Combining Novel Hormonal Therapies with a Poly (ADP-Ribose) Polymerase Inhibitor for Metastatic Castration-Resistant Prostate Cancer: Emerging Evidence
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Study Selection
3.2. Characteristics of Included Trials
3.3. Evidence for Combining PARP Inhibitors and NHT as First-Line Therapy for mCRPC
3.3.1. rPFS
3.3.2. OS
3.3.3. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montgomery, R.B.; Mostaghel, E.A.; Vessella, R.; Hess, D.L.; Kalhorn, T.F.; Higano, C.S.; True, L.D.; Nelson, P.S. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008, 68, 4447–4454. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Eberli, D.; De Meerleer, G.; De Santis, M.; Gillessen, S.; Grummet, J.; Henry, A.M.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. 2023. Available online: https://uroweb.org/guidelines/prostate-cancer (accessed on 27 March 2023).
- Davies, A.H.; Beltran, H.; Zoubeidi, A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat. Rev. Urol. 2018, 15, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Smith, M.R.; Fizazi, K.; Saad, F.; Mulders, P.F.; Sternberg, C.N.; Miller, K.; Logothetis, C.J.; Shore, N.D.; Small, E.J.; et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015, 16, 152–160. [Google Scholar] [PubMed]
- Armstrong, A.J.; Lin, P.; Tombal, B.; Saad, F.; Higano, C.S.; Joshua, A.M.; Parli, T.; Rosbrook, B.; van Os, S.; Beer, T.M. Five-year survival prediction and safety outcomes with enzalutamide in men with chemotherapynaive metastatic castration-resistant prostate cancer from the PREVAIL trial. Eur. Urol. 2020, 78, 347–357. [Google Scholar] [CrossRef]
- Francini, E.; Gray, K.P.; Shaw, G.K.; Evan, C.P.; Hamid, A.A.; Perry, C.E.; Kantoff, P.W.; Taplin, M.-E.; Sweeney, C.J. Impact of new systemic therapies on overall survival of patients with metastatic castration-resistant prostate cancer in a hospital-based registry. Prostate Cancer Prostatic Dis. 2019, 22, 420–427. [Google Scholar] [CrossRef]
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Prostate Cancer (Version 1.2023) [EB/OL]. Fort Washington: NCCN. 2022. Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed on 16 September 2022).
- Shore, N.D.; Laliberté, F.; Ionescu-Ittu, R.; Yang, L.; Mahendran, M.; Lejeune, D.; Yu, L.H.; Burgents, J.; Duh, M.S.; Ghate, S.R. Real-world treatment patterns and overall survival of patients with metastatic castrationresistant prostate cancer in the US prior to PARP inhibitors. Adv. Ther. 2021, 38, 4520–4540. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.-M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.-E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef]
- Fizazi, K.; Piulats, J.M.; Reaume, M.N.; Ostler, P.; McDermott, R.; Gingerich, J.R.; Pintus, E.; Sridhar, S.S.; Bambury, R.M.; Emmenegger, U.; et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N. Engl. J. Med. 2023, 388, 719–732. [Google Scholar] [CrossRef]
- Mateo, J.; Porta, N.; Bianchini, D.; McGovern, U.; Elliott, T.; Jones, R.; Syndikus, I.; Ralph, C.; Jain, S.; Varughese, M.; et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 162–174. [Google Scholar] [CrossRef]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Sautois, B.; Vogelzang, N.J.; Bambury, R.M.; Voog, E.; et al. Rucaparib in Men with Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J. Clin. Oncol. 2020, 38, 3763–3772. [Google Scholar] [CrossRef]
- Hussain, M.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 383, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Mehra, N.; Scagliotti, G.V.; Castro, E.; Dorff, T.; Stirling, A.; Stenzl, A.; Fleming, M.T.; Higano, C.S.; Saad, F.; et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): An open-label, phase 2 trial. Lancet Oncol. 2021, 22, 1250–1264. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Sandhu, S.; Efstathiou, E.; Lara, P.N.; Yu, E.Y.; Saad, F.; Ståhl, O.; Olmos, D.; Mason, G.E.; Espina, B.M.; et al. Niraparib in patients with metastatic castration-resistant prostate cancer and DNA repair gene defects (GALAHAD): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2022, 23, 362–373. [Google Scholar]
- U.S. Food and Drug Administration. LYNPARZA R (Olaparib) Prescribing Information. 2020. Available online: www.azpicentral.com/lynparzatb/lynparzatb.pdf#page=1 (accessed on 10 April 2020).
