Durvalumab-Associated Pneumonitis in Patients with Locally Advanced Non-Small Cell Lung Cancer: A Real-World Population Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Definition and Grading of Pneumonitis
2.3. Outcomes
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Treatment Regimen
3.3. Pneumonitis and Health Utilization Data
3.3.1. Pneumonitis
3.3.2. Other irAEs
3.4. Rechallenge Following Pneumonitis
Health Utilization
3.5. Cancer Responses
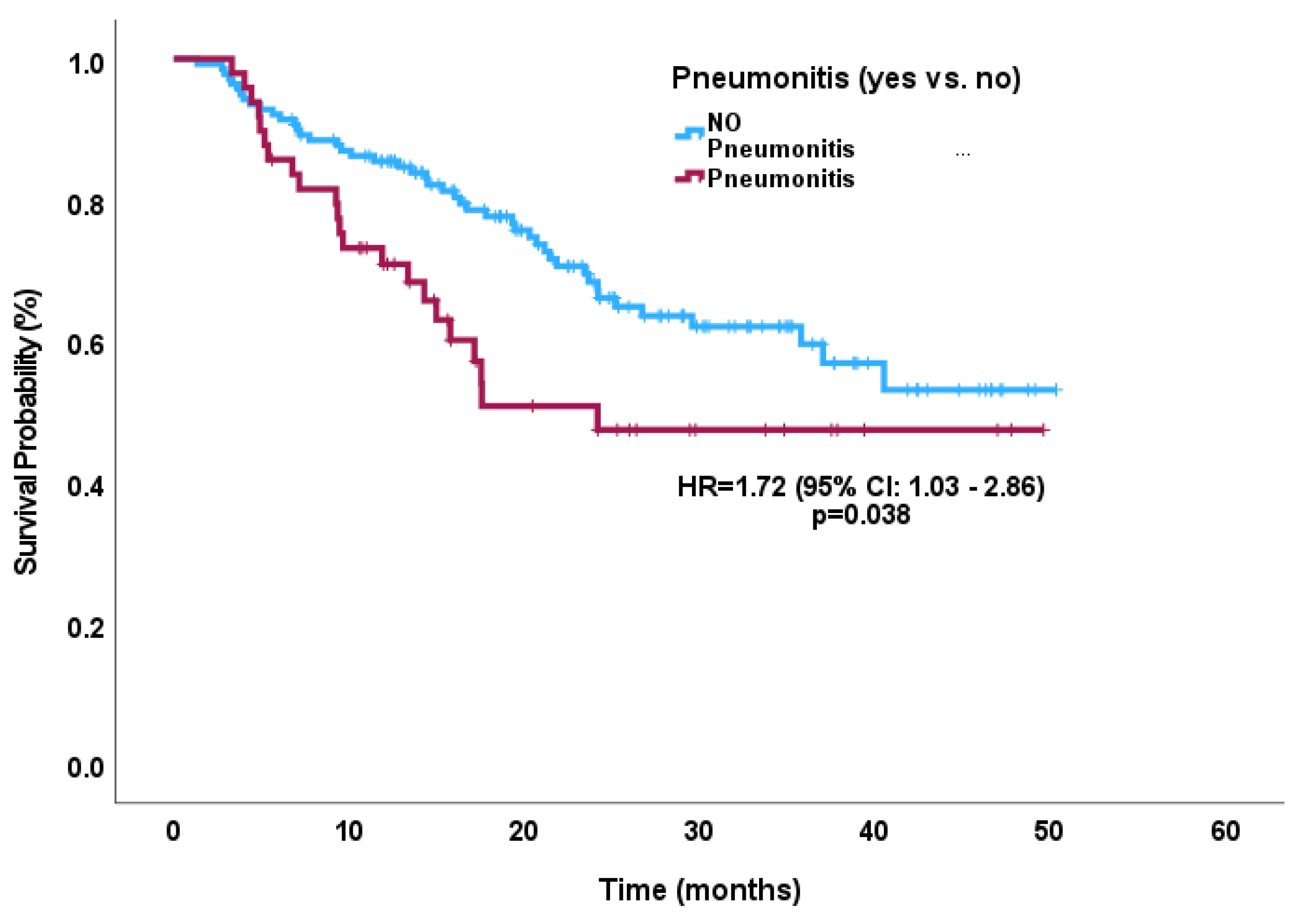
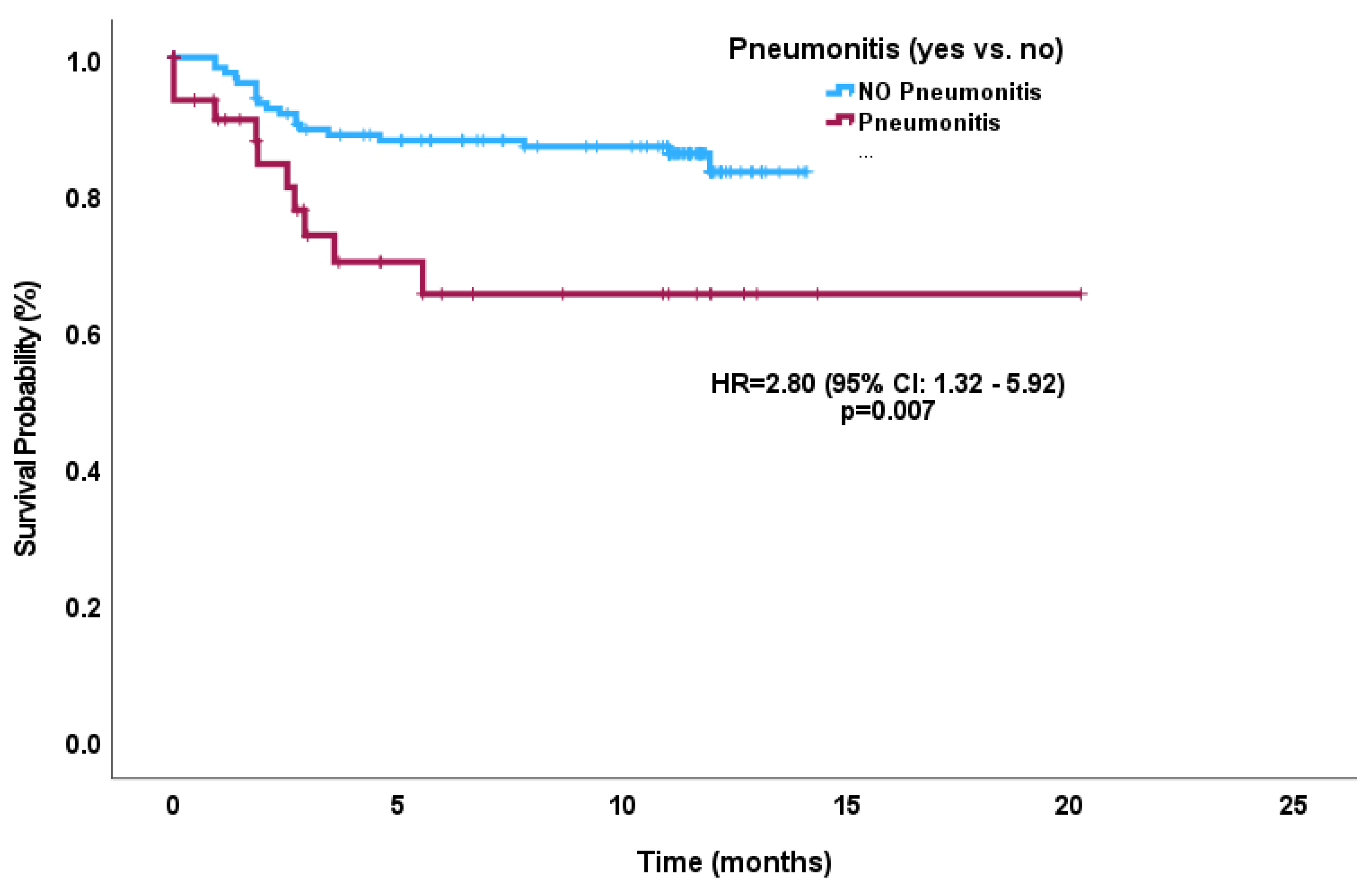
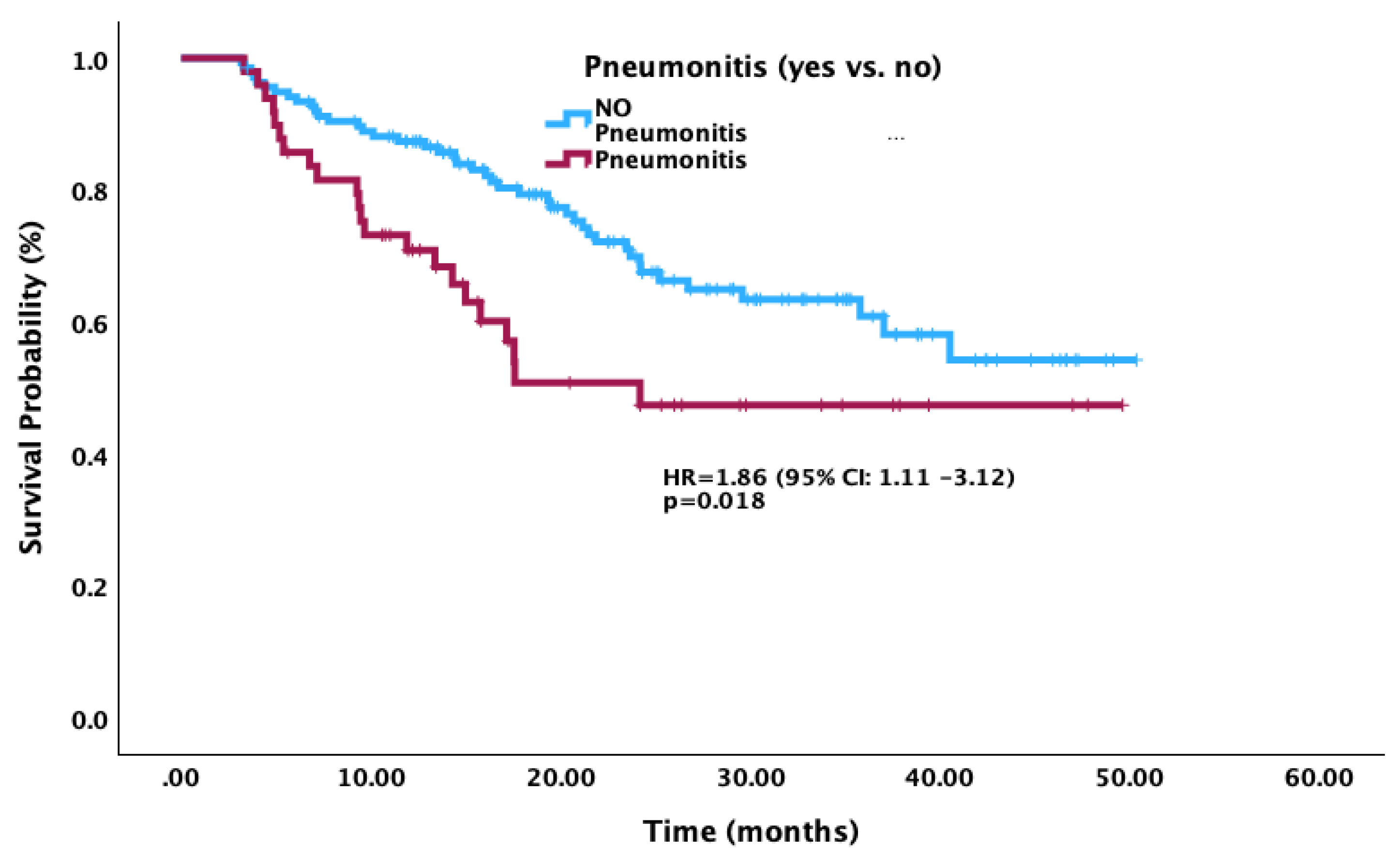
3.6. Survival Outcomes
3.7. Next Line of Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Lee, D.J.; Lee, H.J.; Farmer, J.R.; Reynolds, K.L. Mechanisms Driving Immune-Related Adverse Events in Cancer Patients Treated with Immune Checkpoint Inhibitors. Curr. Cardiol. Rep. 2021, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Aldea, M.; Orillard, E.; Mansi, L.; Marabelle, A.; Scotte, F.; Lambotte, O.; Michot, J.-M. How to Manage Patients with Corticosteroids in Oncology in the Era of Immunotherapy? Eur. J. Cancer 2020, 141, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351. [Google Scholar] [CrossRef]
- Saito, G.; Oya, Y.; Taniguchi, Y.; Kawachi, H.; Daichi, F.; Matsumoto, H.; Iwasawa, S.; Suzuki, H.; Niitsu, T.; Miyauchi, E.; et al. Real-World Survey of Pneumonitis and Its Impact on Durvalumab Consolidation Therapy in Patients with Non-Small Cell Lung Cancer Who Received Chemoradiotherapy after Durvalumab Approval (HOPE-005/CRIMSON). Lung Cancer 2021, 161, 86–93. [Google Scholar] [CrossRef]
- Desilets, A.; Blanc-Durand, F.; Lau, S.; Hakozaki, T.; Kitadai, R.; Malo, J.; Belkaid, W.; Richard, C.; Messaoudene, M.; Cvetkovic, L.; et al. Durvalumab Therapy Following Chemoradiation Compared with a Historical Cohort Treated with Chemoradiation Alone in Patients with Stage III Non–Small Cell Lung Cancer: A Real-World Multicentre Study. Eur. J. Cancer 2021, 142, 83–91. [Google Scholar] [CrossRef]
