Patient Adherence to Oral Anticancer Agents: A Mapping Review of Supportive Interventions
Abstract
:1. Introduction
2. Objectives
3. Methods
3.1. Search Strategy and Eligibility Criteria
3.2. Study Selection and Review
3.3. Data Extraction
4. Results
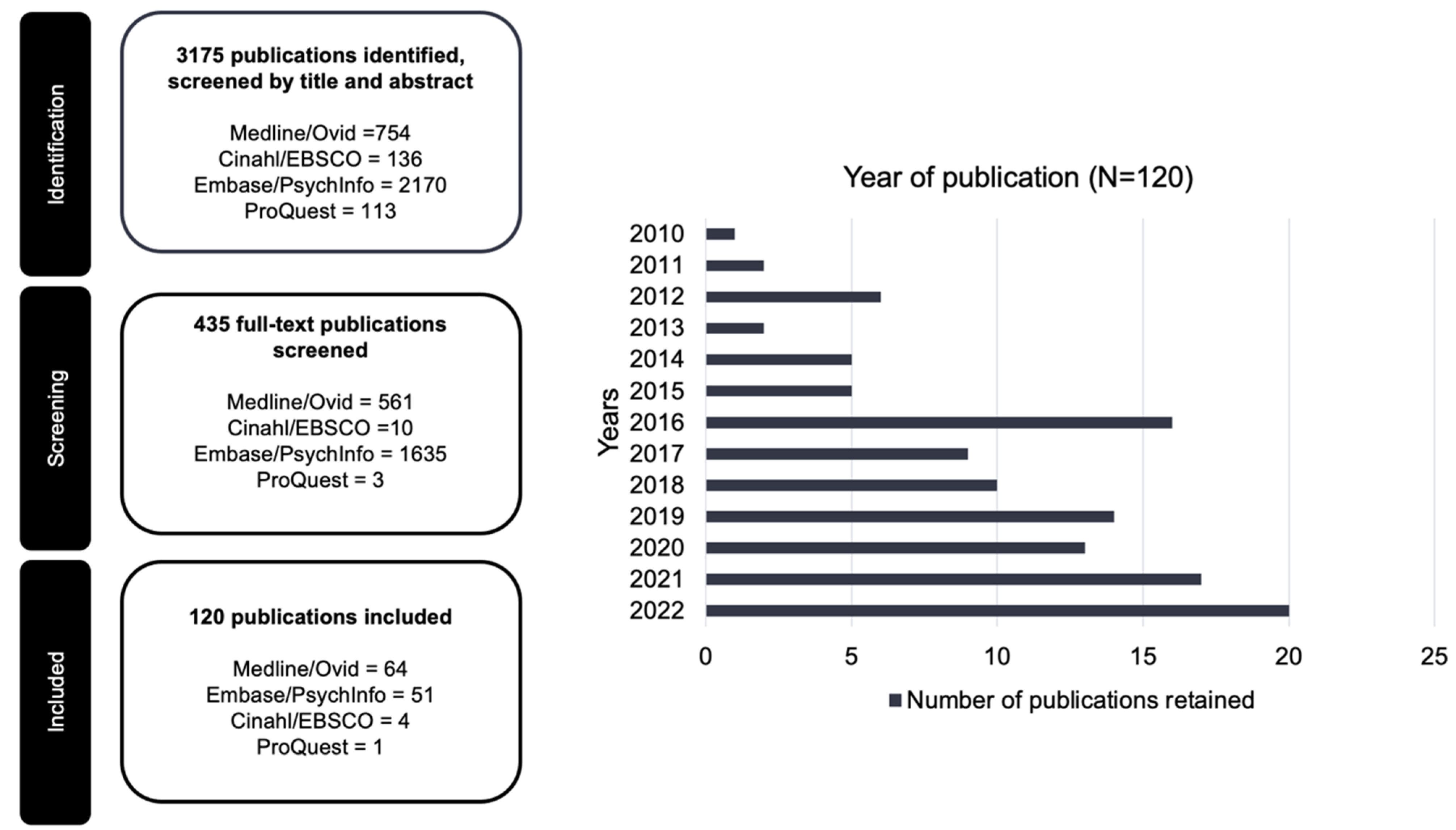
4.1. Year of Publication
4.2. Conceptual or Theoretical Framework
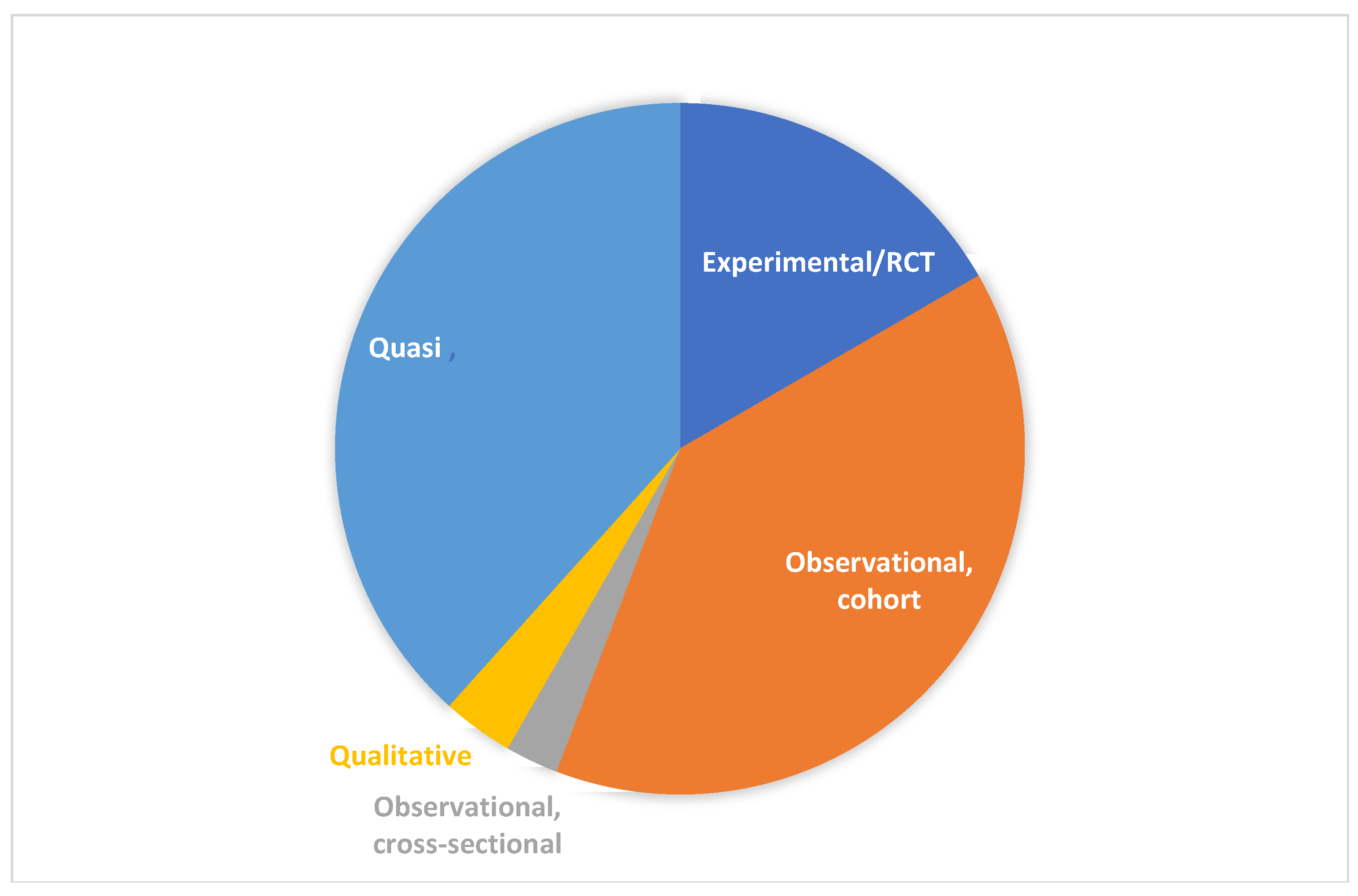