- ESMO. EMA Recommends Extension of Indications for Olaparib. 2020. Available online: www.esmo.org/oncology-news/ema-recommends-extension-of-indications-for-olaparib2 (accessed on 25 September 2020).
- U.S. Food and Drug Administration. RUBRACA R (Rucaparib) Prescribing Information. 2020. Available online: www.accessdata.fda.gov/drugsatfdadocs/label/2020/209115s004lbl.pdf (accessed on 15 October 2020).
- Polkinghorn, W.R.; Parker, J.S.; Lee, M.X.; Kass, E.M.; Spratt, D.E.; Iaquinta, P.J.; Arora, V.K.; Yen, W.-F.; Cai, L.; Zheng, D.; et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013, 3, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Schiewer, M.J.; Goodwin, J.F.; Han, S.; Brenner, J.C.; Augello, M.A.; Dean, J.L.; Liu, F.; Planck, J.L.; Ravindranathan, P.; Chinnaiyan, A.M.; et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012, 2, 1134–1149. [Google Scholar] [CrossRef] [PubMed]
- Asim, M.; Tarish, F.; Zecchini, H.I.; Sanjiv, K.; Gelali, E.; Massie, C.E.; Baridi, A.; Warren, A.Y.; Zhao, W.; Ogris, C.; et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat. Commun. 2017, 8, 374. [Google Scholar] [CrossRef]
- Clarke, N.; Wiechno, P.; Alekseev, B.; Sala, N.; Jones, R.; Kocak, I.; Chiuri, V.E.; Jassem, J.; Fléchon, A.; Redfern, C.; et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018, 19, 975–986. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Knoll, T.; Omar, M.I.; Maclennan, S.; Hernández, V.; Canfield, S.; Yuan, Y.; Bruins, M.; Marconi, L.; Van Poppel, H.; N’dow, J.; et al. Key steps in conducting systematic reviews for underpinning clinical practice guidelines: Methodology of the European Association of Urology. Eur. Urol. 2018, 73, 290–300. [Google Scholar] [CrossRef]
- Chi, K.N.; Rathkopf, D.; Smith, M.R.; Efstathiou, E.; Attard, G.; Olmos, D.; Lee, J.Y.; Small, E.J.; Gomes, A.J.P.d.S.; Roubaud, G.; et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2023, 41, 3339–3351. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.W.; Armstrong, A.J.; Thiery-Vuillemin, A.; Oya, M.; Loredo, E.; Procopio, G.; De Menezes, J.; Girotto, G.; Arslan, C.; Mehra, N.; et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid. 2022, 1, EVIDoa2200043. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.A.; Carles, J.; Fay, A.P.; Matsubara, N.; Heinrich, D.; Szczylik, C.; De Giorgi, U.; Joung, J.Y.; Fong, P.C.C.; et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomised, placebo-controlled, phase 3 trial. Lancet 2023, 402, 291–303, Erratum in Lancet 2023, 402, 290. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. A Clinical Study Evaluating the Benefit of Adding Rucaparib to Enzalutamide for Men with Metastatic Prostate Cancer That Has Become Resistant to Testosterone-Deprivation Therapy (CASPAR). Available online: https://clinicaltrials.gov/ct2/show/NCT04455750 (accessed on 31 August 2023).
