Clinical, Pathological, and Prognostic Features of Male Breast Cancer: A Multicenter Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Biomarkers Evaluation
2.2. Statistical Analysis
3. Results
3.1. Study Population
3.2. Tumor Characteristics
3.3. Surgery and Adjuvant Treatment
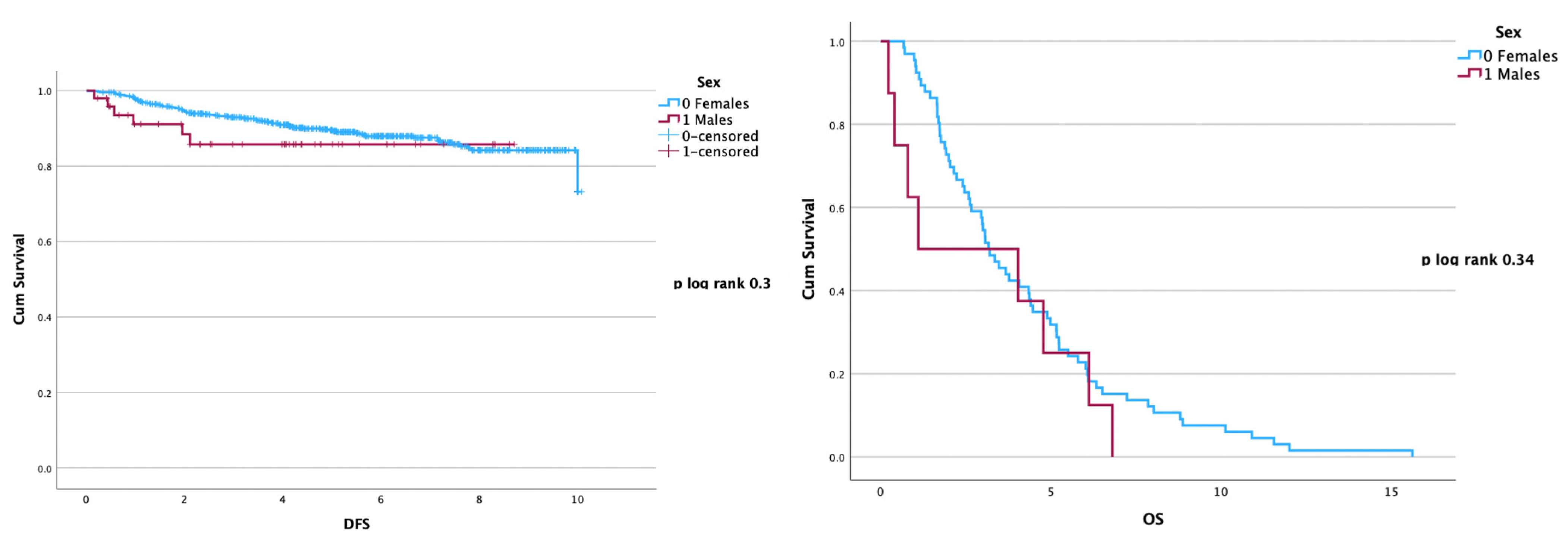
3.4. Disease Recurrence and Overall Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mangone, L.; Ferrari, F.; Mancuso, P.; Carrozzi, G.; Michiara, M.; Falcini, F.; Piffer, S.; Filiberti, R.A.; Caldarella, A.; Vitale, F.; et al. Epidemiology and biological characteristics of male breast cancer in Italy. Breast Cancer 2020, 27, 724–731. [Google Scholar] [CrossRef]
- Humphries, M.P.; Rajan, S.S.; Honarpisheh, H.; Cserni, G.; Dent, J.; Fulford, L.; Jordan, L.B.; Jones, J.L.; Kanthan, R.; Litwiniuk, M.; et al. Characterisation of male breast cancer: A descriptive biomarker study from a large patient series. Sci. Rep. 2017, 7, 45293. [Google Scholar] [CrossRef]
- Sousa, B.; Moser, E.; Cardoso, F. An update on male breast cancer and future directions for research and treatment. Eur. J. Pharmacol. 2013, 717, 71–83. [Google Scholar] [CrossRef]
- Korde, L.A.; Zujewski, J.A.; Kamin, L.; Giordano, S.; Domchek, S.; Anderson, W.F.; Bartlett, J.M.; Gelmon, K.; Nahleh, Z.; Bergh, J.; et al. Multidisciplinary Meeting on Male Breast Cancer: Summary and Research Recommendations. J. Clin. Oncol. 2010, 28, 2114–2122. [Google Scholar] [CrossRef]
- Losurdo, A.; Rota, S.; Gullo, G.; Masci, G.; Torrisi, R.; Bottai, G.; Zuradelli, M.; Gatzemeier, W.; Santoro, A. Controversies in clinicopathological characteristics and treatment strategies of male breast cancer: A review of the literature. Crit. Rev. Oncol. 2017, 113, 283–291. [Google Scholar] [CrossRef]
- Silvestri, V.; Barrowdale, D.; Mulligan, A.M.; Neuhausen, S.L.; Fox, S.; Karlan, B.Y.; Mitchell, G.; James, P.; Thull, D.L.; Zorn, K.K.; et al. Male breast cancer in BRCA1 and BRCA2 mutation carriers: Pathology data from the Consortium of Investigators of Modifiers of BRCA1/2. Breast Cancer Res. 2016, 18, 15. [Google Scholar] [CrossRef]
- Sanguinetti, A.; Polistena, A.; Lucchini, R.; Monacelli, M.; Galasse, S.; Avenia, S.; Triola, R.; Bugiantella, W.; Cirocchi, R.; Rondelli, F.; et al. Male breast cancer, clinical presentation, diagnosis and treatment: Twenty years of experience in our Breast Unit. Int. J. Surg. Case Rep. 2016, 20, 8–11. [Google Scholar] [CrossRef]
