 2023, 13, 382. [Google Scholar] [CrossRef]
- Messmer, A.S.; Que, Y.-A.; Schankin, C.; Banz, Y.; Bacher, U.; Novak, U.; Pabst, T. CAR T-Cell Therapy and Critical Care: A Survival Guide for Medical Emergency Teams. Wien. Klin. Wochenschr. 2021, 133, 1318–1325. [Google Scholar] [CrossRef]
- Iacoboni, G.; Villacampa, G.; Martinez-Cibrian, N.; Bailén, R.; Lopez Corral, L.; Sanchez, J.M.; Guerreiro, M.; Caballero, A.C.; Mussetti, A.; Sancho, J.-M.; et al. Real-World Evidence of Tisagenlecleucel for the Treatment of Relapsed or Refractory Large B-Cell Lymphoma. Cancer Med. 2021, 10, 3214–3223. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene Maraleucel for Patients with Relapsed or Refractory Large B-Cell Lymphomas (TRANSCEND NHL 001): A Multicentre Seamless Design Study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Hunter, B.D.; Redd, R.; Rodig, S.J.; Chen, P.-H.; Wright, K.; Lipschitz, M.; Ritz, J.; Kamihara, Y.; Armand, P.; et al. Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity. J. Clin. Oncol. 2020, 38, 3095–3106. [Google Scholar] [CrossRef]
- Nastoupil, L.J.; Jain, M.D.; Feng, L.; Spiegel, J.Y.; Ghobadi, A.; Lin, Y.; Dahiya, S.; Lunning, M.; Lekakis, L.; Reagan, P.; et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results from the US Lymphoma CAR T Consortium. J. Clin. Oncol. 2020, 38, 3119–3128. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Turtle, C.J.; Hanafi, L.-A.; Berger, C.; Hudecek, M.; Pender, B.; Robinson, E.; Hawkins, R.; Chaney, C.; Cherian, S.; Chen, X.; et al. Immunotherapy of Non-Hodgkin’s Lymphoma with a Defined Ratio of CD8+ and CD4+ CD19-Specific Chimeric Antigen Receptor-Modified T Cells. Sci. Transl. Med. 2016, 8, 355ra116. [Google Scholar] [CrossRef]
- Heini, A.D.; Bacher, U.; Porret, N.; Wiedemann, G.; Legros, M.; Stalder Zeerleder, D.; Seipel, K.; Novak, U.; Daskalakis, M.; Pabst, T. Experiences with Glofitamab Administration Following CAR T Therapy in Patients with Relapsed Mantle Cell Lymphoma. Cells 2022, 11, 2747. [Google Scholar] [CrossRef] [PubMed]
- Kochenderfer, J.N.; Somerville, R.P.T.; Lu, T.; Shi, V.; Bot, A.; Rossi, J.; Xue, A.; Goff, S.L.; Yang, J.C.; Sherry, R.M.; et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated with High Serum Interleukin-15 Levels. J. Clin. Oncol. 2017, 35, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, V.; Seipel, K.; Banz, Y.; Wiedemann, G.; Porret, N.; Bacher, U.; Pabst, T. Glofitamab Treatment in Relapsed or Refractory DLBCL after CAR T-Cell Therapy. Cancers 2022, 14, 2516. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2020, 38, 155–165. [Google Scholar] [CrossRef]
- Saini, N.Y.; Swoboda, D.M.; Greenbaum, U.; Ma, J.; Patel, R.D.; Devashish, K.; Das, K.; Tanner, M.R.; Strati, P.; Nair, R.; et al. Clonal Hematopoiesis Is Associated with Increased Risk of Severe Neurotoxicity in Axicabtagene Ciloleucel Therapy of Large B-Cell Lymphoma. Blood Cancer Discov. 2022, 3, 385–393. [Google Scholar] [CrossRef]
