Seven-Year Single-Center Experience of the Efficacy and Safety of Ferric Carboxymaltose in Cancer Patients with Iron-Deficiency Anemia
Abstract
1. Introduction
2. Materials and Methods
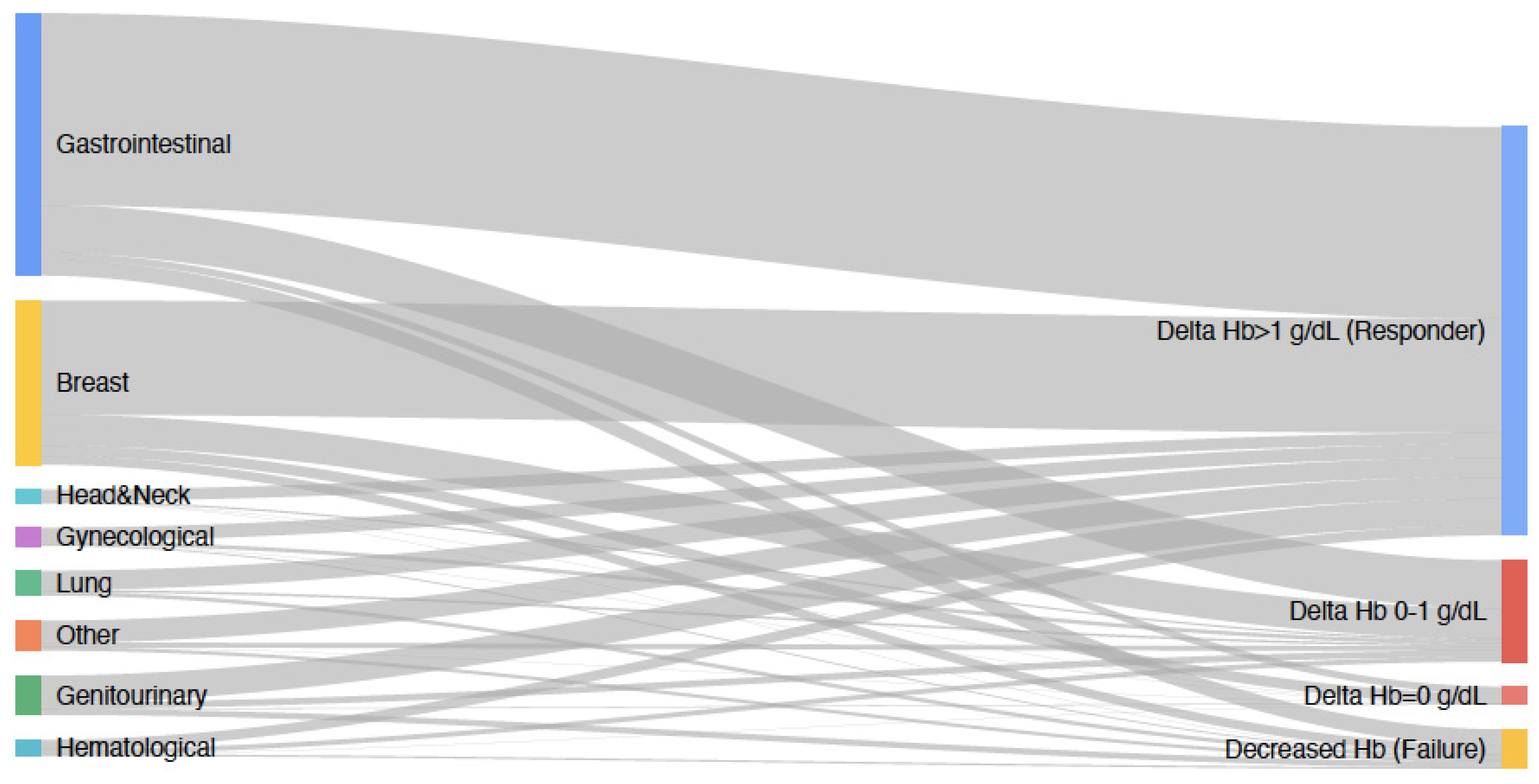
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ludwig, H.; Van Belle, S.; Barrett-Lee, P.; Birgegård, G.; Bokemeyer, C.; Gascón, P.; Kosmidis, P.; Krzakowski, M.; Nortier, J.; Olmi, P.; et al. The European Cancer Anaemia Survey (ECAS): A large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur. J. Cancer 2004, 40, 2293–2306. [Google Scholar] [CrossRef] [PubMed]
- Varlotto, J.; Stevenson, M.A. Anemia, tumor hypoxemia, and the cancer patient. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Family, L.; Xu, L.; Xu, H.; Cannavale, K.; Sattayapiwat, O.; Page, J.H.; Bohac, C.; Chao, C. The effect of chemotherapy-induced anemia on dose reduction and dose delay. Support. Care Cancer 2016, 24, 4263–4271. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.J.; Salas, M.; Ward, A.; Goss, G. Anemia as an independent prognostic factor for survival in patients with cancer: A systemic, quantitative review. Cancer 2001, 91, 2214–2221. [Google Scholar] [CrossRef]
- Gluszak, C.; de Vries-Brilland, M.; Seegers, V.; Baroin, C.; Kieffer, H.; Delva, R.; Cornuault-Foubert, D. Impact of Iron-Deficiency Management on Quality of Life in Patients with Cancer: A Prospective Cohort Study (CAMARA Study). Oncologist 2022, 27, 328–333. [Google Scholar] [CrossRef]
- Madeddu, C.; Gramignano, G.; Astara, G.; Demontis, R.; Sanna, E.; Atzeni, V.; Macciò, A. Pathogenesis and Treatment Options of Cancer Related Anemia: Perspective for a Targeted Mechanism-Based Approach. Front. Physiol. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Neoh, K.; Stanworth, S.; Pasricha, S.-R.; Bennett, M.I. Estimating prevalence of functional iron deficiency anaemia in advanced cancer. Support. Care Cancer 2017, 25, 1209–1214. [Google Scholar] [CrossRef]
- de Castro, J.; Gascón, P.; Casas, A.; Muñoz-Langa, J.; Alberola, V.; Cucala, M.; Barón, F. Iron deficiency in patients with solid tumours: Prevalence and management in clinical practice. Clin. Transl. Oncol. 2014, 16, 823–828. [Google Scholar] [CrossRef]
- Ludwig, H.; Müldür, E.; Endler, G.; Hübl, W. Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann. Oncol. 2013, 24, 1886–1892. [Google Scholar] [CrossRef]
- Buck, I.; Morceau, F.; Grigorakaki, C.; Dicato, M.; Diederich, M. Linking anemia to inflammation and cancer: The crucial role of TNFα. Biochem. Pharmacol. 2009, 77, 1572–1579. [Google Scholar] [CrossRef]
- Hoenemann, C.; Ostendorf, N.; Zarbock, A.; Doll, D.; Hagemann, O.; Zimmermann, M.; Luedi, M. Reticulocyte and Erythrocyte Hemoglobin Parameters for Iron Deficiency and Anemia Diagnostics in Patient Blood Management. A Narrative Review. J. Clin. Med. 2021, 10, 4250. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Nemeth, E.; Swinkels, D.W. Hepcidin in the diagnosis of iron disorders. Blood 2016, 127, 2809–2813. [Google Scholar] [CrossRef] [PubMed]