- Saita, C.; Yamaguchi, T.; Horiguchi, S.-I.; Yamada, R.; Takao, M.; Iijima, T.; Wakaume, R.; Aruga, T.; Tabata, T.; Koizumi, K. Tumor development in Japanese patients with Lynch syndrome. PLoS ONE 2018, 13, e0195572. [Google Scholar] [CrossRef]
- Greif, J.M.; Pezzi, C.M.; Klimberg, V.S.; Bailey, L.; Zuraek, M. Gender Differences in Breast Cancer: Analysis of 13,000 Breast Cancers in Men from the National Cancer Data Base. Ann. Surg. Oncol. 2012, 19, 3199–3204. [Google Scholar] [CrossRef]
- Uslukaya, O.; Gumus, M.; Gumus, H.; Bozdag, Z.; Turkoglu, A. The Management and Outcomes of Male Breast Cancer. J. Breast Heal. 2016, 12, 165–170. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.; Zhang, L.; Li, S.; Shi, Y.; Tong, Z. Poorer Survival of Male Breast Cancer Compared with Female Breast Cancer Patients May Be Due to Biological Differences. Jpn. J. Clin. Oncol. 2013, 43, 954–963. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute Surveillance, Epidemiology and End Results Program. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 10 May 2023).
- Leon-Ferre, R.A.; Giridhar, K.V.; Hieken, T.J.; Mutter, R.W.; Couch, F.J.; Jimenez, R.E.; Hawse, J.R.; Boughey, J.C.; Ruddy, K.J. A contemporary review of male breast cancer: Current evidence and unanswered questions. Cancer Metastasis Rev. 2018, 37, 599–614. [Google Scholar] [CrossRef]
- Hammond, M.E.H.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef] [PubMed]
- NCCN Guidelines Version 4.2023 Breast Cancer. Available online: www.nccn.org (accessed on 10 May 2023).
- Nguyen, P.L.; Taghian, A.G.; Katz, M.S.; Niemierko, A.; Raad, R.F.A.; Boon, W.L.; Bellon, J.R.; Wong, J.S.; Smith, B.L.; Harris, J.R. Breast Cancer Subtype Approximated by Estrogen Receptor, Progesterone Receptor, and HER-2 Is Associated with Local and Distant Recurrence After Breast-Conserving Therapy. J. Clin. Oncol. 2008, 26, 2373–2378. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Gnant, M.; Harbeck, N.; Thomssen, C. St. Gallen 2011: Summary of the Consensus Discussion. Breast Care 2011, 6, 136–141. [Google Scholar] [CrossRef]
- Harbeck, N.; Thomssen, C.; Gnant, M. St. Gallen 2013: Brief Preliminary Summary of the Consensus Discussion. Breast Care 2013, 8, 102–109. [Google Scholar] [CrossRef]
- NCCN Breast Cancer Guidelines 2015. Available online: www.nccn.org (accessed on 5 May 2023).