- Marnell, C.S.; Bick, A.; Natarajan, P. Clonal Hematopoiesis of Indeterminate Potential (CHIP): Linking Somatic Mutations, Hematopoiesis, Chronic Inflammation and Cardiovascular Disease. J. Mol. Cell Cardiol. 2021, 161, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.J.; Lindsley, R.C.; Tchekmedyian, V.; Mar, B.G.; Shi, J.; Jaiswal, S.; Bosworth, A.; Francisco, L.; He, J.; Bansal, A.; et al. Clonal Hematopoiesis Associated with Adverse Outcomes After Autologous Stem-Cell Transplantation for Lymphoma. J. Clin. Oncol. 2017, 35, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.D.; Miller, P.G.; Silver, A.J.; Sellar, R.S.; Bhatt, S.; Gibson, C.; McConkey, M.; Adams, D.; Mar, B.; Mertins, P.; et al. PPM1D-Truncating Mutations Confer Resistance to Chemotherapy and Sensitivity to PPM1D Inhibition in Hematopoietic Cells. Blood 2018, 132, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Hjermind Justesen, E.; Grønbæk, K. Protein Phosphatase, Mg2+/Mn2+-Dependent 1D (PPM1D) Mutations in Haematological Cancer. Br. J. Haematol. 2021, 192, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Zink, F.; Stacey, S.N.; Norddahl, G.L.; Frigge, M.L.; Magnusson, O.T.; Jonsdottir, I.; Thorgeirsson, T.E.; Sigurdsson, A.; Gudjonsson, S.A.; Gudmundsson, J.; et al. Clonal Hematopoiesis, with and without Candidate Driver Mutations, Is Common in the Elderly. Blood 2017, 130, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Lackraj, T.; Ben Barouch, S.; Medeiros, J.J.F.; Pedersen, S.; Danesh, A.; Bakhtiari, M.; Hong, M.; Tong, K.; Joynt, J.; Arruda, A.; et al. Clinical Significance of Clonal Hematopoiesis in the Setting of Autologous Stem Cell Transplantation for Lymphoma. Am. J. Hematol. 2022, 97, 1538–1547. [Google Scholar] [CrossRef]
- Miller, P.G.; Sperling, A.S.; Brea, E.J.; Leick, M.B.; Fell, G.G.; Jan, M.; Gohil, S.H.; Tai, Y.-T.; Munshi, N.C.; Wu, C.J.; et al. Clonal Hematopoiesis in Patients Receiving Chimeric Antigen Receptor T-Cell Therapy. Blood Adv. 2021, 5, 2982–2986. [Google Scholar] [CrossRef]
- Jeong, H.-C.; Gil, N.-Y.; Lee, H.-S.; Cho, S.-J.; Kim, K.; Chun, K.-H.; Cho, H.; Cha, H.-J. Timely Degradation of Wip1 Phosphatase by APC/C Activator Protein Cdh1 Is Necessary for Normal Mitotic Progression. J. Cell Biochem. 2015, 116, 1602–1612. [Google Scholar] [CrossRef]
- Genovese, G.; Kähler, A.K.; Handsaker, R.E.; Lindberg, J.; Rose, S.A.; Bakhoum, S.F.; Chambert, K.; Mick, E.; Neale, B.M.; Fromer, M.; et al. Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence. N. Engl. J. Med. 2014, 371, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Kleiblova, P.; Shaltiel, I.A.; Benada, J.; Ševčík, J.; Pecháčková, S.; Pohlreich, P.; Voest, E.E.; Dundr, P.; Bartek, J.; Kleibl, Z.; et al. Gain-of-Function Mutations of PPM1D/Wip1 Impair the P53-Dependent G1 Checkpoint. J. Cell Biol. 2013, 201, 511–521. [Google Scholar] [CrossRef]
- Lu, X.; Nguyen, T.-A.; Moon, S.-H.; Darlington, Y.; Sommer, M.; Donehower, L.A. The Type 2C Phosphatase Wip1: An Oncogenic Regulator of Tumor Suppressor and DNA Damage Response Pathways. Cancer Metastasis Rev. 2008, 27, 123–135. [Google Scholar] [CrossRef]
- Natrajan, R.; Lambros, M.B.; Rodríguez-Pinilla, S.M.; Moreno-Bueno, G.; Tan, D.S.P.; Marchió, C.; Vatcheva, R.; Rayter, S.; Mahler-Araujo, B.; Fulford, L.G.; et al. Tiling Path Genomic Profiling of Grade 3 Invasive Ductal Breast Cancers. Clin. Cancer Res. 2009, 15, 2711–2722. [Google Scholar] [CrossRef]