4.3. Study Design
4.4. Study Sample
4.5. Sample Size
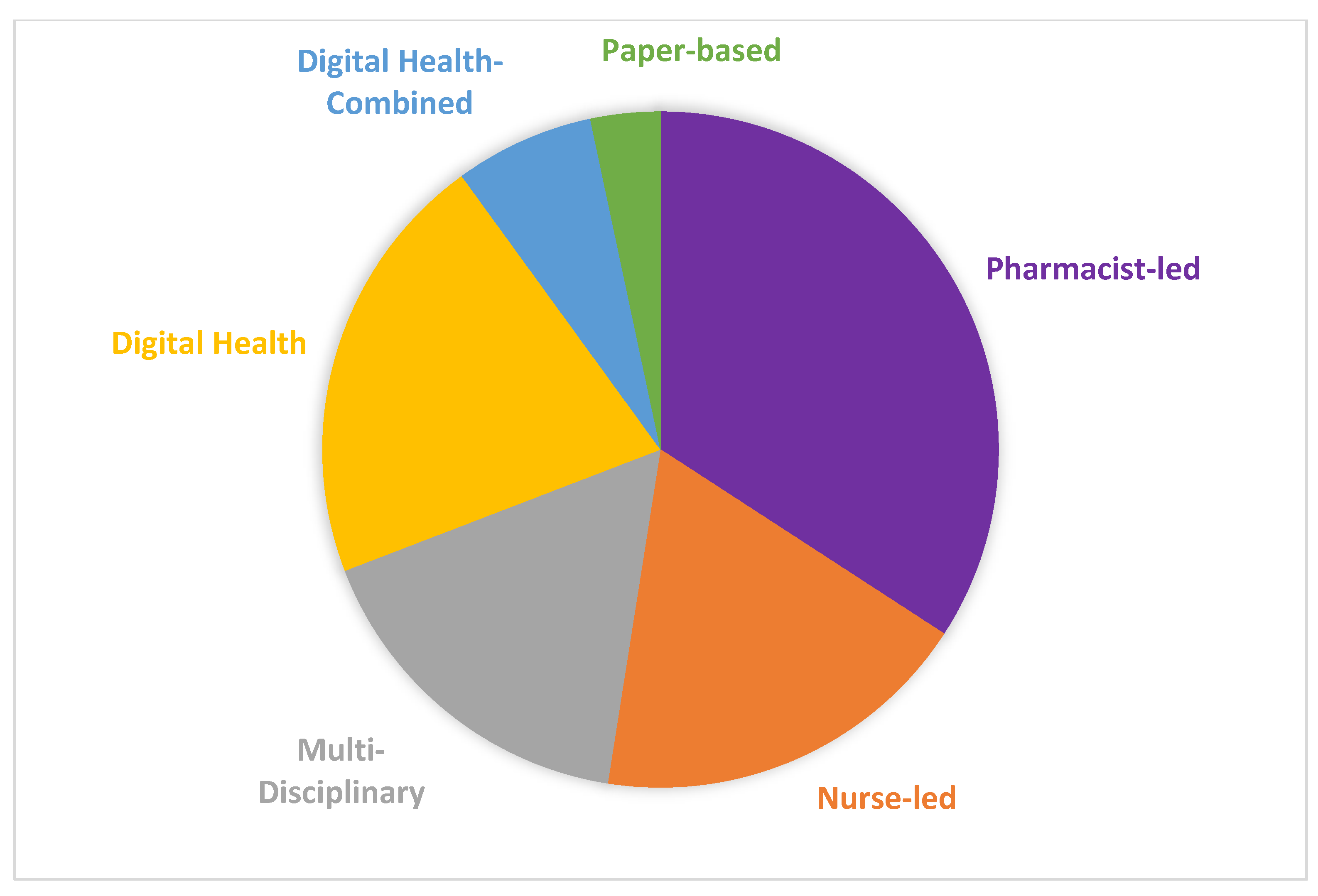
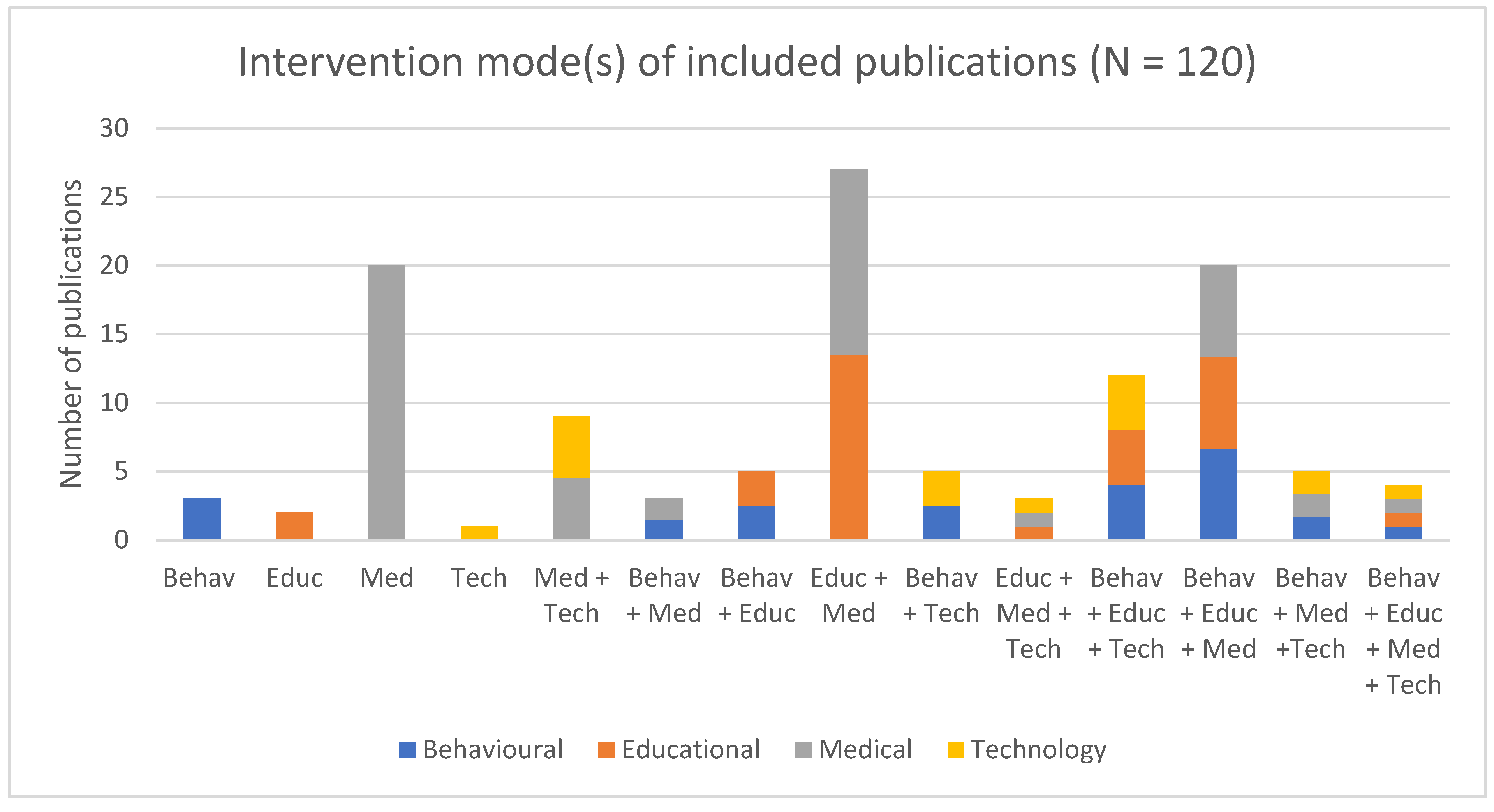
4.6. Intervention Type and Approach
4.7. Primary Outcome(s)
5. Discussion
Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moujaess, E.; Kourie, H.R.; Ghosn, M. Cancer patients and research during COVID-19 pandemic: A systematic review of current evidence. Crit. Rev. Oncol. 2020, 150, 102972. [Google Scholar] [CrossRef] [PubMed]
- Villalba, E.; Pomey, M.-P.; Guemghar, I.; Côté, I. Second Survey Report on the Impact of the Measures Implemented to Counter the COVID-19 Pandemic on Oncology Patients. Coliation Priorité Cancer. 2020. Available online: https://coalitioncancer.com/wp-content/uploads/2020/06/FINAL_REPORT_COVID-CANCER-JUNE2020.pdf (accessed on 9 December 2020).