- Masci, G.; Caruso, M.; Caruso, F.; Salvini, P.; Carnaghi, C.; Giordano, L.; Miserocchi, V.; Losurdo, A.; Zuradelli, M.; Torrisi, R.; et al. Clinicopathological and Immunohistochemical Characteristics in Male Breast Cancer: A Retrospective Case Series. Oncol. 2015, 20, 586–592. [Google Scholar] [CrossRef]
- Humphries, M.P.; Jordan, V.C.; Speirs, V. Obesity and male breast cancer: Provocative parallels? BMC Med. 2015, 13, 134. [Google Scholar] [CrossRef]
- Hill, T.D.; Khamis, H.J.; Tyczynski, J.E.; Berkel, H. Comparison of Male and Female Breast Cancer Incidence Trends, Tumor Characteristics, and Survival. Ann. Epidemiol. 2005, 15, 773–780. [Google Scholar] [CrossRef]
- Liu, D.; Xie, G.; Chen, M. Clinicopathologic characteristics and survival of male breast cancer. Int. J. Clin. Oncol. 2013, 19, 280–287. [Google Scholar] [CrossRef]
- Patten, D.K.; Sharifi, L.K.; Fazel, M. New Approaches in the Management of Male Breast Cancer. Clin. Breast Cancer 2013, 13, 309–314. [Google Scholar] [CrossRef]
- McClurg, D.P.; Urquhart, G.; McGoldrick, T.; Chatterji, S.; Miedzybrodzka, Z.; Speirs, V.; Elsberger, B. Analysis of the Clinical Advancements for BRCA-Related Malignancies Highlights the Lack of Treatment Evidence for BRCA-Positive Male Breast Cancer. Cancers 2022, 14, 3175. [Google Scholar] [CrossRef]
- Cutuli, B.; Le-Nir, C.C.-S.; Serin, D.; Kirova, Y.; Gaci, Z.; Lemanski, C.; De Lafontan, B.; Zoubir, M.; Maingon, P.; Mignotte, H.; et al. Male breast cancer. Evolution of treatment and prognostic factors. Analysis of 489 cases. Crit. Rev. Oncol. 2010, 73, 246–254. [Google Scholar] [CrossRef]
- 30th European Guidelines on Breast Cancer Screening and Diagnosis. Available online: https://healthcare-quality.jrc.ec.europa.eu/ecibc/european-breast-cancer-guidelines (accessed on 15 September 2023).
