A Meta-Analysis of Randomized Clinical Trials Assessing the Efficacy of PARP Inhibitors in Metastatic Castration-Resistant Prostate Cancer
Abstract
:1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- Randomized control trials comparing PARPis with or without androgen receptor pathway inhibitor (ARPI; abiraterone acetate or enzalutamide) against standard of care (ARPI or docetaxel) in prostate cancer patients;
- Studies that reported progression-free survival and overall survival;
- Patient age greater than 18 years;
- Available in the English language without any restrictions on the date or status of the publication.
2.3. Data Extraction
2.4. Trial Selection and Evaluation
2.5. Risk of Bias
2.6. Study Objectives
2.7. Statistical Analysis
3. Progression-Free Survival
3.1. Overview
3.2. Statistical Model
3.3. Mean Effect Size
3.4. Q-Test
3.5. The I2 Statistic
4. Overall Survival
4.1. Overview
4.2. Statistical Model
4.3. Mean Effect Size
4.4. Q-Test
4.5. The I2 Statistic
5. Results
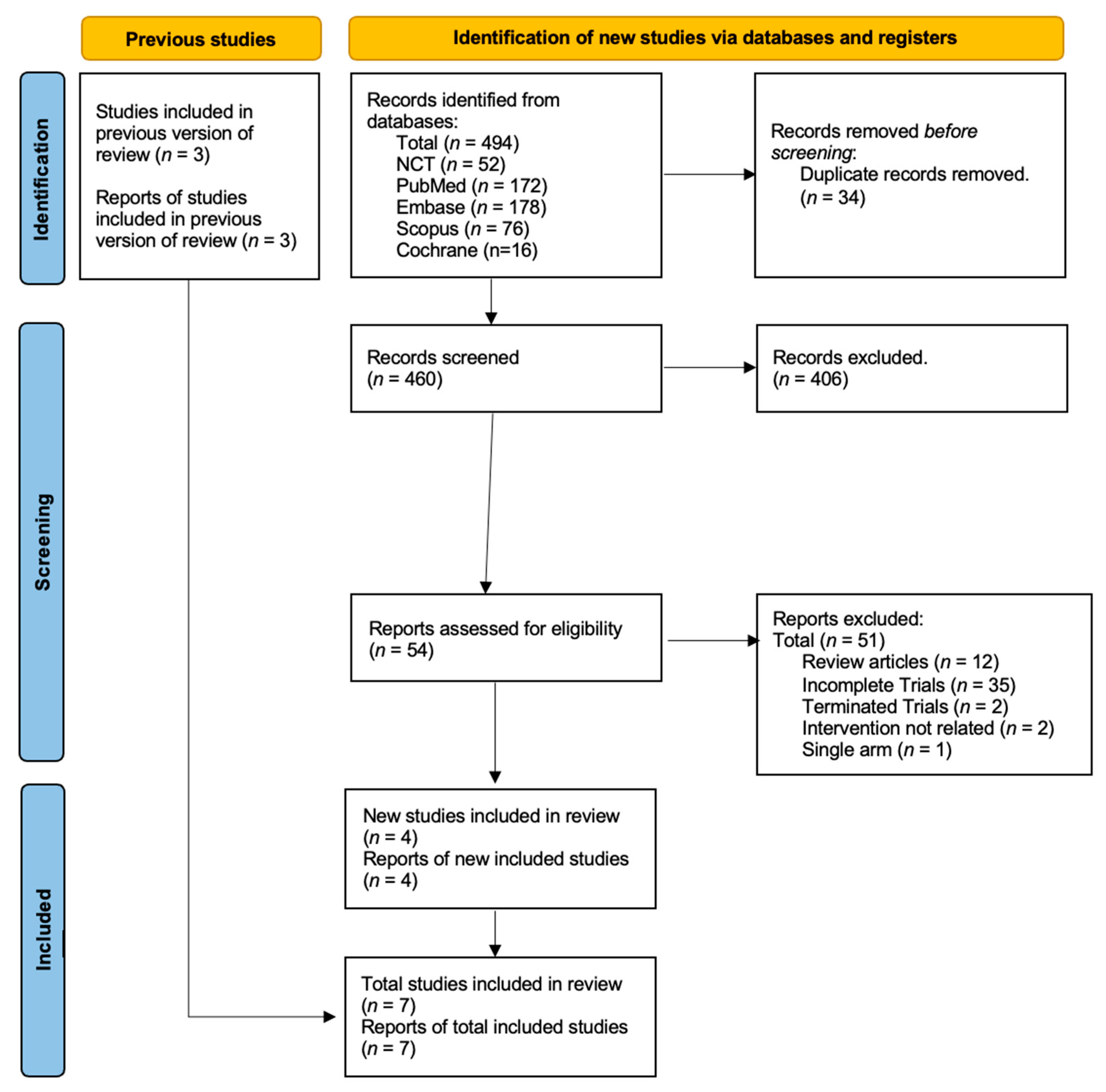
5.1. Study Selection and Characteristics
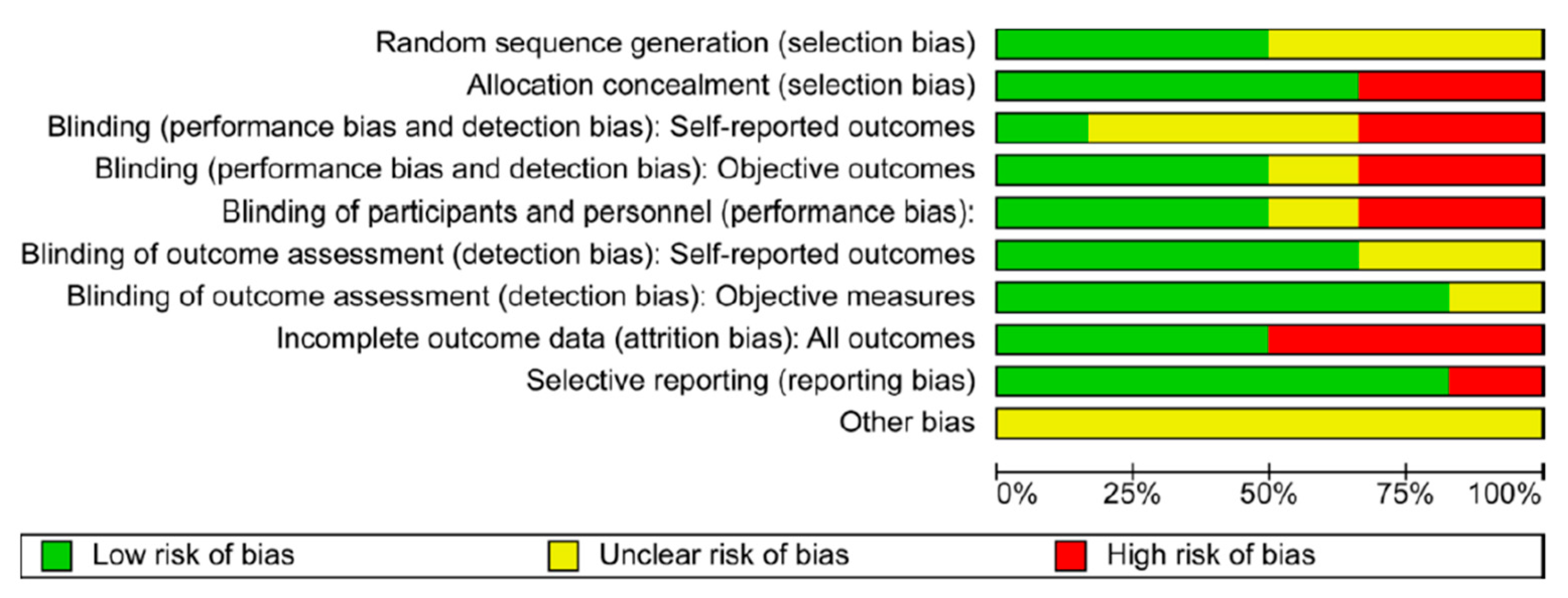
5.2. Quality of Studies
5.3. Result of Quantitative Analysis
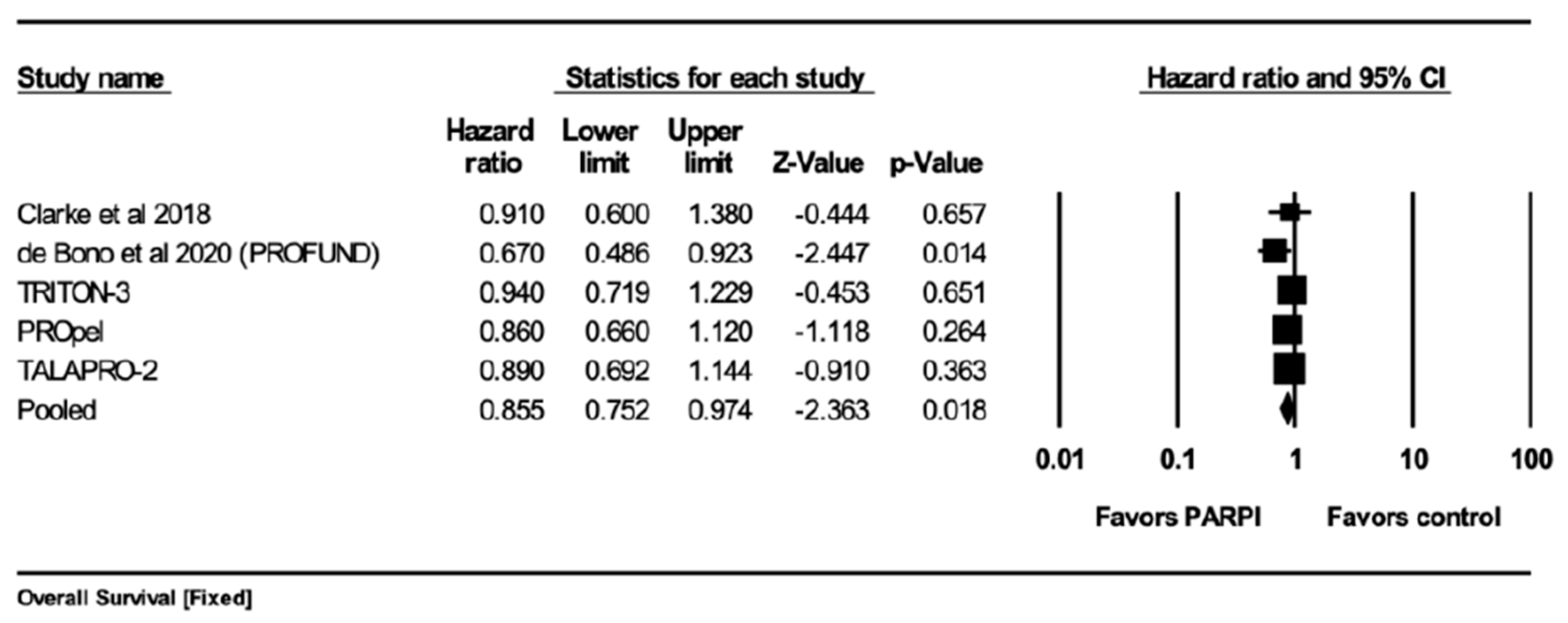
5.3.1. Overall Survival (OS)
5.3.2. Progression-Free Survival (PFS)
5.3.3. Progression-Free Survival (PFS) in Patients without HRR Gene Mutation
6. Discussion
Strengths and Limitations
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rebello, R.J.; Oing, C.; Knudsen, K.E. Prostate cancer. Nat. Rev. Dis. Primers 2021, 7, 8. [Google Scholar]
- Barsouk, A.; Padala, S.A.; Vakiti, A.; Mohammed, A.; Saginala, K.; Thandra, K.C.; Barsouk, A. Epidemiology, Staging and Management of Prostate Cancer. Med. Sci. 2020, 8, 28. [Google Scholar]
- Welch, H.G.; Albertsen, P.C. Reconsidering Prostate Cancer Mortality—The Future of PSA Screening. N. Engl. J. Med. 2020, 382, 1557–1563. [Google Scholar] [PubMed]
- Niazi, M.R.K.; Jahangir, A.; Sahra, S.; Sattar, S.B.A.; Asti, D.; Bershadskiy, A. Efficacy of PARP Inhibitors as Maintenance Therapy for Metastatic Castration-Resistant Prostate Cancer: A Meta-Analysis of Randomized Controlled Trials. Oncology 2021, 35, 708–715. [Google Scholar] [PubMed]