- Government of Canada. Oncology Medicines in Canada: Trends and International Comparisons, 2010–2019. 2020. Available online: https://www.canada.ca/en/patented-medicine-prices-review/services/npduis/analytical-studies/oncology-medicines-trends-international-comparisons.html (accessed on 1 August 2023).
- Iqvia Institute. Global Oncology Trends 2022: Outlook to 2026. 2022. Available online: https://decidehealth.world/system/files/2022-06/iqvia-institute-global-oncology-trends-2022-forweb.pdf (accessed on 1 August 2023).
- Raymond, C.; Leong, C.; Fransoo, R.; Geirnart, M.; Dragan, R.; Rogendran, M.; Thomson, T.; Rajotte, L.; Koseva, I.; Schultz, J.; et al. Outpatient Oral Anticancer Agents in Manitoba. Manitova Centre for Health Policy. 2018. Available online: http://mchp-appserv.cpe.umanitoba.ca/reference/RxOnc_Report_web.pdf (accessed on 21 November 2023).
- Thomas, S.A.; John, T.; Criner, E.; Nguyen, T.M. Challenges to Oral Chemotherapy Adherence. US Pharm. 2019, 44, HS-9–HS-12. [Google Scholar]
- Chakrabarti, S. What’s in a name? Compliance, adherence and concordance in chronic psychiatric disorders. World J. Psychiatry 2014, 4, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: A patient-level meta-analysis of 37,298 women with early breast cancer in 26 randomised trials. Lancet 2019, 393, 1440–1452. [Google Scholar] [CrossRef]
- Vrijens, B.; De Geest, S.; Hughes, D.A.; Przemyslaw, K.; Demonceau, J.; Ruppar, T.; Dobbels, F.; Fargher, E.; Morrison, V.; Lewek, P.; et al. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 2012, 73, 691–705. [Google Scholar] [CrossRef]
- Greer, J.A.; Amoyal, N.; Nisotel, L.; Fishbein, J.N.; MacDonald, J.; Stagl, J.; Lennes, I.; Temel, J.S.; Safren, S.A.; Pirl, W.F. A Systematic Review of Adherence to Oral Antineoplastic Therapies. Oncologist 2016, 21, 354–376. [Google Scholar] [CrossRef]
- Cutler, R.L.; Fernandez-Llimos, F.; Frommer, M.; Benrimoj, C.; Garcia-Cardenas, V. Economic impact of medication non-adherence by disease groups: A systematic review. BMJ Open 2018, 8, e016982. [Google Scholar] [CrossRef]
- DiMatteo, M.R.; Giordani, P.J.; Lepper, H.S.; Croghan, T.W. Patient Adherence and Medical Treatment Outcomes. Med. Care 2002, 40, 794–811. [Google Scholar] [CrossRef]
- Ganesan, P.; Sagar, T.G.; Dubashi, B.; Rajendranath, R.; Kannan, K.; Cyriac, S.; Nandennavar, M. Nonadherence to Imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am. J. Hematol. 2011, 86, 471–474. [Google Scholar] [CrossRef]
- Wu, E.Q.; Johnson, S.; Beaulieu, N.; Arana, M.; Bollu, V.; Guo, A.; Coombs, J.; Feng, W.; Cortes, J. Healthcare resource utilization and costs associated with non-adherence to imatinib treatment in chronic myeloid leukemia patients. Curr. Med. Res. Opin. 2010, 26, 61–69. [Google Scholar] [CrossRef]
- Ross, X.S.; Gunn, K.M.; Suppiah, V.; Patterson, P.; Olver, I. A review of factors influencing non-adherence to oral antineoplastic drugs. Support. Care Cancer 2020, 28, 4043–4050. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.H.; Forkan, A.R.M.; Wickramasinghe, N.; Jayaraman, P.P.; Alexander, M.; Burbury, K.; Schofield, P. Investigation of Intervention Solutions to Enhance Adherence to Oral Anticancer Medicines in Adults: Overview of Reviews. JMIR Cancer 2022, 8, e34833. [Google Scholar] [CrossRef] [PubMed]
- Lafata, J.E.; Nguyen, B.; Staresinic, C.; Johnson, M.; Gratie, D.; Muluneh, B. Interpersonal communication-, education- and counselling-based interventions to support adherence to oral anticancer therapy: A systematic review. J. Oncol. Pharm. Pr. 2023, 29, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Waseem, H. Interventions to Support Adherence to Oral Anticancer Medications: Systematic Review and Meta-Analysis. Oncol. Nurs. Forum 2022, 49, E4–E16. [Google Scholar] [CrossRef]
- Booth, A. EVIDENT Guidance for Reviewing the Evidence: A compendium of methodological literature and websites. Methodology 2016, 13. in press. [Google Scholar] [CrossRef]