- Steensma, D.P.; Sasu, B.J.; Sloan, J.A.; Tomita, D.K.; Loprinzi, C.L. Serum hepcidin levels predict response to intravenous iron and darbepoetin in chemotherapy-associated anemia. Blood 2015, 125, 3669–3671. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.; Beguin, Y.; Bokemeyer, C.; Dicato, M.; Gascón, P.; Glaspy, J.; Hofmann, A.; Link, H.; Littlewood, T.; Ludwig, H.; et al. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv96–iv110. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef]
- Kulnigg, S.; Stoinov, S.; Simanenkov, V.; Dudar, L.V.; Karnafel, W.; Garcia, L.C.; Sambuelli, A.M.; D’Haens, G.; Gasche, C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: The ferric carboxymaltose (FERINJECT) randomized controlled trial. Am. J. Gastroenterol. 2008, 103, 1182–1192. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Bock, A.H.; Carrera, F.; Eckardt, K.-U.; Gaillard, C.; Van Wyck, D.; Roubert, B.; Nolen, J.G.; Roger, S.D.; on behalf of the FIND-CKD Study Investigators. FIND-CKD: A randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol. Dial. Transplant. 2014, 29, 2075–2084. [Google Scholar] [CrossRef]
- Keeler, B.D.; Simpson, J.A.; Ng, O.; Padmanabhan, H.; Brookes, M.J.; Acheson, A.G.; Banerjea, A.; Walter, C.; Maxwell-Armstrong, C.; Williams, J.; et al. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. Br. J. Surg. 2017, 104, 214–221. [Google Scholar] [CrossRef]
- Steinmetz, T.; Tschechne, B.; Harlin, O.; Klement, B.; Franzem, M.; Wamhoff, J.; Tesch, H.; Rohrberg, R.; Marschner, N. Clinical experience with ferric carboxymaltose in the treatment of cancer- and chemotherapy-associated anaemia. Ann. Oncol. 2013, 24, 475–482. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, Y.; Park, S.; Kim, K.; Kim, S.J.; Kim, W.S.; Jung, C.W.; Lee, J.; Lee, S.H. Efficacy of intravenous iron treatment for chemotherapy-induced anemia: A prospective Phase II pilot clinical trial in South Korea. PLoS Med. 2020, 17, e1003091. [Google Scholar] [CrossRef]
- Toledano, A.; Luporsi, E.; Morere, J.F.; Scotté, F.; Laribi, K.; Barrière, J.; Huot-Marchand, P.; Duvillié, L.; Concas, V.H.; Bugat, R. Clinical use of ferric carboxymaltose in patients with solid tumours or haematological malignancies in France. Support. Care Cancer 2016, 24, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghe, L.; Bruyneel, L.; Stragier, E.; Ferrante, M.; Dierickx, D.; Prenen, H. The effectiveness of intravenous iron for iron deficiency anemia in gastrointestinal cancer patients: A retrospective study. Ann. Gastroenterol. 2017, 30, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Makharadze, T.; Boccia, R.; Krupa, A.; Blackman, N.; Henry, D.H.; Gilreath, J.A. Efficacy and safety of ferric carboxymaltose infusion in reducing anemia in patients receiving chemotherapy for nonmyeloid malignancies: A randomized, placebo-controlled study (IRON-CLAD). Am. J. Hematol. 2021, 96, 1639–1646. [Google Scholar] [CrossRef]