- Castro, E.; Chi, K.N.; Sandhu, S.; Olmos, D.; Attard, G.; Saad, M.; Gomes, A.J.; Rathkopf, D.E.; Smith, M.R.; Kang, T.W.; et al. Impact of run-in treatment with abiraterone acetate and prednisone (AAP) in the MAGNITUDE study of niraparib (NIRA) and AAP in patients (pts) with metastatic castrationresistant prostate cancer (mCRPC) and homologous recombination repair (HRR) gene alterations. J. Clin. Oncol. 2023, 41 (Suppl. S6), 172. [Google Scholar]
- Efstathiou, E.; Smith, M.R.; Sandhu, S.; Attard, G.; Saad, M.; Olmos, D.; Castro, E.; Roubaud, G.; Gomes, A.J.; Small, E.J.; et al. Niraparib (NIRA) with abiraterone acetate and prednisone (AAP) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and homologous recombination repair (HRR) gene alterations: Second interim analysis (IA2) of MAGNITUDE. J. Clin. Oncol. 2023, 41 (Suppl. S6), 170. [Google Scholar] [CrossRef]
- Clarke, N.W.; Armstrong, A.J.; Thiery-Vuillemin, A.; Oya, M.; Shore, N.D.; Procopio, G.; Guedes, J.D.C.; Arslan, C.; Mehra, N.; Parnis, F.; et al. Final overall survival (OS) in PROpel: Abiraterone (abi) and olaparib (ola) versus abiraterone and placebo (pbo) as first-line (1L) therapy for metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2023, 41 (Suppl. S6), LBA16. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.; Carles, J.; Fay, A.P.; Matsubara, N.; Heinrich, D.; Szczylik, C.; De Giorgi, U.; Joung, J.Y.; Fong, P.C.; et al. TALAPRO-2: Phase 3 study of talazoparib (TALA) + enzalutamide (ENZA) versus placebo (PBO) + ENZA as first-line (1L) treatment in patients (pts) with metastatic castrationresistant prostate cancer (mCRPC). J. Clin. Oncol. 2023, 41 (Suppl. S6), LBA17. [Google Scholar] [CrossRef]