- Giordano, S.H.; Perkins, G.H.; Broglio, K.; Garcia, S.G.; Middleton, L.P.; Buzdar, A.U.; Hortobagyi, G.N. Adjuvant systemic therapy for male breast carcinoma. Cancer 2005, 104, 2359–2364. [Google Scholar] [CrossRef]
- Johansson, I.; Killander, F.; Linderholm, B.; Hedenfalk, I. Molecular profiling of male breast cancer—Lost in translation? Int. J. Biochem. Cell Biol. 2014, 53, 526–535. [Google Scholar] [CrossRef]
- Fentiman, I.S. Surgical options for male breast cancer. Breast Cancer Res. Treat. 2018, 172, 539–544. [Google Scholar] [CrossRef]
- Rossi, L.; McCartney, A.; De Santo, I.; Risi, E.; Moretti, E.; Malorni, L.; Biganzoli, L.; Di Leo, A. The optimal duration of adjuvant endocrine therapy in early luminal breast cancer: A concise review. Cancer Treat. Rev. 2019, 74, 29–34. [Google Scholar] [CrossRef]
- Villasco, A.; Accomasso, F.; D’alonzo, M.; Agnelli, F.; Sismondi, P.; Biglia, N. Evaluation of the ability of the Clinical Treatment Score at 5 years (CTS5) compared to other risk stratification methods to predict the response to an extended endocrine therapy in breast cancer patients. Breast Cancer 2021, 28, 1131–1140. [Google Scholar] [CrossRef]
- Curigliano, G.; Burstein, H.J.; Winer, E.P.; Gnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.M.; Piccart-Gebhart, M.; Senn, H.-J.; et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1700–1712. [Google Scholar] [CrossRef]
- Rosso, R.; D’alonzo, M.; Bounous, V.E.; Actis, S.; Cipullo, I.; Salerno, E.; Biglia, N. Adherence to Adjuvant Endocrine Therapy in Breast Cancer Patients. Curr. Oncol. 2023, 30, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Severson, T.M.; Zwart, W. A review of estrogen receptor/androgen receptor genomics in male breast cancer. Endocr.-Relat. Cancer 2017, 24, R27–R34. [Google Scholar] [CrossRef] [PubMed]
- Gucalp, A.; Traina, T.A.; Eisner, J.R.; Parker, J.S.; Selitsky, S.R.; Park, B.H.; Elias, A.D.; Baskin-Bey, E.S.; Cardoso, F. Male breast cancer: A disease distinct from female breast cancer. Breast Cancer Res. Treat. 2019, 173, 37–48. [Google Scholar] [CrossRef]
- Liu, N.; Johnson, K.J.; Ma, C.X. Male Breast Cancer: An Updated Surveillance, Epidemiology, and End Results Data Analysis. Clin. Breast Cancer 2018, 18, e997–e1002. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Verkooijen, H.M.; Chia, K.-S.; Bouchardy, C.; Pukkala, E.; Larønningen, S.; Mellemkjær, L.; Czene, K.; Hartman, M. Incidence and Outcome of Male Breast Cancer: An International Population-Based Study. J. Clin. Oncol. 2011, 29, 4381–4386. [Google Scholar] [CrossRef] [PubMed]
- El-Tamer, M.B.; Komenaka, I.K.; Troxel, A.; Li, H.; Joseph, K.-A.; Ditkoff, B.-A.; Schnabel, F.R.; Kinne, D.W. Men with Breast Cancer Have Better Disease-Specific Survival Than Women. Arch. Surg. 2004, 139, 1079–1082. [Google Scholar] [CrossRef]
- Marchal, F.; Salou, M.; Marchal, C.; Lesur, A.; Desandes, E. Men with Breast Cancer Have Same Disease-Specific and Event-Free Survival as Women. Ann. Surg. Oncol. 2009, 16, 972–978. [Google Scholar] [CrossRef]