- Ferraro, S.; Biganzoli, G.; Bussetti, M.; Castaldi, S.; Biganzoli, E.M.; Plebani, M. Managing the impact of inter-method bias of prostate specific antigen assays on biopsy referral: The key to move towards precision health in prostate cancer management. Clin. Chem. Lab. Med. 2023, 61, 142–153. [Google Scholar] [PubMed]
- Ng, S.P.; Duchesne, G.; Tai, K.-H.; Foroudi, F.; Kothari, G.; Williams, S. Support for the use of objective comorbidity indices in the assessment of noncancer death risk in prostate cancer patients. Prostate Int. 2017, 5, 8–12. [Google Scholar] [PubMed]
- Cooperberg, M.R.; Carroll, P.R.; Klotz, L. Active Surveillance for Prostate Cancer: Progress and Promise. J. Clin. Oncol. 2011, 29, 3669–3676. [Google Scholar] [PubMed]
- Wolff, R.F.; Ryder, S.; Bossi, A.; Briganti, A.; Crook, J.; Henry, A.; Karnes, J.; Potters, L.; de Reijke, T.; Stone, N.; et al. A systematic review of randomised controlled trials of radiotherapy for localised prostate cancer. Eur. J. Cancer 2015, 51, 2345–2367. [Google Scholar]
- Corn, P.G.; I Heath, E.; Zurita, A.; Ramesh, N.; Xiao, L.; Sei, E.; Li-Ning-Tapia, E.; Tu, S.-M.; Subudhi, S.K.; Wang, J.; et al. Cabazitaxel plus carboplatin for the treatment of men with metastatic castration-resistant prostate cancers: A randomised, open-label, phase 1–2 trial. Lancet Oncol. 2019, 20, 1432–1443. [Google Scholar]
- Rathi, N.; McFarland, T.R.; Nussenzveig, R.; Agarwal, N.; Swami, U. Evolving Role of Immunotherapy in Metastatic Castration Refractory Prostate Cancer. Drugs 2021, 81, 191–206. [Google Scholar]
- Garcia, J.A. Sipuleucel-T in patients with metastatic castration-resistant prostate cancer: An insight for oncologists. Ther. Adv. Med. Oncol. 2011, 3, 101–108. [Google Scholar] [CrossRef]
- Wu, K.; Liang, J.; Shao, Y.; Xiong, S.; Feng, S.; Li, X. Evaluation of the Efficacy of PARP Inhibitors in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 777663. [Google Scholar] [CrossRef]
- Wang, S.S.Y.; Jie, Y.E.; Cheng, S.W.; Ling, G.L.; Ming, H.V.Y. PARP Inhibitors in Breast and Ovarian Cancer. Cancers 2023, 15, 2357. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Duan, Y.; Zhao, Y.; Li, Y.; Liu, J.; Zhang, C.; He, S. PARP inhibitors in breast and ovarian cancer with BRCA mutations: A meta-analysis of survival. Aging 2021, 13, 8975–8988. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, K.; Takebayashi, S.-I.; Taguchi, H.; Takeda, S.; Okumura, K. PARP-1 ensures regulation of replication fork progression by homologous recombination on damaged DNA. J. Cell Biol. 2008, 183, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Konecny, G.E.; Kristeleit, R.S. PARP inhibitors for BRCA1/2-mutated and sporadic ovarian cancer: Current practice and future directions. Br. J. Cancer 2016, 115, 1157–1173. [Google Scholar] [CrossRef]
- Slade, D. PARP and PARG inhibitors in cancer treatment. Genes. Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef]
- Pham, M.M.; Ngoi, N.Y.; Peng, G.; Tan, D.S.; Yap, T.A. Development of poly(ADP-ribose) polymerase inhibitor and immunotherapy combinations: Progress, pitfalls, and promises. Trends Cancer 2021, 7, 958–970. [Google Scholar] [CrossRef]
- Clarke, N.; Wiechno, P.; Alekseev, B.; Sala, N.; Jones, R.; Kocak, I.; Chiuri, V.E.; Jassem, J.; Fléchon, A.; Redfern, C. {Rebello, 2021 #22}{Rebello, 2021 #22} Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018, 19, 975–986. [Google Scholar]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Hussain, M. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Hussain, M.; Daignault-Newton, S.; Twardowski, P.W.; Albany, C.; Stein, M.N.; Kunju, L.P.; Chinnaiyan, A.M. Targeting Androgen Receptor and DNA Repair in Metastatic Castration-Resistant Prostate Cancer: Results From NCI 9012. J. Clin. Oncol. 2018, 36, 991–999. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.; Carles, J.; Fay, A.P.; Matsubara, N.; Heinrich, D.; Szczylik, C.; De Giorgi, U.; Joung, J.Y.; Fong, P.C.; et al. TALAPRO-2: Phase 3 study of talazoparib (TALA) + enzalutamide (ENZA) versus placebo (PBO) + ENZA as first-line (1L) treatment in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2023, 41 (Suppl. S6), LBA17. [Google Scholar] [CrossRef]
- Fizazi, K.; Piulats, J.M.; Reaume, M.N.; Ostler, P.; McDermott, R.; Gingerich, J.R.; Pintus, E.; Sridhar, S.S.; Bambury, R.M.; Emmenegger, U.; et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N. Engl. J. Med. 2023, 388, 719–732. [Google Scholar] [CrossRef]