- Campbell, F.; Tricco, A.C.; Munn, Z.; Pollock, D.; Saran, A.; Sutton, A.; White, H.; Khalil, H. Mapping reviews, scoping reviews, and evidence and gap maps (EGMs): The same but different—The “Big Picture” review family. Syst. Rev. 2023, 12, 45. [Google Scholar] [CrossRef]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Khalil, H.; Tricco, A.C. Differentiating between mapping reviews and scoping reviews in the evidence synthesis ecosystem. J. Clin. Epidemiol. 2022, 149, 175–182. [Google Scholar] [CrossRef]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, A12–A13. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. he PRISMA 2020 statement: An updated guideline for reporting systematic reviews Systematic reviews and Meta-Analyses. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Mednis, D. The definition of “medical intervention” biomedical aspects. MOJ Public Health 2020, 9, 1–3. [Google Scholar] [CrossRef]
- Ricci, L.; Villegente, J.; Loyal, D.; Ayav, C.; Kivits, J.; Rat, A. Tailored patient therapeutic educational interventions: A patient-centred communication model. Health Expect. 2022, 25, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.B.; Bulatao, R.A.; Cohen, B.; National Research Council (US). Critical Perspectives on Racial and Ethnic Differences in Health in Late Life; Panel on Race, Ethnicity, and Health in Later Life, Ed.; National Academies Press (US): Cambridge, MA, USA, 2004. [Google Scholar]
- De Witte, N.A.J.; Joris, S.; Van Assche, E.; Van Daele, T. Technological and Digital Interventions for Mental Health and Wellbeing: An Overview of Systematic Reviews. Front. Digit. Health 2021, 3, 754337. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Approves First Cancer Drug through New Oncology Review Pilot That Enables Greater Development Efficiency. 2018. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-cancer-drug-through-new-oncology-review-pilot-enables-greater-development (accessed on 2 August 2022).
- Levin, J. FDA Approves First Generic Capecitabine to Treat Colorectal and Breast Cancers. 2013. Available online: https://www.fiercepharma.com/pharma/fda-approves-first-generic-capecitabine-to-treat-colorectal-and-breast-cancers (accessed on 2 August 2022).
- Pfizer. Pfizer Receives U.S. FDA Accelerated Approval of IBRANCE® (Palbociclib). 2013. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer_receives_u_s_fda_accelerated_approval_of_ibrance_palbociclib (accessed on 2 August 2022).
- Mir, T.H. Adherence Versus Compliance. HCA Health J. Med. 2023, 4, 22–220. [Google Scholar] [CrossRef]
- World Health Organization. Aherence to Long-Term Therapies: Evidence for Action. 2003. Available online: https://www.who.int/chp/knowledge/publications/adherence_report/en/ (accessed on 2 May 2022).
- Rosenberg, S.M.; Petrie, K.J.; Stanton, A.L.; Ngo, L.; Finnerty, E.; Partridge, A.H. Interventions to Enhance Adherence to Oral Antineoplastic Agents: A Scoping Review. J. Natl. Cancer Inst. 2020, 112, 443–465. [Google Scholar] [CrossRef]
- Spoelstra, S.; Sansoucie, H. Putting Evidence Into Practice: Evidence-Based Interventions for Oral Agents for Cancer. CJON 2015, 19 (Suppl. 3), 60–72. [Google Scholar] [CrossRef]
- Kil, T. Effects of the multimodal intervention program including animal-assisted therapy on depression and self-esteem among university students. J. Anim. Sci. Technol. 2021, 63, 1443–1452. [Google Scholar] [CrossRef]
- Irwin, M. Theoretical Foundations of Adherence Behaviors: Synthesis and Application in Adherence to Oral Oncology Agents. CJON 2015, 19 (Suppl. 3), 31–35. [Google Scholar] [CrossRef]
- Kahwati, L.; Viswanathan, M.; Golin, C.E.; Kane, H.; Lewis, M.; Jacobs, S. Identifying configurations of behavior change techniques in effective medication adherence interventions: A qualitative comparative analysis. Syst. Rev. 2016, 5, 83. [Google Scholar] [CrossRef]
- Knudsen, S.V.; Laursen, H.V.B.; Johnsen, S.P.; Bartels, P.D.; Ehlers, L.H.; Mainz, J. Can quality improvement improve the quality of care? A systematic review of reported effects and methodological rigor in plan-do-study-act projects. BMC Health Serv. Res. 2019, 19, 683. [Google Scholar] [CrossRef] [PubMed]
- Agency for Healthcare Research and Quality. Plan-Do-Study-Act (PDSA) Directions and Examples. 2020. Available online: https://www.ahrq.gov/health-literacy/improve/precautions/tool2b.html (accessed on 21 November 2023).