- Shaverdian, N.; Thor, M.; Shepherd, A.F.; Offin, M.D.; Jackson, A.; Wu, A.J.; Gelblum, D.Y.; Yorke, E.D.; Simone, C.B.; Chaft, J.E.; et al. Radiation Pneumonitis in Lung Cancer Patients Treated with Chemoradiation plus Durvalumab. Cancer Med. 2020, 9, 4622–4631. [Google Scholar] [CrossRef]
- Jung, H.A.; Noh, J.M.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Ahn, M.-J.; Pyo, H.; Ahn, Y.C.; Park, K. Real World Data of Durvalumab Consolidation after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. Lung Cancer 2020, 146, 23–29. [Google Scholar] [CrossRef]
- Bruni, A.; Scotti, V.; Borghetti, P.; Vagge, S.; Cozzi, S.; D’Angelo, E.; Giaj Levra, N.; Fozza, A.; Taraborrelli, M.; Piperno, G.; et al. A Real-World, Multicenter, Observational Retrospective Study of Durvalumab After Concomitant or Sequential Chemoradiation for Unresectable Stage III Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 744956. [Google Scholar] [CrossRef]
- LeClair, J.N.; Merl, M.Y.; Cohenuram, M.; Luon, D. Real-World Incidence of Pneumonitis in Patients Receiving Durvalumab. Clin. Lung Cancer 2022, 23, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Ono, A.; Wakuda, K.; Kawabata, T.; Yabe, M.; Miyawaki, T.; Miyawaki, E.; Kodama, H.; Nishioka, N.; Mamesaya, N.; et al. Prognostic Impact of Pneumonitis after Durvalumab Therapy in Patients with Locally Advanced Non-Small Cell Lung Cancer. Investig. New Drugs 2022, 40, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, Y.; Mizumoto, M.; Sekino, Y.; Maruo, K.; Ishida, T.; Sumiya, T.; Nakamura, M.; Ohkawa, A.; Takizawa, D.; Okumura, T.; et al. Risk Factor of Pneumonitis on Dose-Volume Relationship for Chemoradiotherapy with Durvalumab: Multi-Institutional Research in Japan. Clin. Transl. Radiat. Oncol. 2021, 29, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Chamorro, R.J.; Rishi, A.; Liveringhouse, C.; Bryant, J.M.M.; Perez, B.A.; Rosenberg, S.A.; Dilling, T.J. Real World Rates and Predictive Factors of Pneumonitis in Advanced, Non-Resectable NSCLC Treated with Concurrent Chemoradiation and Durvalumab. Int. J. Radiat. Oncol. 2022, 114, e370. [Google Scholar] [CrossRef]