- Tan, D.S.P.; Lambros, M.B.K.; Rayter, S.; Natrajan, R.; Vatcheva, R.; Gao, Q.; Marchiò, C.; Geyer, F.C.; Savage, K.; Parry, S.; et al. PPM1D Is a Potential Therapeutic Target in Ovarian Clear Cell Carcinomas. Clin. Cancer Res. 2009, 15, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- Bulavin, D.V.; Demidov, O.N.; Saito, S.; Kauraniemi, P.; Phillips, C.; Amundson, S.A.; Ambrosino, C.; Sauter, G.; Nebreda, A.R.; Anderson, C.W.; et al. Amplification of PPM1D in Human Tumors Abrogates P53 Tumor-Suppressor Activity. Nat. Genet. 2002, 31, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.I.; Dayaram, T.; Tovy, A.; De Braekeleer, E.; Jeong, M.; Wang, F.; Zhang, J.; Heffernan, T.P.; Gera, S.; Kovacs, J.J.; et al. PPM1D Mutations Drive Clonal Hematopoiesis in Response to Cytotoxic Chemotherapy. Cell Stem Cell 2018, 23, 700–713.e6. [Google Scholar] [CrossRef] [PubMed]
- Al Hinai, A.S.A.; Grob, T.; Rijken, M.; Kavelaars, F.G.; Zeilemaker, A.; Erpelinck-Verschueren, C.A.J.; Sanders, M.A.; Löwenberg, B.; Jongen-Lavrencic, M.; Valk, P.J.M. PPM1D Mutations Appear in Complete Remission after Exposure to Chemotherapy without Predicting Emerging AML Relapse. Leukemia 2021, 35, 2693–2697. [Google Scholar] [CrossRef] [PubMed]
- Burocziova, M.; Danek, P.; Oravetzova, A.; Chalupova, Z.; Alberich-Jorda, M.; Macurek, L. Ppm1d Truncating Mutations Promote the Development of Genotoxic Stress-Induced AML. Leukemia 2023, 37, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Bolton, K.L.; Ptashkin, R.N.; Gao, T.; Braunstein, L.; Devlin, S.M.; Kelly, D.; Patel, M.; Berthon, A.; Syed, A.; Yabe, M.; et al. Cancer Therapy Shapes the Fitness Landscape of Clonal Hematopoiesis. Nat. Genet. 2020, 52, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Gillis, N.K.; Ball, M.; Zhang, Q.; Ma, Z.; Zhao, Y.; Yoder, S.J.; Balasis, M.E.; Mesa, T.E.; Sallman, D.A.; Lancet, J.E.; et al. Clonal Haemopoiesis and Therapy-Related Myeloid Malignancies in Elderly Patients: A Proof-of-Concept, Case-Control Study. Lancet Oncol. 2017, 18, 112–121. [Google Scholar] [CrossRef]
- Miller, P.G.; Sathappa, M.; Moroco, J.A.; Jiang, W.; Qian, Y.; Iqbal, S.; Guo, Q.; Giacomelli, A.O.; Shaw, S.; Vernier, C.; et al. Allosteric Inhibition of PPM1D Serine/Threonine Phosphatase via an Altered Conformational State. Nat. Commun. 2022, 13, 3778. [Google Scholar] [CrossRef]
- Eskelund, C.W.; Husby, S.; Favero, F.; Klausen, T.W.; Rodriguez-Gonzalez, F.G.; Kolstad, A.; Pedersen, L.B.; Räty, R.K.; Geisler, C.H.; Jerkeman, M.; et al. Clonal Hematopoiesis Evolves from Pretreatment Clones and Stabilizes after End of Chemotherapy in Patients with MCL. Blood 2020, 135, 2000–2004. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Kim, T.; Jeong, J.-Y.; Jo, J.-C.; Lee, W.S.; Shin, H.-J.; Lee, J.H.; Lee, H.S. Poor Prognostic Impact of High Serum Ferritin Levels in Patients with a Lower Risk of Diffuse Large B Cell Lymphoma. Int. J. Hematol. 2020, 111, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Sesques, P.; Ferrant, E.; Safar, V.; Wallet, F.; Tordo, J.; Dhomps, A.; Karlin, L.; Brisou, G.; Vercasson, M.; Hospital-Gustem, C.; et al. Commercial Anti-CD19 CAR T Cell Therapy for Patients with Relapsed/Refractory Aggressive B Cell Lymphoma in a European Center. Am. J. Hematol. 2020, 95, 1324–1333. [Google Scholar] [CrossRef] [PubMed]