- Rao, A.; Heller, G.; Ryan, C.J.; VanderWeele, D.J.; Lewis, L.D.; Tan, A.; Watt, C.; Chen, R.C.; Kohli, M.; Barata, P.C.; et al. Alliance A031902 (CASPAR): A randomized phase (ph) 3 trial of enzalutamide with rucaparib/placebo in first-line metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2023, 41 (Suppl. S6), TPS277. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Evans, C.P.; Kim, C.S.; Kimura, G.; et al. Enzalutamide in Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur. Urol. 2017, 71, 151–154. [Google Scholar] [CrossRef]
- Saad, F.; Chi, K.N.; Shore, N.D.; Graff, J.N.; Posadas, E.M.; Lattouf, J.-B.; Espina, B.M.; Zhu, E.; Yu, A.; Hazra, A.; et al. Niraparib with androgen receptor-axis-targeted therapy in patients with metastatic castration-resistant prostate cancer: Safety and pharmacokinetic results from a phase 1b study (BEDIVERE). Cancer Chemother. Pharmacol. 2021, 88, 25–37. [Google Scholar] [CrossRef]
- Annala, M.; Vandekerkhove, G.; Khalaf, D.; Taavitsainen, S.; Beja, K.; Warner, E.W.; Sunderland, K.; Kollmannsberger, C.; Eigl, B.J.; Finch, D.; et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2018, 8, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Romero-Laorden, N.; del Pozo, A.; Lozano, R.; Medina, A.; Puente, J.; Piulats, J.M.; Lorente, D.; Saez, M.I.; Morales-Barrera, R.; et al. PROREPAIR-B: A prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2019, 37, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Azad, A.A.; Iguchi, T.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Alcaraz, A.; Alekseev, B.; Shore, N.D.; et al. Improved Survival with Enzalutamide in Patients with Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2022, 40, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Abida, W. Combining Poly(ADP)-Ribose Polymerase Inhibitors with Abiraterone in Castration-Resistant Prostate Cancer: Is Biomarker Testing Necessary? J. Clin. Oncol. 2023, 41, 3291–3294. [Google Scholar] [CrossRef] [PubMed]
- Sathianathen, N.J.; Koschel, S.; Thangasamy, I.A.; Teh, J.; Alghazo, O.; Butcher, G.; Howard, H.; Kapoor, J.; Lawrentschuk, N.; Siva, S.; et al. Indirect Comparisons of Efficacy between Combination Approaches in Metastatic Hormone-sensitive Prostate Cancer: A Systematic Review and Network Meta-analysis. Eur. Urol. 2020, 77, 365–372. [Google Scholar] [CrossRef]