- Yao, N.; Shi, W.; Liu, T.; Siyin, S.T.; Wang, W.; Duan, N.; Xu, G.; Qu, J. Clinicopathologic characteristics and prognosis for male breast cancer compared to female breast cancer. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Foerster, R.; Foerster, F.G.; Wulff, V.; Schubotz, B.; Baaske, D.; Wolfgarten, M.; Kuhn, W.C.; Rudlowski, C. Matched-pair analysis of patients with female and male breast cancer: A comparative analysis. BMC Cancer 2011, 11, 335. [Google Scholar] [CrossRef]
- Scomersi, S.; Giudici, F.; Cacciatore, G.; Losurdo, P.; Fracon, S.; Cortinovis, S.; Ceccherini, R.; Zanconati, F.; Tonutti, M.; Bortul, M. Comparison between male and female breast cancer survival using propensity score matching analysis. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]


| Variables | FBC (n = 680) | Male BC (n = 49) | p-Value |
|---|---|---|---|
| Age at diagnosis (years; SD) | 66.6 (±11.2) | 68.6 (±10.1) | 0.25 |
| Type of surgery | |||
| Conservative | 434 (63.8%) | 0 | <0.05 |
| Mastectomy | 246 (36.2%) | 49 (100%) | <0.05 |
| Tumor diameter (mm; SD) | 21.5 (±12.1) | 17.3 (±7.8) | <0.05 |
| pT | |||
| pT1 | 375 (54.3%) | 26 (53.1%) | 0.88 |
| pT2 | 251 (36.4%) | 16 (32.7%) | 0.64 |
| pT3 | 22 (3.2%) | 0 | 0.39 |
| pT4 | 32 (6.1%) | 7 (14.2%) | 0.01 |
| Nodal status (N) | |||
| pN0 | 440 (64.7%) | 33 (67.3%) | 0.72 |
| pN+ | 215 (31.6%) | 15 (30.6%) | 0.79 |
| pNx | 25 (3.7%) | 1 (2.1%) | 0.89 |
| Tumor grade (G) | |||
| G1 | 71 (10.4%) | 8 (16.3%) | 0.22 |
| G2 | 253 (37.2%) | 19 (38.8%) | 0.87 |
| G3 | 356 (52.4%) | 22 (44.9%) | 0.31 |
| Ki67 > 20% | 372 (53.9%) | 29 (59.1%) | 0.3 |
| Histological type | |||
| NST | 500 (72.5%) | 36 (73.5%) | 0.81 |
| Lobular | 69 (10%) | 2 (4.1%) | <0.05 |
| Other | 121 (17.5%) | 11 (22.4%) | 0.23 |
| Molecular subtypes | |||
| Luminal A | 300 (43.5%) | 18 (36.7%) | 0.07 |
| Luminal B | 169 (24.5%) | 26 (53.1%) | 0.04 |
| Luminal B HER2+/HER2+ | 160 (23.1%) | 5 (10.2%) | 0.09 |
| Basal-like | 51 (8.9%) | 0 (0%) | 0.06 |
| AET | 592 (87%) | 46 (93.9%) | |
| Tamoxifen | 36 (5.3%) | 36 (73.5%) | <0.05 |
| Aromatase Inhibitors | 556 (81.8%) | 4 (8.2%) | <0.05 |
| None | 88 (12.9%) | 6 (12.3%) | 0.25 |
| AET drop-out rate | 48 (7.6%) | 8 (16.3%) | 0.04 |
| Adjuvant radiotherapy | |||
| Breast | 434 (63.8%) | 0 | <0.05 |
| Thoracic wall/nodes | 28 (4.1%) | 8 (16.3%) | <0.05 |
| None | 218 (32.1%) | 41 (83.7%) | <0.05 |
| Adjuvant chemotherapy | 281 (40.7%) | 20 (40.8%) | 1 |
| (a) Females | (b) Males | |||||
|---|---|---|---|---|---|---|
| Variables | HR | CI | p Value | HR | CI | p Value |
| Tumor stage | ||||||
| pT1 | 1 | 1 | ||||
| pT2 | 1.62 | 1.1–2.4 | 0.03 | 1.97 | 1.4–2.7 | <0.05 |
| pT3 | 2.02 | 0.81–4.98 | 0.13 | - | - | - |
| pT4 | 4.95 | 2.78–8.82 | <0.05 | 1.48 | 1.3–2.02 | 0.04 |
| Nodal involvement | 2.22 | 1.8–2.02 | 0.03 | 2.55 | 1.8–2.02 | 0.02 |
| Grade | ||||||
| G1 | 1 | 1 | ||||
| G2 | 1.23 | 0.84–2.56 | 0.73 | 1.32 | 0.73–3.45 | 0.34 |
| G3 | 2.71 | 1.71–4.34 | <0.05 | 1.84 | 1.2–3.6 | 0.04 |
| Ki67 value | ||||||
| Ki67 < 20% | 1 | 1 | ||||