- Tsukita, Y.; Yamamoto, T.; Mayahara, H.; Hata, A.; Takeda, Y.; Nakayama, H.; Tanaka, S.; Uchida, J.; Usui, K.; Toyoda, T.; et al. Intensity-Modulated Radiation Therapy with Concurrent Chemotherapy Followed by Durvalumab for Stage III Non-Small Cell Lung Cancer: A Multi-Center Retrospective Study. Radiother. Oncol. 2021, 160, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Ellison, C.; Martens, M.; Alvarez Argote, J.; Benz, S.; Currey, A.; Johnstone, C.; Klawikowski, S.; Livingston, K.; Longo, J.M.; Menon, S.; et al. High-Grade Pneumonitis Events in Unresectable, Locally Advanced Non-Small Cell Lung Cancer Patients Treated with Definitive Chemoradiation Followed by Adjuvant Durvalumab. JTO Clin. Res. Rep. 2023, 100537. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Huang, Y.; Li, W.; Zhao, J.; Yang, Y.; Li, C.; Wang, L.; Bi, N. Real-World Safety and Efficacy of Consolidation Durvalumab After Chemoradiation Therapy for Stage III Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. 2022, 112, 1154–1164. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; Naidoo, J.; Faivre-Finn, C.; Özgüroğlu, M.; Villegas, A.; Daniel, D.; Murakami, S.; Hui, R.; Lee, K.; Cho, B.C.; et al. MA05.02 PACIFIC Subgroup Analysis: Pneumonitis in Stage III, Unresectable NSCLC Patients Treated with Durvalumab vs. Placebo After CRT. J. Thorac. Oncol. 2018, 13, S370–S371. [Google Scholar] [CrossRef]
- Frost, N.; Unger, K.; Blum, T.G.; Misch, D.; Kurz, S.; Lüders, H.; Olive, E.; Raspe, M.; Hilbrandt, M.; Koch, M.; et al. Management, Risk Factors and Prognostic Impact of Checkpoint-Inhibitor Pneumonitis (CIP) in Lung Cancer—A Multicenter Observational Analysis. Lung Cancer 2023, 179, 107184. [Google Scholar] [CrossRef]