- Health Quality Ontario. PDSA Cycles. 2023. Available online: https://www.hqontario.ca/portals/0/documents/qi/rf-document-pdsa-cycles-en.pdf (accessed on 21 November 2023).
- Taylor, M.J.; McNicholas, C.; Nicolay, C.; Darzi, A.; Bell, D.; E Reed, J. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMJ Qual. Saf. 2014, 23, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Center for Substance Abuse Treatment. Enhancing Motivation for Change in Substance Abuse Treatment; Chapter 3—Motivational Interviewing as a Counseling Style; Substance Abuse and Mental Health Services Administration (US): Rockville, MD, USA, 1999.
- Bischof, G.; Bischof, A.; Rumpf, H.-J. Motivational Interviewing: An Evidence-Based Approach for Use in Medical Practice. Dtsch. Aerzteblatt Online 2021, 118, 109–115. [Google Scholar] [CrossRef]
- Rubak, S.; Sandbaek, A.; Lauritzen, T.; Christensen, B. Motivational interviewing: A systematic review and meta-analysis. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2005, 55, 305–312. [Google Scholar]
- Bandura, A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef]
- Náfrádi, L.; Nakamoto, K.; Schulz, P.J. Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PLoS ONE 2017, 12, e0186458. [Google Scholar] [CrossRef] [PubMed]
- Marcus, A. Pay up or Retract? Survey Creator’s Demands for Money Rile Some Health Researchers. 2017. Available online: https://www.science.org/content/article/pay-or-retract-survey-creators-demands-money-rile-some-health-researchers (accessed on 21 November 2023).
- Cuevas, C.D.L.; Peñate, W. Psychometric properties of the eight-item Morisky Medication Adherence Scale (MMAS-8) in a psychiatric outpatient setting. Int. J. Clin. Health Psychol. 2015, 15, 121–129. [Google Scholar] [CrossRef]
- Office of Research Services at the University of Pennsylvania. Consider Alternatives to the Morisky Medication Adherence Scale (MMAS-4 and MMAS-8). 2020. Available online: https://researchservices.upenn.edu/2020/02/24/consider-alternatives-to-the-morisky-medication-adherence-scale-mmas-4-and-mmas-8/ (accessed on 21 November 2023).
- Chan, A.H.Y.; Horne, R.; Hankins, M.; Chisari, C. The Medication Adherence Report Scale: A measurement tool for eliciting patients’ reports of nonadherence. Br. J. Clin. Pharmacol. 2020, 86, 1281–1288. [Google Scholar] [CrossRef]