| Classification | Locus Chr7 | VAF | NT Change | AA Change |
|---|---|---|---|---|
| indel | 60,663,077 | 0.079 | AT/A | L450fs * |
| nonsense | 60,663,083 | 0.023 | T/G | L450 * |
| missense | 60,663,085 | 0.036 | G/A | E451K |
| nonsense | 60,663,106 | 0.032 | C/T | R458 * |
| nonsense | 60,663,157 | 0.026 | G/T | E475 * |
| nonsense | 60,663,185 | 0.018 | T/A | L484 * |
| indel | 60,663,224 | 0.046 | del 17 | L498fs * |
| missense | 60,663,259 | 0.015 | G/A | D509N |
| indel | 60,663,262 | 0.217, 0.121, 0.017, 0.017 | C/CA | N512fs * |
| indel | 60,663,269 | 0.025 | AT/A | L513fs * |
| indel | 60,663,307 | 0.044 | GA/G | I526fs * |
| missense | 60,663,334 | 0.081, 0.078 | T/G | F543V |
| indel | 60,663,336 | 0.054 | TA/T | R536fs * |
| indel | 60,663,340 | 0.042 | AG/A | R536fs * |
| nonsense | 60,663,347 | 0.018 | T/G | L538 * |
| Cohort (n = 85) | PPM1Dwt (n = 67) | PPM1Dmut (n = 18) | p-Value | |
|---|---|---|---|---|
| Sex (female: male) | 34:51 | 26:40 | 7:11 | >0.99 |
| Median age at ID (range) | 61 (34–79) | 61 (34–79) | 61 (41–78) | 0.85 |
| Median age at CAR-T (range) | 66 (35–82) | 65 (35–82) | 69 (52–79) | 0.47 |
| DLBCL, de novo | 51 (60%) | 39 (58%) | 12 (67%) | 0.42 |
| DLBCL, transformed | 33 (39%) | 27 (40%) | 5 (28%) | |
| Initial Disease Stage | 0.73 | |||
| I | 3 (3%) | 2 (3%) | 1 (5%) | |
| II | 14 (16%) | 12 (18%) | 2 (11%) | |
| III | 14 (16%) | 9 (14%) | 5 (28%) | |
| IV | 33 (39%) | 26 (39%) | 6 (33%) | |
| NR | 21 (25%) | 17 (25%) | 4 (22%) | |
| Prognostic Index (IPI) | 0.98 | |||
| 1 | 1 (1%) | 1 (2%) | 0 | |
| 2 | 8 (9%) | 6 (9%) | 2 (11%) | |
| 3 | 36 (42%) | 28 (42%) | 8 (44%) | |
| 4 | 33 (39%) | 25 (37%) | 8 (44%) | |
| NR | 7 (8%) | 7 (10%) | 0 | |
| Number of treatment lines | >0.99 | |||
| prior to CAR-T therapy | ||||
| 1 | 2 (2%) | 2 (3%) | 0 | |
| 2 | 28 (33%) | 22 (33%) | 6 (33%) | |
| ≥3 | 55 (65%) | 43 (64%) | 12 (67%) | |
| Bridging chemotherapy | 35 (41%) | 27 (40%) | 8 (44%) | 0.99 |
| Bridging radiotherapy | 16 (19%) | 14 (21%) | 2 (11%) | 0.51 |
| Stem Cell Therapy (SCT) | 42 (49%) | 30 (45%) | 12 (67%) | 0.31 |
| autologous | 41 | 30 | 11 | |
| allogeneic | 1 | 0 | 1 | |
| Number of CR prior to | 0.59 | |||
| CAR-T therapy | ||||
| 0 | 35 (41%) | 28 (42%) | 6 (33%) | |
| 1 | 35 (41%) | 27 (40%) | 8 (44%) | |
| 2 | 13 (15%) | 9 (14%) | 4 (22%) | |
| ≥3 | 1 (1%) | 1 (2%) | 0 | |
| Number of PR prior | 0.77 | |||
| to CAR-T therapy | ||||
| 0 | 32 (38%) | 24 (36%) | 8 (44%) | |
| 1 | 40 (47%) | 31 (46%) | 8 (44%) | |
| 2 | 9 (11%) | 7 (11%) | 2 (11%) | |
| ≥3 | 3 (4%) | 3 (5%) | 0 |
| Cohort (n = 85) | PPM1Dwt (n = 67) | PPM1Dmut (n = 18) | p-Value | |
|---|---|---|---|---|
| CAR-T Product | ||||
| Kymriah®:Yescarta®:Celgene® | 53:26:6 | 40:22:5 | 13:4:1 | 0.4 |
| Target antigen variants | 0.79 0.26 | |||
| CD19 +/−rs2904880 | 48:37 (56%) | 37:30 (55%) | 11:7 (61%) | |
| Stage at CAR-T cell infusion | ||||
| CR | 5 (5.9%) | 5 (7.5%) | 0 | |
| PR | 30 (35.3%) | 21 (31%) | 9 (50%) | |
| SD | 4 (4.7%) | 3 (4.5%) | 1 (5.5%) | |
| PD | 46 (54.1%) | 38 (57%) | 8 (44%) | |