- Chen, K.; O’Brien, J.; McVey, A.; Jenjitranant, P.; Kelly, B.D.; Kasivisvanathan, V.; Lawrentschuk, N.; Murphy, D.G.; Azad, A.A. Combination treatment in metastatic prostate cancer: Is the bar too high or have we fallen short? Nat. Rev. Urol. 2023, 20, 116–123. [Google Scholar] [CrossRef]
- Alaimo, A.; Lorenzoni, M.; Ambrosino, P.; Bertossi, A.; Bisio, A.; Macchia, A.; Zoni, E.; Genovesi, S.; Cambuli, F.; Foletto, V.; et al. Calcium cytotoxicity sensitizes prostate cancer cells to standard-of-care treatments for locally advanced tumors. Cell Death Dis. 2020, 11, 1039. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, A.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef] [PubMed]

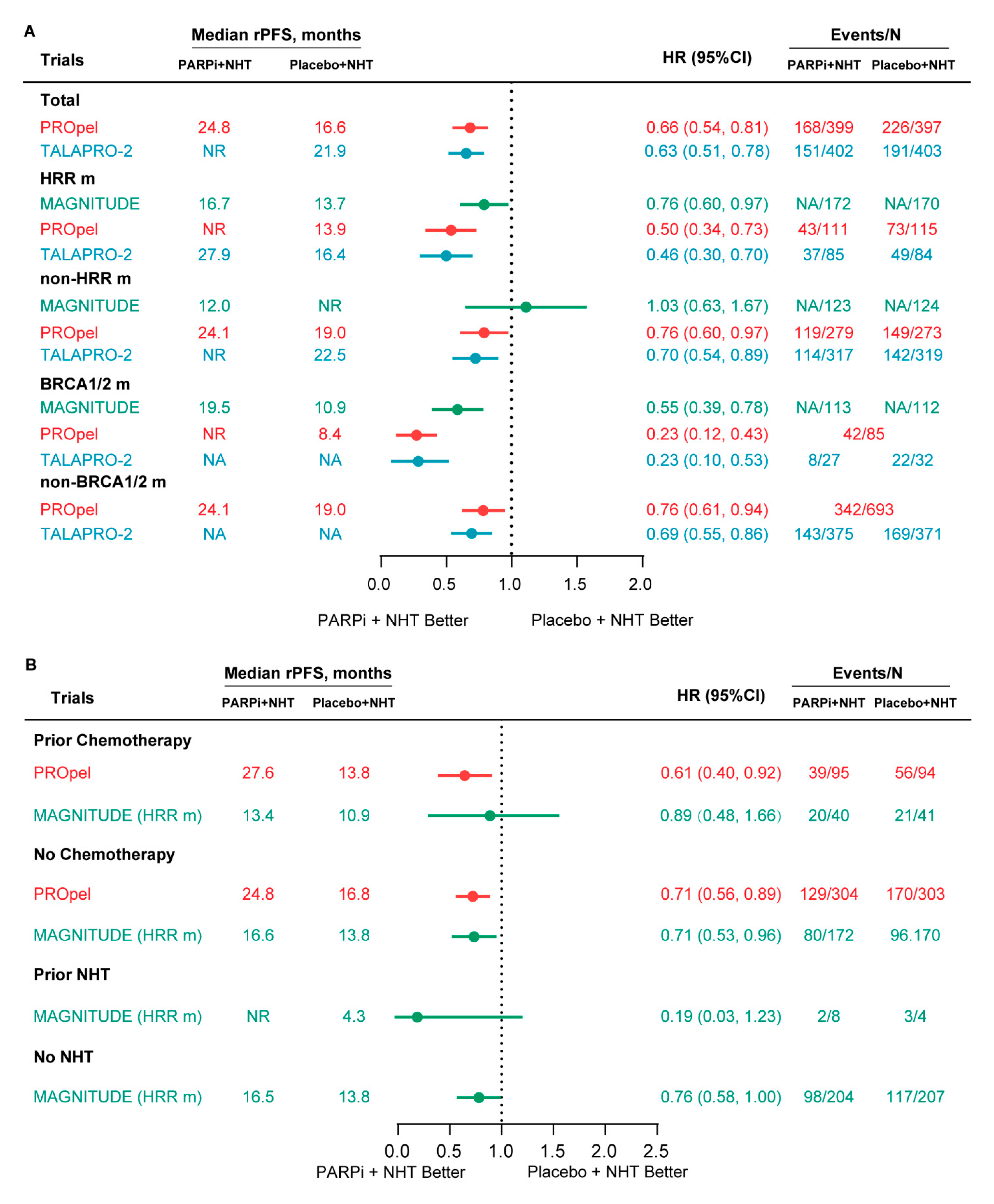
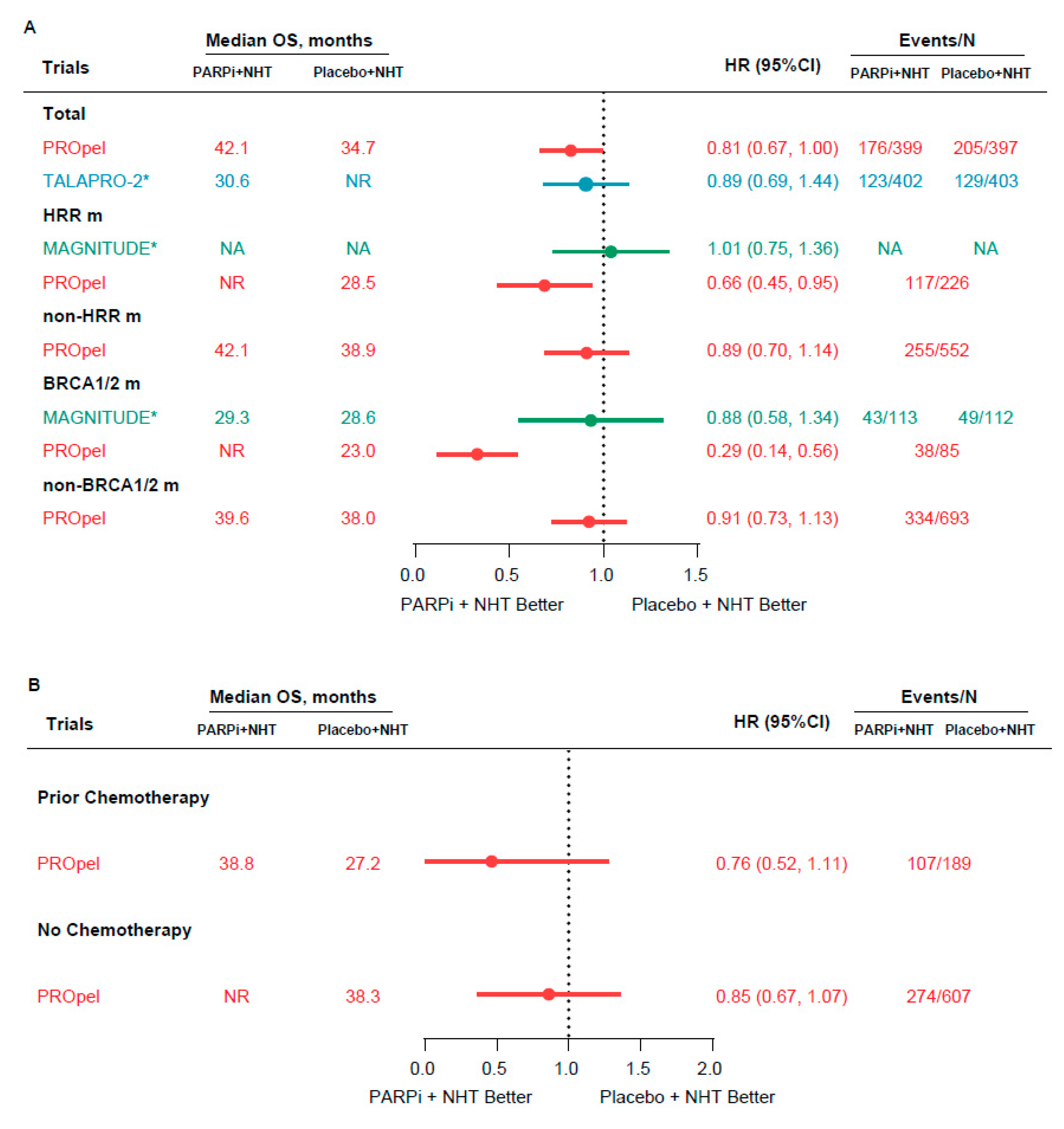
| Clinical Trial | Treatment Arms | Patients | HRR Gene Panel | HRR m Status | Primary End Points | Other Reported End Points | |
|---|---|---|---|---|---|---|---|
| Trials reported data | |||||||
| MAGNITUDE (NCT03748641) [26,30,31] | Niraparib + AAP (n = 212 in HRR m cohort; n = 123 in non-HRR m cohort) | Placebo + AAP (n = 211 in HRR m cohort; n = 124 in non-HRR m cohort) | mCRPC, unselected patients, allowed ≦4 months first-line AAP (41 in non-HRR m cohort and 98 in HRR m cohort) in the mCRPC first-line setting; 3.1% (n = 31) and 20.1% (n = 85) included patients have NHT and taxane-based chemotherapy in mCSPC and/or nmCRPC stage in HRR m cohort, respectively. | Tissue and/or blood samples: ATM, BRCA1, BRCA2, BRIP1, CDK12, CHEK2, FANCA, HDAC2, PALB2 | For HRR+ cohort: Niraparib + AAP: 46.3% (n = 98) BRCA1/2 m, 53.7% (n = 114) non-BRCA1/2 m; Placebo + AAP: 43.6% (n = 92) BRCA1/2 m, 56.4% (n = 119) non-BRCA1/2 m. | rPFS | Immature OS (at second interim analysis); AEs |