| Ki67 > 20% | 2.02 | 1.28–3.17 | <0.05 | 4.24 | 0.49–36.3 | 0.19 |
| Molecular subtypes | ||||||
| Luminal A | 1 | 1 | ||||
| Luminal B | 2.55 | 1.69–3.85 | <0.05 | 2.47 | 1.07–5.73 | 0.03 |
| Luminal B HER2+/HER2+ | 0.78 | 0.47–1.28 | 0.32 | 1.49 | 0.49–4.54 | 0.47 |
| Basal-like | 2.54 | 1.38–4.67 | <0.05 | - | - | - |
| AET drop-out | 1.29 | 0.65–2.58 | 0.47 | 2.45 | 0.45–13.3 | 0.29 |
| (a) Females | (b) Males | |||||
|---|---|---|---|---|---|---|
| Variables | HR | CI | p Value | HR | CI | p Value |
| pT ≥ 2 | 1.54 | 1.24–1.91 | <0.05 | 1.50 | 1.15–1.94 | 0.02 |
| Nodal involvement | 6.66 | 3.98–11.15 | <0.05 | 6.43 | 3.55–11.67 | <0.05 |
| G3 | 1.67 | 0.97–2.71 | 0.06 | 1.94 | 1.08–3.51 | 0.01 |
| Luminal B | 1.15 | 0.76–1.29 | 0.73 | 1.22 | 0.66–1.21 | 0.4 |
| Basal-like | 1.74 | 0.84–3.78 | 0.07 | - | - | - |
| (a) Females | (b) Males | |||||
|---|---|---|---|---|---|---|
| Variables | HR | CI | p Value | HR | CI | p Value |
| Tumor stage | ||||||
| pT1 | 1 | 1 | ||||
| pT2 | 1.40 | 0.80–2.43 | 0.23 | 1.12 | 0.88–1.66 | 0.24 |
| pT3 | 2.5 | 1.06–5.89 | 0.03 | - * | - | - |
| pT4 | 1.02 | 0.79–1.31 | 0.89 | 1.01 | 0.79–1.30 | 0.90 |
| Nodal involvement | 1.32 | 0.82–2.11 | 0.25 | 1.65 | 0.34–8.34 | 0.55 |
| Grade | ||||||
| G1 | 1 | 1 | ||||
| G2 | 4.09 | 1.32–12.6 | 0.02 | 3.94 | 1.29–12.2 | 0.02 |
| G3 | 2.88 | 1.11–7.47 | 0.03 | 2.93 | 1.13–7.57 | 0.03 |
| Ki67 value | ||||||
| Ki67 < 20% | ref | 1 | ||||
| Ki67 > 20% | 1.22 | 0.74–1.98 | 0.44 | 4.14 | 0.73–23.5 | 0.11 |
| Molecular subtypes | ||||||
| Luminal A | 1 | 1 | ||||
| Luminal B | 1.24 | 0.69–2.19 | 0.46 | 1.27 | 0.79–2.02 | 0.31 |
| Luminal B HER2+/HER2+ | 0.93 | 0.67–1.27 | 0.69 | 0.93 | 0.68–1.28 | 0.67 |
| Basal-like | 1.42 | 1.06–1.90 | <0.05 | - * | - | - |
| AET drop-out | 1.02 | 0.24–4.2 | 0.97 | 0.32 | 0.03–2.71 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accomasso, F.; Actis, S.; Minella, C.; Rosso, R.; Granaglia, C.; Ponzone, R.; Biglia, N.; Bounous, V.E. Clinical, Pathological, and Prognostic Features of Male Breast Cancer: A Multicenter Study. Curr. Oncol. 2023, 30, 9860-9871. https://doi.org/10.3390/curroncol30110716
Accomasso F, Actis S, Minella C, Rosso R, Granaglia C, Ponzone R, Biglia N, Bounous VE. Clinical, Pathological, and Prognostic Features of Male Breast Cancer: A Multicenter Study. Current Oncology. 2023; 30(11):9860-9871. https://doi.org/10.3390/curroncol30110716
Chicago/Turabian StyleAccomasso, Francesca, Silvia Actis, Carola Minella, Roberta Rosso, Claudia Granaglia, Riccardo Ponzone, Nicoletta Biglia, and Valentina Elisabetta Bounous. 2023. "Clinical, Pathological, and Prognostic Features of Male Breast Cancer: A Multicenter Study" Current Oncology 30, no. 11: 9860-9871. https://doi.org/10.3390/curroncol30110716
APA StyleAccomasso, F., Actis, S., Minella, C., Rosso, R., Granaglia, C., Ponzone, R., Biglia, N., & Bounous, V. E. (2023). Clinical, Pathological, and Prognostic Features of Male Breast Cancer: A Multicenter Study. Current Oncology, 30(11), 9860-9871. https://doi.org/10.3390/curroncol30110716