- Talens, A.; López-Pintor, E.; Guilabert, M.; Cantó-Sancho, N.; Aznar, M.T.; Lumbreras, B. Validation of a scale to assess adherence to oral chemotherapy based on the experiences of patients and healthcare professionals (EXPAD-ANEO). Front. Pharmacol. 2023, 14, 1113898. [Google Scholar] [CrossRef]
- Pharmacy Times. Do You Know the Difference between These Adherence Measures? 2015. Available online: https://www.pharmacytimes.com/view/do-you-know-the-difference-between-these-adherence-measures (accessed on 2 August 2023).
| Concept 1 Patient/Population | Concept 2 Intervention/Exposure | Concept 3 Outcome |
|---|---|---|
| Oral anticancer agent | Supportive intervention | Medication adherence |
| Theory/Framework | Number of Publications N = 120, n (%) |
|---|---|
| Plan-Do-Study-Act (PDSA) Model | 4 (3.3) |
| Self-efficacy | 3 (2.5) |
| Motivational interviewing | 2 (1.6) |
| Self-efficacy and motivational interviewing | 1 (0.83) |
| Acceptance and Commitment Therapy (ACT) and self-affirmation theory | 1 (0.83) |
| Concordance and shared decision-making | 1 (0.83) |
| Conceptual framework created to study med adherence | 1 (0.83) |
| Health belief model and the stress process model | 1 (0.83) |
| Intervention for Symptom Management Model | 1 (0.83) |
| Motivation Theory | 1 (0.83) |
| Self-Care-Deficient Nursing Theory | 1 (0.83) |
| Self-Regulatory Model of Antiretroviral Adherence | 1 (0.83) |
| Social Representation (SR) theory | 1 (0.83) |
| Synergy Model of Patient Care and Ottawa Model of Research Use | 1 (0.83) |
| UK Medical Research Council’s Self-Management Framework | 1 (0.83) |
| Study Design | Number of Publications, N = 120, n (%) |
|---|---|
| Observational, cohort | 47 (39.2) |
| Quasi-experimental | 46 (38.3) |
| Experimental | 20 (16.7) |
| Qualitative | 4 (3.3) |
| Observational, cross-sectional | 3 (2.5) |
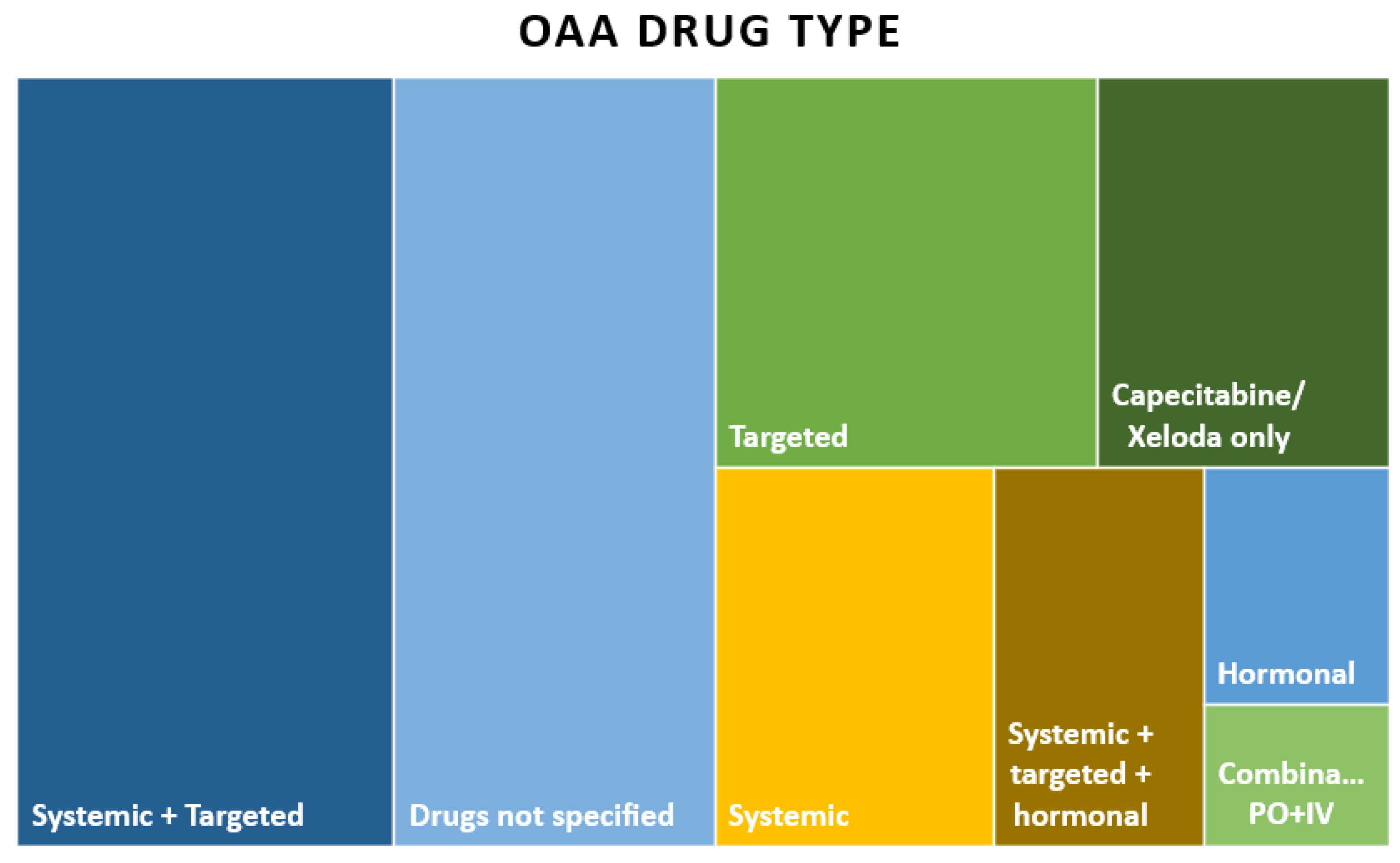
| OAA Drug Types | Number of Publications, N = 120, n (%) |
|---|---|
| Systemic and targeted | 33 (27.5) |
| Drugs not specified | 28 (23.3) |
| Targeted | 17 (14.2) |
| Capecitabine/Xeloda only | 13 (10.8) |
| Systemic, targeted, and hormonal | 3 (2.5) |
| Hormonal | 5 (4.2) |