| PROpel (NCT03732820) [27,32] | Olaparib + AAP (n = 399) | Placebo + AAP (n = 397) | mCRPC, unselected patients, no prior systemic treatment for mCRPC; Only 1 patient received NHT at mCSPC stage; 22.6% and 22.4% patients received docetaxel at mCSPC stage in the combined arm and placebo arm, respectively. | Tissue and/or blood samples: ATM, BRCA1, BRCA2, BARD1, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, RAD51B, RAD51C, RAD51D, RAD54L | Olaparib + AAP: 27.8% (n = 111) HRR m, 69.9% (n = 279) non-HRR m, 11.8% (n = 47) BRCA1/2 m; Placebo + AAP: 29.0% (n = 115) HRR m, 68.8% (n = 273) non-HRR m, 9.6% (n = 38) BRCA1/2 m. | rPFS | Final OS; AEs |
| TALAPRO-2 (NCT03395197) [28,33] | Talazoparib + ENZA (n = 402) | Placebo + ENZA (n = 403) | mCRPC, unselected patients, no prior systemic treatment for mCRPC; 5.2% (n = 21) and 6.2% (n = 25) patients received abiraterone at mCSPC stage in the combined arm and placebo arm; 21.4% (n = 86) and 23.1% (n = 93) patients received docetaxel at mCSPC stage in the combined arm and placebo arm. | Tissue and/or blood samples: BRCA1, BRCA2, PALB2, ATM, ATR, CHEK2, FANCA, RAD51C, NBN, MLH1, MRE11A, CDK12 | Talazoparib + ENZA: 21.1% (n = 85) HRR m, 78.9% (n = 317) non-HRR m, 6.9% (n = 28) BRCA1/2 m; Placebo + ENZA: 20.3% (n = 82) HRR m, 79.7% (n = 321) non-HRR m, 7.9% (n = 32) BRCA1/2 m; | rPFS | Immature OS; AEs |
| Trials not reported data | |||||||
| CASPAR (NCT04455750) [29,34] | Rucaparib + ENZA (n = 492) | Placebo + ENZA (n = 492) | mCRPC, unselected patients, no prior treatment for mCRPC; Prior NHT (except ENZA) and/or docetaxel chemotherapy at mCSPC and/or nmCRPC stage was allowed. | Tissue samples: NA | - | rPFS and OS | - |
| AEs, n (%) | MAGNITUDE (HRR m) | PROpel | TALAPRO-2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Niraparib + AAP | Placebo + AAP | Olaparib + AAP | Placebo + AAP | Talazoparib + ENZA | Placebo + ENZA | |||||||
| All Grades | Grade ≥ 3 | All Grades | Grade ≥ 3 | All Grades | Grade ≥ 3 | All Grades | Grade ≥ 3 | All Grades | Grade ≥ 3 | All Grades | Grade ≥ 3 | |