- Suresh, K.; Psoter, K.J.; Voong, K.R.; Shankar, B.; Forde, P.M.; Ettinger, D.S.; Marrone, K.A.; Kelly, R.J.; Hann, C.L.; Levy, B.; et al. Impact of Checkpoint Inhibitor Pneumonitis on Survival in NSCLC Patients Receiving Immune Checkpoint Immunotherapy. J. Thorac. Oncol. 2019, 14, 494–502. [Google Scholar] [CrossRef]
- Barrón, F.; Sánchez, R.; Arroyo-Hernández, M.; Blanco, C.; Zatarain-Barrón, Z.L.; Catalán, R.; Ramos-Ramírez, M.; Cardona, A.F.; Flores-Estrada, D.; Arrieta, O. Risk of Developing Checkpoint Immune Pneumonitis and Its Effect on Overall Survival in Non-Small Cell Lung Cancer Patients Previously Treated With Radiotherapy. Front. Oncol. 2020, 10, 570233. [Google Scholar] [CrossRef]
- Hassanzadeh, C.; Sita, T.; Savoor, R.; Samson, P.P.; Bradley, J.; Gentile, M.; Roach, M.; Mohindra, N.; Waqar, S.; Kruser, T.J.; et al. Implications of Pneumonitis after Chemoradiation and Durvalumab for Locally Advanced Non-Small Cell Lung Cancer. J. Thorac. Dis. 2020, 12, 6690–6700. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Durán-Pacheco, G.; Maiya, V.; Socinski, M.; Sonpavde, G.P.; Puente, J.; Essioux, L.; Carter, C.A.; Cardona, J.V.; Mohindra, R.; et al. 2032MO Correlation of Safety and Efficacy in Atezolizumab Therapy across Indications. Ann. Oncol. 2023, 34, S1079. [Google Scholar] [CrossRef]
- Gao, R.W.; Day, C.N.; Yu, N.Y.; Bush, A.; Amundson, A.C.; Prodduturvar, P.; Majeed, U.; Butts, E.; Oliver, T.; Schwecke, A.J.; et al. Dosimetric Predictors of Pneumonitis in Locally Advanced Non-Small Cell Lung Cancer Patients Treated with Chemoradiation Followed by Durvalumab. Lung Cancer 2022, 170, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Ono, A.; Kawabata, T.; Mamesaya, N.; Kawamura, T.; Kobayashi, H.; Omori, S.; Wakuda, K.; Kenmotsu, H.; Naito, T.; et al. Clinical and Radiation Dose-Volume Factors Related to Pneumonitis after Treatment with Radiation and Durvalumab in Locally Advanced Non-Small Cell Lung Cancer. Investig. New Drugs 2020, 38, 1612–1617. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Trends in Prevalence of Tobacco Use 2000–2025, 3rd ed.; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-000003-2. [Google Scholar]