| Combination treatment of IV and PO | 3 (2.5) |
| Intervention Modality | Number of Publications, N = 120, n (%) |
|---|---|
| Pharmacist-led | 41 (34.2) |
| Nurse-led | 22 (18.3) |
| Digital health | 25 (20.8) |
| Multi-disciplinary | 20 (16.6) |
| Digital health/healthcare provider combined | 8 (6.7) |
| Paper-based | 4 (3.3) |
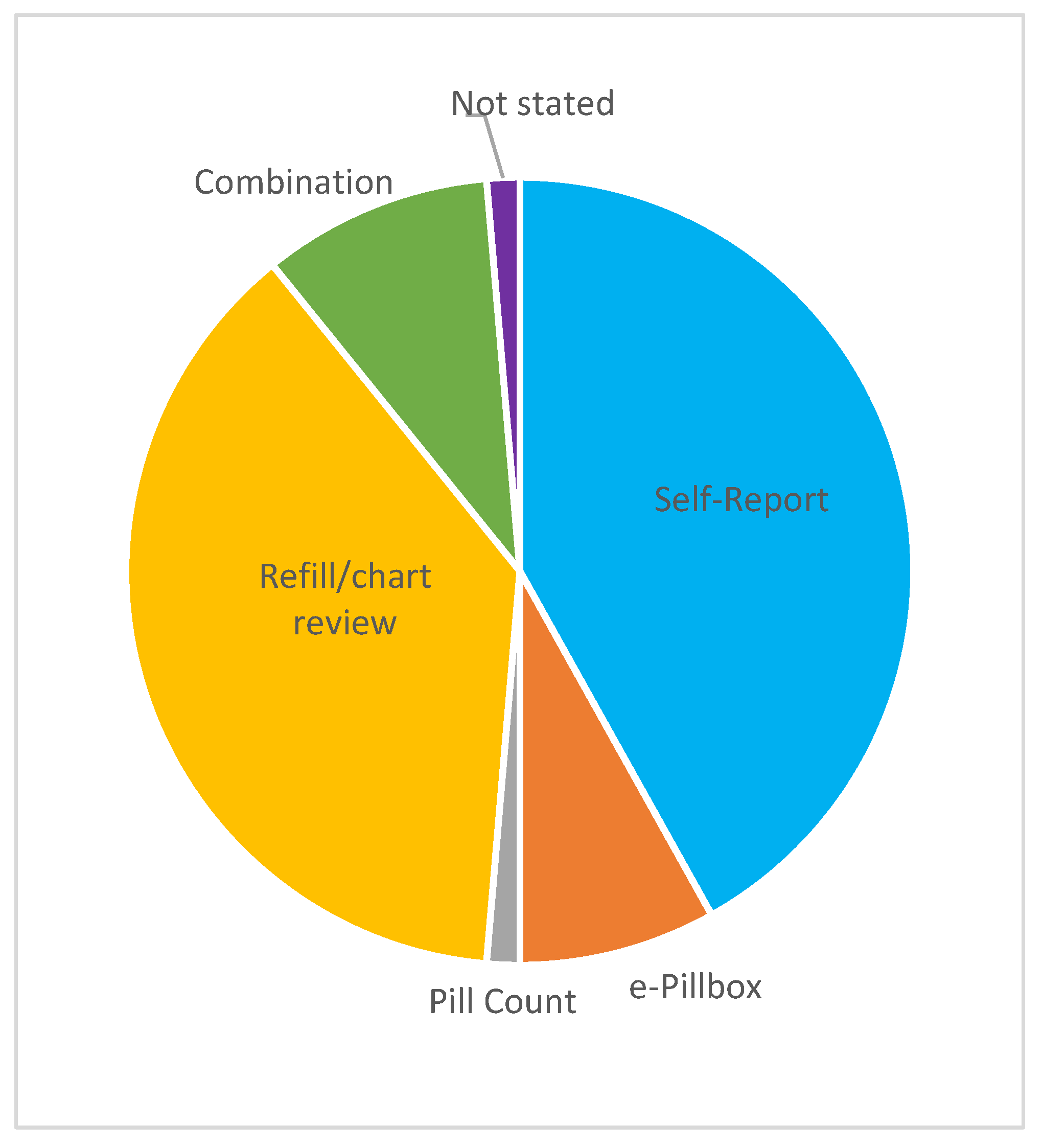
| Self-Report (n = 31) | Refill/Chart (n = 28) | Combination (n = 7) |
|---|---|---|
| Study-specific questionnaire (n = 16) | Medication possession ratio: MPR (n = 16) | Diary + pill count (n = 1) |
| Morisky Medication Adherence Scale: MMAS-8 (n = 7) | Details not available: NA (n = 7) | Self-report (Basel Assessment of Adherence Scale: BAAS) + pill count (n = 1) |
| Via telephone (n = 2) | Proportion of days covered: PDC (n = 2) | Self-report (MARS-5) + e-pillbox (n = 1) |
| Medication Adherence Report Scale: MARS-5 (n = 1) | Relative dose intensity: RDI (n = 2), | Self-report (MMAS-8) + e-pillbox (n = 1) |
| Oral Chemotherapy Adherence Scale: OCAS (n = 1) | MPR/PDC/TTT (n = 1) | Self-report (study-specific) + refill (NA) (n = 1) |
| Medication Adherence Questionnaire: MAQ (n = 1) | Plasma drug concentration + self-report (study-specific) (n = 1) | |
| Morisky Green Levine Medication Adherence Scale (n = 1) | RDI + pill count (n = 1) | |
| Patient diary (n = 1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.; Loiselle, C.G. Patient Adherence to Oral Anticancer Agents: A Mapping Review of Supportive Interventions. Curr. Oncol. 2023, 30, 10224-10236. https://doi.org/10.3390/curroncol30120744
Ahmed S, Loiselle CG. Patient Adherence to Oral Anticancer Agents: A Mapping Review of Supportive Interventions. Current Oncology. 2023; 30(12):10224-10236. https://doi.org/10.3390/curroncol30120744
Chicago/Turabian StyleAhmed, Saima, and Carmen G. Loiselle. 2023. "Patient Adherence to Oral Anticancer Agents: A Mapping Review of Supportive Interventions" Current Oncology 30, no. 12: 10224-10236. https://doi.org/10.3390/curroncol30120744
APA StyleAhmed, S., & Loiselle, C. G. (2023). Patient Adherence to Oral Anticancer Agents: A Mapping Review of Supportive Interventions. Current Oncology, 30(12), 10224-10236. https://doi.org/10.3390/curroncol30120744