- Saad, F.; Armstrong, A.J.; Thiery-Vuillemin, A.; Oya, M.; Loredo, E.; Procopio, G.; de Menezes, J.J.; Girotto, G.C.; Arslan, C.; Mehra, N.; et al. PROpel: Phase III trial of olaparib (ola) and abiraterone (abi) versus placebo (pbo) and abi as first-line (1L) therapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2022, 40 (Suppl. S6), 11. [Google Scholar] [CrossRef]
- Chi, K.; Sandhu, S.; Smith, M.; Attard, G.; Saad, M.; Olmos, D.; Castro, E.; Roubaud, G.; Gomes, A.P.d.S.; Small, E.; et al. Niraparib plus abiraterone acetate with prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: Second interim analysis of the randomized phase III MAGNITUDE trial. Ann. Oncol. 2023, 34, 772–782. [Google Scholar] [CrossRef]
- Iannantuono, G.M.; Chandran, E.; Floudas, C.S.; Choo-Wosoba, H.; Butera, G.; Roselli, M.; Gulley, J.L.; Karzai, F. Efficacy and safety of PARP inhibitors in metastatic castration-resistant prostate cancer: A systematic review and meta-analysis of clinical trials. Cancer Treat Rev. 2023, 120, 102623. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Sautois, B.; Vogelzang, N.J.; Bambury, R.M.; Voog, E.; et al. Rucaparib in Men with Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J. Clin. Oncol. 2020, 38, 3763–3772. [Google Scholar] [CrossRef]
- Hussain, M.H.A.; Kocherginsky, M.; Agarwal, N.; Zhang, J.; Adra, N.; Paller, C.J.; Picus, J.; Reichert, Z.R.; Szmulewitz, R.Z.; Tagawa, S.T.; et al. BRCAAWAY: A randomized phase 2 trial of abiraterone, olaparib, or abiraterone + olaparib in patients with metastatic castration-resistant prostate cancer (mCRPC) with DNA repair defects. J. Clin. Oncol. 2022, 40 (Suppl. S16), 5018. [Google Scholar] [CrossRef]
- Stopsack, K.H. Efficacy of PARP Inhibition in Metastatic Castration-resistant Prostate Cancer is Very Different with Non-BRCA DNA Repair Alterations: Reconstructing Prespecified Endpoints for Cohort B from the Phase 3 PROfound Trial of Olaparib. Eur. Urol. 2021, 79, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Congregado, B.; Rivero, I.; Osmán, I.; Sáez, C.; López, R.M. PARP Inhibitors: A New Horizon for Patients with Prostate Cancer. Biomedicines 2022, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Asim, M.; Tarish, F.; Zecchini, H.I.; Sanjiv, K.; Gelali, E.; Massie, C.E.; Baridi, A.; Warren, A.Y.; Zhao, W.; Ogris, C.; et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat. Commun. 2017, 8, 374. [Google Scholar] [CrossRef] [PubMed]