- Abdel-Razeq, H.; Saadeh, S.S.; Malhis, R.; Yasser, S.; Abdulelah, H.; Eljaber, R.; Kleib, A.; Ismael, R. Treatment of anemia in cancer patients undergoing chemotherapy with intravenous ferric carboxymaltose without erythropoiesis-stimulating agents. Ther. Adv. Med. Oncol. 2020, 12, 1758835920953292. [Google Scholar] [CrossRef] [PubMed]
- Talboom, K.; Borstlap, W.A.A.; Roodbeen, S.X.; Bruns, E.R.J.; Buskens, C.J.; Hompes, R.; Tytgat, K.M.A.J.; Tuynman, J.B.; Consten, E.C.J.; Heuff, G.; et al. Ferric carboxymaltose infusion versus oral iron supplementation for preoperative iron deficiency anaemia in patients with colorectal cancer (FIT): A multicentre, open-label, randomised, controlled trial. Lancet Haematol. 2023, 10, e250–e260. [Google Scholar] [CrossRef]
- Abdel-Razeq, H.; Abbasi, S.; Saadi, I.; Jaber, R.; Abdelelah, H. Intravenous iron monotherapy for the treatment of non-iron-deficiency anemia in cancer patients undergoing chemotherapy: A pilot study. Drug Des. Dev. Ther. 2013, 7, 939–944. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laso-Morales, M.J.; Vives, R.; Bisbe, E.; García-Erce, J.A.; Muñoz, M.; Martínez-López, F.; Carol-Boeris, F.; Pontes-García, C. Single-dose intravenous ferric carboxymaltose infusion versus multiple fractionated doses of intravenous iron sucrose in the treatment of post-operative anaemia in colorectal cancer patients: A randomised controlled trial. Blood Transfus. 2022, 20, 310–318. [Google Scholar] [CrossRef]
- Bach, M.; Geisel, T.; Martin, J.; Schulze, B.; Schaefer, R.; Virgin, G.; Stein, J. Efficacy and Safety of Intravenous Ferric Carboxymaltose in Geriatric Inpatients at a German Tertiary University Teaching Hospital: A Retrospective Observational Cohort Study of Clinical Practice. Anemia 2015, 2015, 647930. [Google Scholar] [CrossRef]
- Ning, S.; Zeller, M.P. Management of iron deficiency. Hematology 2019, 2019, 315–322. [Google Scholar] [CrossRef]
- Calleja, J.L.; Delgado, S.; del Val, A.; Hervás, A.; Larraona, J.L.; Terán, Á.; Cucala, M.; Mearin, F.; on behalf of the Colon Cancer Study, G. Ferric carboxymaltose reduces transfusions and hospital stay in patients with colon cancer and anemia. Int. J. Color. Dis. 2016, 31, 543–551. [Google Scholar] [CrossRef]
- Marinho, J.; Leão, I.; Custódio, S.; Dias, E.; Moreira Pinto, A.; Costa, T.; Capela, A.; Dias, M.; Coelho, H.; Cunha, Â.; et al. Ferric Carboxymaltose in the treatment of chemotherapy-induced anaemia: An effective, safe and cost- sparing alternative to blood transfusion. Sci. Rep. 2019, 9, 20410. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, C.; Schiller, B.; Moran, J. Reticulocyte hemoglobin equivalent (Ret He) and assessment of iron-deficient states. Clin. Lab. Haematol. 2006, 28, 303–308. [Google Scholar] [CrossRef]