| Any AEs | 210 (99.1) | 142 (66.9) | 199 (94.3) | 98 (46.5) | 387 (97.2) | 188 (47.2) | 376 (94.9) | 152 (38.4) | 392 (98.0) | 299 (75.0) | 379 (95.0) | 181 (45.0) |
| Interruption due to adverse event | - | - | - | - | 178 (44.7) | - | 100 (25.3) | - | 300 (75.0) | - | 94 (23.0) | - |
| Dose reduction due to adverse event | 42 (19.8) | - | 7 (3.3) | - | 80 (20.1) | - | 22 (5.6) | - | 223 (56.0) | - | 29 (7.0) | - |
| Discontinuation due to adverse event | 23 (10.8) | - | 10 (4.7) | - | 55 (13.8) | - | 31 (7.8) | - | 76 (19.0) | - | 49 (12.0) | - |
| Blood and lymphatic system disorders | ||||||||||||
| Anemia | 98 (46.2) | 63 (29.7) | 43 (20.4) | 16 (7.6) | 183 (46.0) | 60 (15.1) | 65 (16.4) | 13 (3.3) | 262 (66.0) | 185 (46.0) | 70 (17.0) | 17 (4.0) |
| Thrombocytopenia | 45 (21.2) | 14 (6.6) | 18 (8.5) | 5 (2.4) | - | - | - | - | 98 (25.0) | 29 (7.0) | 14 (3.0) | 4 (1.0) |
| Neutropenia | 29 (13.7) | 14 (6.6) | 12 (5.7) | 3 (1.4) | - | - | - | - | 142 (36.0) | 73 (18.0) | 28 (7.0) | 6 (1.0) |
| Leukopenia | 22 (10.4) | 4 (1.9) | 5 (2.4) | 1 (0.5) | - | - | - | - | 88 (22.0) | 25 (6.0) | 18 (4.0) | 0 (0) |
| Cardiac disorders | ||||||||||||
| Hypertension | 66 (31.1) | 31 (14.6) | 44 (20.9) | 26 (12.3) | 50 (12.6) | 14 (3.5) | 65 (16.4) | 13 (3.3) | 55 (14.0) | 21 (5.0) | 62 (15.0) | 30(7.0) |
| Arrhythmia | 27 (12.7) | 6 (2.8) | - | - | - | - | - | - | - | - | - | - |
| General disorders | ||||||||||||
| Fatigue | 56 (26.4) | 7 (3.3) | 35 (16.6) | 9 (4.3) | 148 (37.2) | 9 (2.3) | 112 (28.3) | 6 (1.5) | 134 (34.0) | 16 (4.0) | 118 (29.0) | 8 (2.0) |
| Gastrointestinal disorders | ||||||||||||
| Constipation | 65 (30.7) | 0 (0) | 29 (13.7) | 0 (0) | 69 (17.3) | 0 (0) | 55 (13.9) | 1 (0.3) | 72 (18.0) | 1 (<1.0) | 68 (17.0) | 2 (<1.0) |
| Nausea | 50 (23.6) | 1 (0.5) | 29 (13.7) | 0 (0) | 112 (28.1) | 1 (0.3) | 50 (12.6) | 1 (0.3) | 82 (21.0) | 2 (<1.0) | 50 (12.0) | 3 (<1.0) |
| Diarrhea | - | - | - | - | 69 (17.3) | 3 (0.8) | 37 (9.3) | 1 (0.3) | 57 (14.0) | 1 (<1.0) | 55 (14.0) | 0 (0) |