| Study Name | Treatment Drugs | Study Characteristic | Inclusion | Exclusion | Primary Outcome |
|---|---|---|---|---|---|
| Clarke et al. (NCT01972217) [19] | olaparib (300 mg bid) + abiraterone (1000 mg/od) (n = 71) vs. abiraterone (1000 mg/od) alone (n = 71) | mCRPC patients previously treated with docetaxel and candidates for abiraterone treatment | Age >18 with mCRPC. ≤2 prior lines of chemotherapy, testosterone <50 ng/dL, no previous exposure to second-generation ARPI, candidates for abiraterone treatment, life expectancy ≥12 weeks, ECOG performance status of 0–2. | Previous treatment with PARPis, or cytotoxic chemotherapy. Other malignancies (including MGUS and MDS) within the last 5 years | Percentage of patients experiencing adverse events Number of patients with dose-limiting toxicities Median (rPFS) time percentage of patients with progression events or death |
| De Bono et al. PROfound study (NCT029 87543) [20] | olaparib (300 mg bid) vs. enzalutamide (160 mg/od) OR aberaterone (1000 mg/od) + Prednisone (5 mg/bid) | mCRPC patients with disease progression on treatment with enzalutamide or abiraterone Cohort A = Pts with at least one alteration in BRCA1, BRCA2, or ATM Cohort B = Pts with alteration in any of the other 12 genes | men (≥18 years of age) with mCRPC. ≤ 2 prior lines of chemotherapy, no previous exposure to second-generation antihormonal agents, candidates for abiraterone treatment, life expectancy ≥12 weeks, ECOG performance status of 0–2. | Previous treatment with PARPis, or cytotoxic chemotherapy. Other malignancies (including MGUS and MDS) within the last 5 years | PFS via RECIST (v1.1) for soft tissue, as a 20% increase in the sum of diameters of target lesions |
| Hussain et al. NCT01576172 [21]. | Arm A = abiraterone (1000 mg) + prednisone (5 mg/bid) Arm B = veliparib (300 mg/bid)+ abiraterone (1000 mg) + prednisone (5 mg/bid) | pts stratified by ETS fusion status (positive or negative), randomly assigned to Arm (A) and (B) | Men with mCRPC, testosterone <50 ng/dL, ECOG status of 0 to 2, no prior exposure to abiraterone, and up to two prior chemotherapy regimens. | Chemotherapy, radiotherapy, history of active seizures, pituitary or adrenal dysfunction, active or symptomatic viral hepatitis, chronic liver disease, brain metastases | Confirmed PSA response rate time frame: up to 3 years |