- Aung, W.Y.; Lee, C.-S.; Morales, J.; Rahman, H.; Seetharamu, N. Safety and Efficacy of Immune Checkpoint Inhibitors in Cancer Patients and Preexisting Autoimmune Disease: A Systematic Review and Meta-Analysis in Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2023, 24, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Hellyer, J.A.; Aredo, J.V.; Das, M.; Ramchandran, K.; Padda, S.K.; Neal, J.W.; Wakelee, H.A. Role of Consolidation Durvalumab in Patients With EGFR- and HER2-Mutant Unresectable Stage III NSCLC. J. Thorac. Oncol. 2021, 16, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Mouri, A.; Kaira, K.; Yamaguchi, O.; Shiono, A.; Hashimoto, K.; Nishihara, F.; Shinomiya, S.; Akagami, T.; Murayama, Y.; et al. Chemoradiotherapy Followed by Durvalumab in Patients with Unresectable Advanced Non-small Cell Lung Cancer: Management of Adverse Events. Thorac. Cancer 2020, 11, 1280–1287. [Google Scholar] [CrossRef]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]
- Scott, S.C.; Pennell, N.A. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Fucà, G.; Galli, G.; Poggi, M.; Lo Russo, G.; Proto, C.; Imbimbo, M.; Ferrara, R.; Zilembo, N.; Ganzinelli, M.; Sica, A.; et al. Modulation of Peripheral Blood Immune Cells by Early Use of Steroids and Its Association with Clinical Outcomes in Patients with Metastatic Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. ESMO Open 2019, 4, e000457. [Google Scholar] [CrossRef] [PubMed]