| Median interval ID to CAR-T cell infusion in months (range) | 26 | 25 | 33 | 0.28 |
| (6–312) | (6–312) | (8–277) | ||
| Median LDH U/L prior CAR-T | 490 | 461 | 602 | 0.075 |
| (range) | (249–3949) | (249–2355) | (344–3949) |
| Total Cohort (n = 85) | PPM1Dwt (n = 67) | PPM1Dmut (n = 18) | p-Value | |
|---|---|---|---|---|
| CRS | 68 (80%) | 52 (77%) | 16 (89%) | 0.57 |
| grade 1 | 42 (62%) | 32 (62%) | 10 (62%) | |
| grade 2 | 22 (32%) | 17 (33%) | 5 (31%) | |
| grade 3 | 3 (4.4%) | 3 (5.8%) | 0 | |
| grade 4 | 1 (1.5%) | 0 | 1 (6%) | |
| ICANS | 31 (36%) | 23 (34%) | 8 (44%) | 0.19 |
| grade 1 | 9 (29%) | 7 (30%) | 2 (25%) | |
| grade 2 | 6 (19%) | 6 (26%) | 0 | |
| grade 3 | 11 (35%) | 7 (30%) | 4 (50%) | |
| grade 4 | 5 (16%) | 3 (13%) | 2 (25%) | |
| Median Peak CRP | 41.5 | 30 | 64 | 0.105 |
| mg/L (range) | (3–328) | (3–323) | (3–328) | |
| Median Peak IL-6 | 556 | 443 | 559 | 0.58 |
| pg/mL (range) | (4–157,117) | (4–157,117) | (7–42,209) | |
| Median Peak Ferritin μg/L (range) | 1265 (99–13,393) | 1209 (99–13,393) | 1772 (290–12,398) | 0.064 |
| Admissions to IMC/ICU | 16 | 11 | 5 | 0.34 |
| Hospitalization time in days (range) | 21 (14–68) | 21 (14–68) | 23 (18–41) | 0.34 |
| Best remission status | n = 79 | n = 64 | n = 15 | 0.044 |
| post CAR T-cell therapy | ||||
| CR | 41 (52%) | 36 (56%) | 5 (33%) | |
| PR | 26 (33%) | 17 (27%) | 9 (60%) | |
| SD | 4 (5%) | 3 (5%) | 1 (7%) | |
| PD | 8 (10%) | 8 (13%) | 0 | |
| Median Survival time Progression free (PFS) | 12 |
| PFS | OS | |||
|---|---|---|---|---|
| Predictors | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| PPM1Dmut | 1.41 (0.72, 2.74) | 0.3 | 2.37 (1.18, 4.77) | 0.016 |
| Age > 65 | 1.63 (0.92, 2.90) | 0.094 | 2.25 (1.19, 4.26) | 0.012 |
| Sex | 0.81 (0.43, 1.51) | 0.5 | 0.89 (0.44, 1.80) | 0.7 |
| Transformed DLBCL | 0.56 (0.29, 1.06) | 0.074 | 0.44 (0.21, 0.91) | 0.027 |
| Peak ferritin | 1.02 (1.01, 1.03) | <0.001 | 1.03 (1.02, 1.04) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seipel, K.; Frey, M.; Nilius, H.; Akhoundova, D.; Banz, Y.; Bacher, U.; Pabst, T. Low-Frequency PPM1D Gene Mutations Affect Treatment Response to CD19-Targeted CAR T-Cell Therapy in Large B-Cell Lymphoma. Curr. Oncol. 2023, 30, 10463-10476. https://doi.org/10.3390/curroncol30120762
Seipel K, Frey M, Nilius H, Akhoundova D, Banz Y, Bacher U, Pabst T. Low-Frequency PPM1D Gene Mutations Affect Treatment Response to CD19-Targeted CAR T-Cell Therapy in Large B-Cell Lymphoma. Current Oncology. 2023; 30(12):10463-10476. https://doi.org/10.3390/curroncol30120762
Chicago/Turabian StyleSeipel, Katja, Michèle Frey, Henning Nilius, Dilara Akhoundova, Yara Banz, Ulrike Bacher, and Thomas Pabst. 2023. "Low-Frequency PPM1D Gene Mutations Affect Treatment Response to CD19-Targeted CAR T-Cell Therapy in Large B-Cell Lymphoma" Current Oncology 30, no. 12: 10463-10476. https://doi.org/10.3390/curroncol30120762
APA StyleSeipel, K., Frey, M., Nilius, H., Akhoundova, D., Banz, Y., Bacher, U., & Pabst, T. (2023). Low-Frequency PPM1D Gene Mutations Affect Treatment Response to CD19-Targeted CAR T-Cell Therapy in Large B-Cell Lymphoma. Current Oncology, 30(12), 10463-10476. https://doi.org/10.3390/curroncol30120762