| TRITON-3, NCT02975934 | Arm A = oral rucaparib (600 mg twice daily). Arm B = physician’s choice control (docetaxel or a second-generation ARPI (abiraterone acetate or enzalutamide)) | Men with mCRPC and a BRCA or ATM alteration + disease progression on previous second-generation ARPI. Previous taxane-based chemotherapy for castration-sensitive disease was permitted. | men (≥18 years of age), with mCRPC, molecular evidence of BRCA1/2 or ATM gene mutation. ECOG 0–1. Disease progression on prior ARPI | Active second malignancy, prior treatment with any PARPi, prior chemotherapy for mCRPC, metastasis to CNS | Assess the efficacy of rucaparib on the basis of rPFS in MCRPC patients with HRD who progressed on prior AR-directed therapy |
| Propel study NCT03732820 | Arm A = oral abiraterone (1000 mg once daily) + olaparib (300 mg twice daily) + prednisone or prednisolone. Arm B = abiraterone + prednisolone + placebo | double-blind, randomized Phase III trial of abiraterone and olaparib versus abiraterone and placebo in first-line treatment of patients with mCRPC regardless of HRR status. | men (≥18 years of age), who are treatment naïve at mCRPC stage, ECOG 0–1, previous treatment with ARPI was allowed if it was at least 4 weeks before randomization | Active second malignancy, MDS or AML, prior treatment with any PARPi. | To determine the efficacy of the combination of olaparib and abiraterone vs. placebo and abiraterone by assessment of rPFS in patients with mCRPC who have received no prior cytotoxic chemotherapy or ARPI at mCRPC stage |
| TALAPRO-2 (NCT03395197) | Arm A = talazoparib 0.5 mg + enzalutamide 160 mg/daily, Arm B = placebo + enzalutamide 160 mg | pts randomized according to prior abiraterone or docetaxel for CSPC and HRR gene alteration status | Mildly or asymptomatic mCRPC with disease progression at study entry, ECOG PS ≤1, ongoing androgen deprivation therapy, no prior life-prolonging therapy for CRPC | Patients who received treatment at the CRPC stage, prior treatment with PARPis, ARPI, cytotoxic chemotherapy, Brain metastasis | To assess radiologic progression-free survival (rPFS) by BICR per RECIST (v.1.1) |