- Tiu, B.C.; Zubiri, L.; Iheke, J.; Pahalyants, V.; Theodosakis, N.; Ugwu-Dike, P.; Seo, J.; Tang, K.; Sise, M.E.; Sullivan, R.; et al. Real-World Incidence and Impact of Pneumonitis in Patients with Lung Cancer Treated with Immune Checkpoint Inhibitors: A Multi-Institutional Cohort Study. J. Immunother. Cancer 2022, 10, e004670. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Gong, J.; Sun, X.; Yu, L.; Liao, B.; Chen, X.; Li, Y. Identification and Prediction of Immune Checkpoint Inhibitors-Related Pneumonitis by Machine Learning. Front. Immunol. 2023, 14, 1138489. [Google Scholar] [CrossRef]
- Nuñez, N.G.; Berner, F.; Friebel, E.; Unger, S.; Wyss, N.; Gomez, J.M.; Purde, M.-T.; Niederer, R.; Porsch, M.; Lichtensteiger, C.; et al. Immune Signatures Predict Development of Autoimmune Toxicity in Patients with Cancer Treated with Immune Checkpoint Inhibitors. Med 2023, 4, 113–129.e7. [Google Scholar] [CrossRef]
| Demographic | Total (n = 189) | Pneumonitis (n = 49) | No Pneumonitis (n = 140) | p-Value |
|---|---|---|---|---|
| Median age (years) | 67 (range 30–84) | 67 (range 31–81) | 67 (range 30–84) | 0.999 |
| Sex | 0.546 | |||
| Male | 88 (47%) | 21 (43%) | 67 (48%) | |
| Female | 101 (53%) | 28 (57%) | 73 (52%) | |
| ECOG | 0.861 | |||
| ECOG 0 | 40 (21%) | 11 (22%) | 29 (21%) | |
| ECOG 1 | 132 (70%) | 35 (71%) | 97 (69%) | |
| ECOG 2–3 | 17 (9%) | 3 (6%) | 13 (9%) | |
| Histology | 0.087 | |||
| Adenocarcinoma | 106 (56%) | 34 (69%) | 72 (51%) | |
| Squamous cell | 70 (37%) | 14 (29%) | 56 (40%) | |
| Others * | 13 (7%) | 5 (10%) | 12 (9%) | |
| Tumour characteristics | 0.331 | |||
| PDL-1 ≥ 50% | 59 (31%) | 15 (31%) | 44 (31%) | |
| PDL-1 1–49% | 45 (24%) | 12 (24%) | 33 (24%) | |
| PDL-1 < 1% | 36 (19%) | 13 (27%) | 23 (16%) | |
| Unknown | 49 (26%) | 9 (18%) | 40 (29%) | |
| Molecular characteristics | 0.233 | |||
| Epidermal growth factor receptor (EGFR) | 8 (4%) | 4 (8%) | 4 (3%) | |
| B-raf proto-oncogene (BRAF [V600E)] | 2 (1%) | 1 (2%) | 1 (1%) | |
| K-Ras proto-oncogene (KRAS) | 24 (13%) | 6 (12%) | 18 (13%) | |
| Anaplastic lymphoma kinase (ALK) fusion | 1 (1%) | 0 (0%) | 1 (1%) | |
| ROS proto-oncogene 1 (ROS1) | 1 (1%) | 1 (2%) | 0 (0%) | |