- Sasu, B.J.; Li, H.; Rose, M.J.; Arvedson, T.L.; Doellgast, G.; Molineux, G. Serum hepcidin but not prohepcidin may be an effective marker for anemia of inflammation (AI). Blood Cells Mol. Dis. 2010, 45, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Drakou, A.; Margeli, A.; Theodorakopoulou, S.; Agrogiannis, I.; Poziopoulos, C.; Papassotiriou, I.; Vlahakos, D.V. Assessment of serum bioactive hepcidin-25, soluble transferrin receptor and their ratio in predialysis patients: Correlation with the response to intravenous ferric carboxymaltose. Blood Cells Mol. Dis. 2016, 59, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Hershko, C.; Camaschella, C. How I treat unexplained refractory iron deficiency anemia. Blood 2014, 123, 326–333. [Google Scholar] [CrossRef]
- Grimmelt, A.C.; Cohen, C.D.; Fehr, T.; Serra, A.L.; Wuethrich, R.P. Safety and tolerability of ferric carboxymaltose (FCM) for treatment of iron deficiency in patients with chronic kidney disease and in kidney transplant recipients. Clin. Nephrol. 2009, 71, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Decruyenaere, A.; Kortbeek, K.; Delanghe, S.; Rottey, S.; Denys, H.; Lapeire, L. Incidence, evolution and risk factors of hypophosphatemia in patients with solid tumors receiving ferric carboxymaltose: A retrospective cohort study. Acta Clin. Belg. 2023, 78, 298–307. [Google Scholar] [CrossRef]
| N = 1102 | |
|---|---|
| Age (years, median (Q1–Q3)) | 60 (48–68) |
| Female, n (%) | 696 (63.2%) |
| Cancer type, n (%) | |
| Gastrointestinal | 510 (46.3%) |
| Breast | 319 (28.9%) |
| Genitourinary | 76 (6.9%) |
| Lung | 48 (4.4%) |
| Gynecological | 35 (3.2%) |
| Hematological | 31 (2.8%) |
| Head and Neck | 25 (2.3%) |
| Other | 58 (5.3) |
| Active chemotherapy, n (%) | 870 (78.9%) |
| Anemia Grade n (%) | |
| 1 | 764 (69.3%) |
| 2 | 306 (27.8%) |
| 3 | 32 (2.9%) |
| N = 1102 | |
|---|---|
| Baseline hemoglobin | |
| Mean (SD) | 10.4 (±1.1) g/dL |
| Median (Q1–Q3) | 10.7 (9.7–11.4) |
| Leucocyte (Median (Q1–Q3)) | 6200 (4400–8100) |
| Thrombocyte (Median (Q1–Q3)) | 295,000 (220,000–382,000) |
| Ferritin (Median (Q1–Q3)) | 23.7 (16.7–37.8) |
| Transferrin saturation (Median (Q1–Q3)) | 8 (5–12) |
| Week-12 hemoglobin | |
| Mean (SD) | 12.2 (±1.4) g/dL |
| Median (Q1–Q3) | 12.3 (11.3–13.3) |
| Δ hemoglobin | |
| Mean (SD) | 1.81 (±0.14) g/dL |
| Median (Q1–Q3) | 1.60 (0.8–2.8) |
| Treatment Response, % (Increase in Hb ≥ 1 g/dL) | 794 (72.1%) |
| Δ Hemoglobin (±SD), g/dL | p | Response Rate, % | p | |
|---|---|---|---|---|
| Gender | 0.87 | 0.018 | ||
| Female | 1.81 (±1.27) | 74.5% | ||
| Male | 1.79 (±1.62) | 67.7% | ||
| Age | 0.35 | 0.70 | ||
| <65 | 1.83 (±1.41) | 72.4% | ||
| ≥65 | 1.75 (±1.41) | 71.4% | ||
| Tumor Type | 0.67 | 0.41 | ||
| Gastrointestinal | 1.76 (±1.32) | 72.9% | ||
| Breast | 1.83 (±1.48) | 69.9% | ||
| Genitourinary | 1.63 (±1.49) | 68.4% | ||
| Lung | 2.09 (±1.67) | 77.0% | ||
| Gynecological | 1.92 (±1.29) | 74.2% | ||
| Hematological | 1.73 (±1.66) | 61.2% | ||