| MAGNI-TUDE TRIAL | niraparib 200 mg + abiraterone acetate 1000 mg plus prednisone 10 mg or placebo + AAP | Phase III, randomized, double-blind, placebo-controlled, multicenter study. The efficacy of Niraparib was assessed in HRR+ and HRR-negative patients | Pt who had used ARPI for less than 4 months, prior systemic therapy (docetaxel, enzalutamide, apalutamide, darolutamide) for metastatic castration-sensitive prostate cancer or non-metastatic castration-resistant prostate cancer. No prior use of PARPis | Prior use of PARPis, Use of AAP more than 2–4 months prior to randomization, History of CAD, brain metastasis, or MDS/AML | To evaluate the effectiveness of niraparib and AAP compared to AAP and placebo, as determined by radiographic progression-free survival (rPFS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alameddine, Z.; Niazi, M.R.K.; Rajavel, A.; Behgal, J.; Keesari, P.R.; Araji, G.; Mustafa, A.; Wei, C.; Jahangir, A.; Terjanian, T.O. A Meta-Analysis of Randomized Clinical Trials Assessing the Efficacy of PARP Inhibitors in Metastatic Castration-Resistant Prostate Cancer. Curr. Oncol. 2023, 30, 9262-9275. https://doi.org/10.3390/curroncol30100669
Alameddine Z, Niazi MRK, Rajavel A, Behgal J, Keesari PR, Araji G, Mustafa A, Wei C, Jahangir A, Terjanian TO. A Meta-Analysis of Randomized Clinical Trials Assessing the Efficacy of PARP Inhibitors in Metastatic Castration-Resistant Prostate Cancer. Current Oncology. 2023; 30(10):9262-9275. https://doi.org/10.3390/curroncol30100669
Chicago/Turabian StyleAlameddine, Zakaria, Muhammad Rafay Khan Niazi, Anisha Rajavel, Jai Behgal, Praneeth Reddy Keesari, Ghada Araji, Ahmad Mustafa, Chapman Wei, Abdullah Jahangir, and Terenig O Terjanian. 2023. "A Meta-Analysis of Randomized Clinical Trials Assessing the Efficacy of PARP Inhibitors in Metastatic Castration-Resistant Prostate Cancer" Current Oncology 30, no. 10: 9262-9275. https://doi.org/10.3390/curroncol30100669
APA StyleAlameddine, Z., Niazi, M. R. K., Rajavel, A., Behgal, J., Keesari, P. R., Araji, G., Mustafa, A., Wei, C., Jahangir, A., & Terjanian, T. O. (2023). A Meta-Analysis of Randomized Clinical Trials Assessing the Efficacy of PARP Inhibitors in Metastatic Castration-Resistant Prostate Cancer. Current Oncology, 30(10), 9262-9275. https://doi.org/10.3390/curroncol30100669