| Smoker/past smoker | 177 (94%) | 45 (92%) | 132 (94%) | 0.545 |
| Obese (BMI ≥ 30) at diagnosis | 46 (24%) | 15 (31%) | 31 (22%) | 0.234 |
| Good LIPI score | 98 (52%) | 26 (53%) | 72 (51%) | 0.844 |
| Reason to discontinue durvalumab | 0.103 | |||
| Progression | 41 (22%) | 6 (12%) | 34 (24%) | |
| Toxicity | 43 (23%) | 6 (12%) | 10 (7%) | |
| Death/Poor ECOG | 7 (4%) | 4 (8%) | 7 (5%) | |
| Best Response post-durvalumab | 0.108 | |||
| Partial Response | 50 (26%) | 8 (16%) | 42 (30%) | |
| Stable Disease | 104 (55%) | 28 (57%) | 76 (54%) | |
| Progressive Disease | 30 (16%) | 11 (22%) | 19 (14%) | |
| Pneumonitis Cohort (n = 49) | |
|---|---|
| Grade of pneumonitis | |
| Grade 1 | 5 (10%) |
| Grade 2 | 27 (55%) |
| Grade 3 | 14 (29%) |
| Grade 4 | 3 (6%) |
| Corticosteroid use | 42 (86%) |
| Median dose (converted to prednisone equivalent, mg) | 50 |
| Median days used (days) | 62 |
| ED visit from pneumonitis | 23 (47%) |
| ED visit within first month of starting durvalumab | 7 (14%) |
| Hospitalization from pneumonitis | 17 (35%) |
| Hospitalization within first month of starting durvalumab | 4 (8%) |
| Intensive care unit (ICU) admission | 2 (4%) |
| Hospice utilization | 0 (0%) |
| In-hospital death from complication of pneumonitis | 5 (13%) |
| Other immune related adverse events (irAE) | 14 (29%) |
| Colitis | 2 (4%) |
| Thyroiditis | 7 (14%) |
| Dermatitis | 2 (4%) |
| Other (arthritis and hepatitis) | 4 (8%) |
| Variable | Hazard Ratio | p | |
|---|---|---|---|
| Sex | Male Female | 0.67 (0.30–1.52) Reference | 0.34 |
| Autoimmune condition | Yes No | 1.54 (0.64–3.71) Reference | 0.34 |
| Vital Statistics | Dead Alive | 1.80 (0.79–4.10) Reference | 0.16 |
| V20 Gy (%) | 1.02 (1.00–1.04) | 0.05 |
| Variable | Hazard Ratio | p | |
|---|---|---|---|
| Age | 0.98 (0.95–1.02) | 0.27 | |
| Sex | Male Female | 2.97 (1.58–5.58) Reference | 0.001 |
| Autoimmune condition | Yes No | 1.20 (0.59–2.42) Reference | 0.61 |