| Decreased appetite | 30 (14.2) | 1 (0.5) | 13 (6.2) | 1 (0.5) | 58 (14.6) | 4 (1.0) | 23 (5.8) | 0 (0) | 86 (22.0) | 5 (1.0) | 63 (16.0) | 4 (1.0) |
| Hepatotoxicity | 25 (11.8) | 4 (1.9) | - | - | - | - | - | - | - | - | - | - |
| Back pain | 31 (14.6) | 5 (2.4) | 44 (20.9) | 2 (0.9) | 67 (17.1) | 3 (0.8) | 73 (18.4) | 4 (1.0) | 88 (22.0) | 10 (3.0) | 72 (18.0) | 4 (1.0) |
| Arthralgia | 28 (13.2) | 2 (1.0) | 20 (9.5) | 1 (0.5) | 51 (12.8) | 0 (0) | 70 (17.7) | 2 (0.5) | 58 (15.0) | 2 (<1.0) | 79 (20.0) | 2 (<1.0) |
| Urinary tract infection | - | - | - | - | 41 (10.3) | 8 (2.0) | 31 (7.8) | 4 (1.0) | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Xiong, X.; Zheng, W.; Liao, X.; Xu, H.; Yang, L.; Wei, Q. Combining Novel Hormonal Therapies with a Poly (ADP-Ribose) Polymerase Inhibitor for Metastatic Castration-Resistant Prostate Cancer: Emerging Evidence. Curr. Oncol. 2023, 30, 10311-10324. https://doi.org/10.3390/curroncol30120751
Yang J, Xiong X, Zheng W, Liao X, Xu H, Yang L, Wei Q. Combining Novel Hormonal Therapies with a Poly (ADP-Ribose) Polymerase Inhibitor for Metastatic Castration-Resistant Prostate Cancer: Emerging Evidence. Current Oncology. 2023; 30(12):10311-10324. https://doi.org/10.3390/curroncol30120751
Chicago/Turabian StyleYang, Jie, Xingyu Xiong, Weitao Zheng, Xinyang Liao, Hang Xu, Lu Yang, and Qiang Wei. 2023. "Combining Novel Hormonal Therapies with a Poly (ADP-Ribose) Polymerase Inhibitor for Metastatic Castration-Resistant Prostate Cancer: Emerging Evidence" Current Oncology 30, no. 12: 10311-10324. https://doi.org/10.3390/curroncol30120751
APA StyleYang, J., Xiong, X., Zheng, W., Liao, X., Xu, H., Yang, L., & Wei, Q. (2023). Combining Novel Hormonal Therapies with a Poly (ADP-Ribose) Polymerase Inhibitor for Metastatic Castration-Resistant Prostate Cancer: Emerging Evidence. Current Oncology, 30(12), 10311-10324. https://doi.org/10.3390/curroncol30120751