| Head and Neck | 2.6 (±1.23) | 88.5% | ||
| Other | 1.81 (±1.88) | 74.1% | ||
| Anemia Grade | <0.005 | <0.005 | ||
| Grade 1 | 1.52 (±1.24) | 68.1% | ||
| Grade 2 | 2.39 (±1.53) | 79.4% | ||
| Grade 3 | 3.06 (±1.66) | 93.7% | ||
| Active Treatment | 0.04 | 0.24 | ||
| Yes | 1.74 (±1.30) | 72.9% | ||
| No | 2.01 (±1.73) | 69.0% |
| N = 510 | |
|---|---|
| Age (years, median (Q1–Q3)) | 57 (45–68) |
| Female, n (%) | 252 (49.4%) |
| Cancer type, n (%) | |
| Upper GI | 147 (28.8%) |
| HPB | 80 (15.7%) |
| Lower GI | 283 (55.5%) |
| Active chemotherapy, n (%) | 423 (82.9%) |
| Baseline hemoglobin | |
| Mean (SD) | 10.4 (±1.7) g/dL |
| Median (Q1–Q3) | 10.7 (9.8–11.4) |
| Δ hemoglobin | |
| Mean (SD) | 1.76 (±0.34) g/dL |
| Median (Q1–Q3) | 1.60 (0.9–2.7) |
| Treatment response, % (increase in Hb ≥ 1 g/dL) | 372 (72.9%) |
| Δ Hemoglobin (±SD), g/dL | p | Response Rate, % | p | |
|---|---|---|---|---|
| Gender | 0.81 | 0.21 | ||
| Female | 1.77 (±1.22) | 74.6% | ||
| Male | 1.74 (±1.52) | 69.2% | ||
| Age | 0.87 | 0.54 | ||
| <65 | 1.77 (±1.35) | 72.1% | ||
| ≥65 | 1.75 (±1.26) | 74.6% | ||
| Tumor Location | 0.91 | 0.40 | ||
| Upper GI | 1.80 (±1.36) | 72.1% | ||
| HPB | 1.78 (±1.32) | 67.5% | ||
| Lower GI | 1.74 (±1.30) | 74.9% | ||
| Active Treatment | 0.03 | 0. 04 | ||
| Yes | 1.71 (±1.25) | 72.9% | ||
| No | 2.04 (±1.57) | 84.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aktaş, B.Y.; Ata, E.B.; Çeşmeci, E.; Çakır, İ.Y.; Coşkunpınar, M.; Tahillioğlu, Y.; Güner, G.; Güven, D.C.; Arık, Z.; Kertmen, N.; et al. Seven-Year Single-Center Experience of the Efficacy and Safety of Ferric Carboxymaltose in Cancer Patients with Iron-Deficiency Anemia. Curr. Oncol. 2023, 30, 9689-9700. https://doi.org/10.3390/curroncol30110703
Aktaş BY, Ata EB, Çeşmeci E, Çakır İY, Coşkunpınar M, Tahillioğlu Y, Güner G, Güven DC, Arık Z, Kertmen N, et al. Seven-Year Single-Center Experience of the Efficacy and Safety of Ferric Carboxymaltose in Cancer Patients with Iron-Deficiency Anemia. Current Oncology. 2023; 30(11):9689-9700. https://doi.org/10.3390/curroncol30110703
Chicago/Turabian StyleAktaş, Burak Yasin, Emine Büşra Ata, Engin Çeşmeci, İbrahim Yahya Çakır, Muharrem Coşkunpınar, Yağmur Tahillioğlu, Gürkan Güner, Deniz Can Güven, Zafer Arık, Neyran Kertmen, and et al. 2023. "Seven-Year Single-Center Experience of the Efficacy and Safety of Ferric Carboxymaltose in Cancer Patients with Iron-Deficiency Anemia" Current Oncology 30, no. 11: 9689-9700. https://doi.org/10.3390/curroncol30110703
APA StyleAktaş, B. Y., Ata, E. B., Çeşmeci, E., Çakır, İ. Y., Coşkunpınar, M., Tahillioğlu, Y., Güner, G., Güven, D. C., Arık, Z., Kertmen, N., Dizdar, Ö., Yalçın, Ş., & Aksoy, S. (2023). Seven-Year Single-Center Experience of the Efficacy and Safety of Ferric Carboxymaltose in Cancer Patients with Iron-Deficiency Anemia. Current Oncology, 30(11), 9689-9700. https://doi.org/10.3390/curroncol30110703