| Smoking status | Yes No | 1.86 (0.76–4.59) Reference | 0.18 |
| Pneumonitis development | Had pneumonitis No pneumonitis | 1.51 (0.80–2.85) Reference | 0.20 |
| V20 Gy (%) | 1.02 (1.01–1.04) | 0.007 |
| Next Line of Therapy | Disease Progression (n = 34) | Non-Pneumonitis Toxicities (n = 10) | Completion of Durvalumab (n = 86) |
|---|---|---|---|
| Platinum-containing doublet chemotherapy | 3 | 1 | 6 |
| Single-agent chemotherapy | 3 | 0 | 0 |
| Chemoimmunotherapy | 1 | 1 | 1 |
| Immunotherapy alone (pembrolizumab) | 4 | 1 | 3 |
| Tyrosine kinase inhibitors * | 3 | 0 | 0 |
| None | 26 | 5 | 76 |
| Rechallenged | 0 | 2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, C.A.; Ghosh, S.; Morrison, H.; Meyers, D.; Stukalin, I.; Kerba, M.; Hao, D.; Pabani, A. Durvalumab-Associated Pneumonitis in Patients with Locally Advanced Non-Small Cell Lung Cancer: A Real-World Population Study. Curr. Oncol. 2023, 30, 10396-10407. https://doi.org/10.3390/curroncol30120757
Lim CA, Ghosh S, Morrison H, Meyers D, Stukalin I, Kerba M, Hao D, Pabani A. Durvalumab-Associated Pneumonitis in Patients with Locally Advanced Non-Small Cell Lung Cancer: A Real-World Population Study. Current Oncology. 2023; 30(12):10396-10407. https://doi.org/10.3390/curroncol30120757
Chicago/Turabian StyleLim, Chloe Ahryung, Sunita Ghosh, Hali Morrison, Daniel Meyers, Igor Stukalin, Marc Kerba, Desiree Hao, and Aliyah Pabani. 2023. "Durvalumab-Associated Pneumonitis in Patients with Locally Advanced Non-Small Cell Lung Cancer: A Real-World Population Study" Current Oncology 30, no. 12: 10396-10407. https://doi.org/10.3390/curroncol30120757
APA StyleLim, C. A., Ghosh, S., Morrison, H., Meyers, D., Stukalin, I., Kerba, M., Hao, D., & Pabani, A. (2023). Durvalumab-Associated Pneumonitis in Patients with Locally Advanced Non-Small Cell Lung Cancer: A Real-World Population Study. Current Oncology, 30(12), 10396-10407. https://doi.org/10.3390/curroncol30120757






