Unraveling the Mysteries of Perineural Invasion in Benign and Malignant Conditions
Abstract
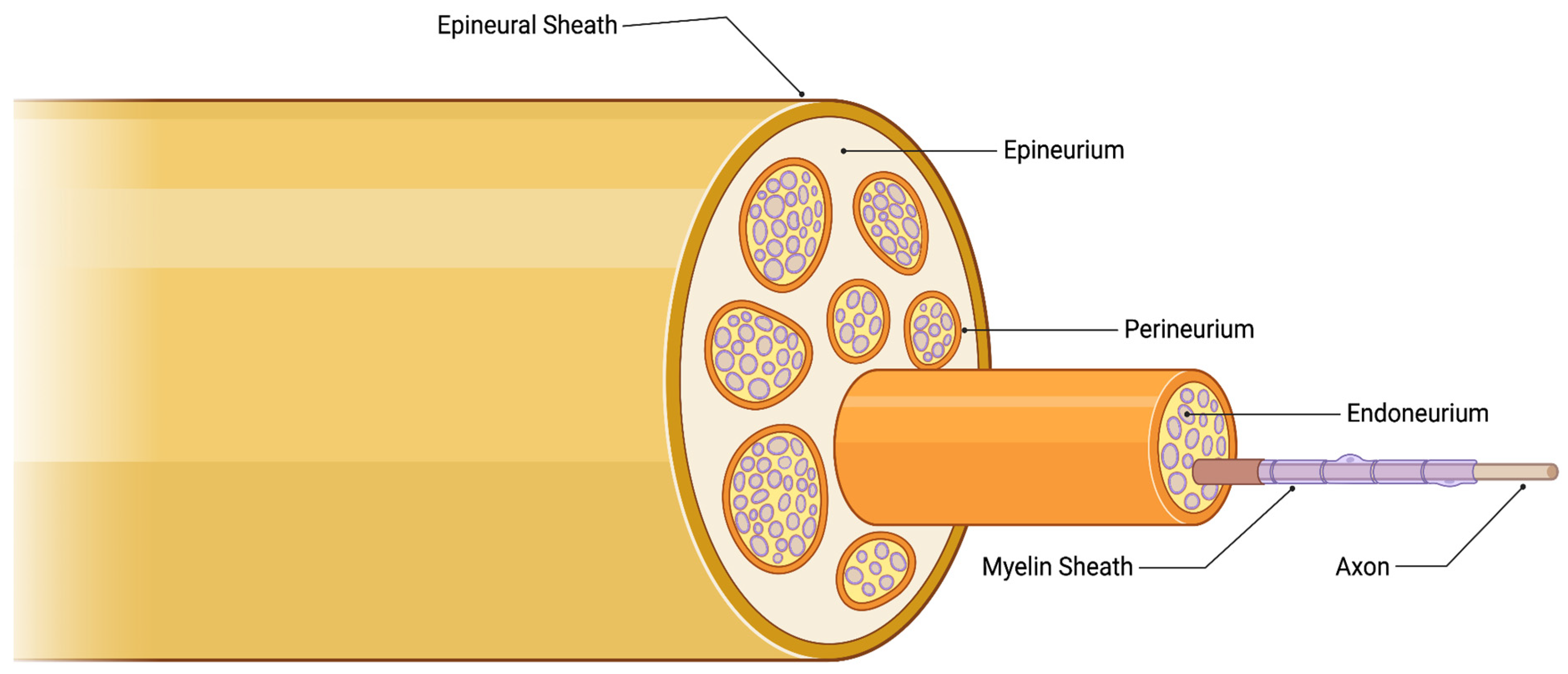
1. Introduction
2. Signaling Pathways Involved in Inducing Perineural Invasion
2.1. PI3K/Akt Pathway
2.2. MAPK Pathway
2.3. JAK/STAT Pathway
2.4. Other Pathways
3. Neurotrophic Factors and Their Functions and Expression Patterns in Cancer
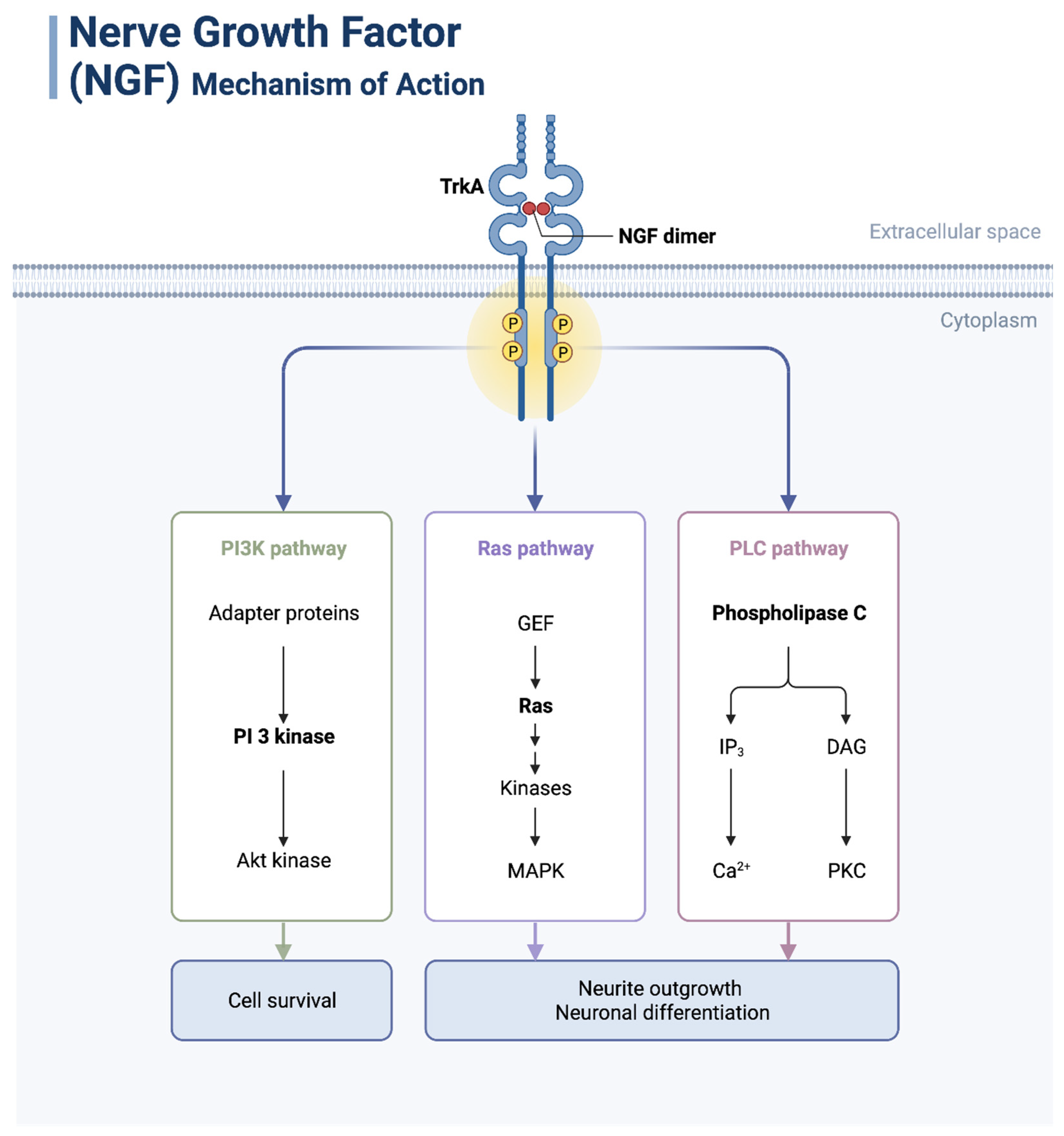
3.1. NGF
3.2. GDNF
3.3. BDNF
3.4. NT-3
4. Experimental Models of Perineural Invasion in Cancer
5. Clinical Significance of Perineural Invasion in Cancer
5.1. Perineural Invasion as a Diagnostic Marker of Malignancy in Certain Tumors
5.1.1. Prostate Cancer
5.1.2. Skin Cancers
5.1.3. Gastric Cancer
5.1.4. Pancreatic Ductal Adenocarcinoma
5.1.5. Gallbladder Cancer
5.1.6. Colorectal Cancer
5.1.7. Other Tumors
5.2. Perineural Invasion as an Independent Predictor of Poor Prognosis in Certain Cancers
5.2.1. Cutaneous Squamous Cell Carcinoma
5.2.2. Colorectal Cancer
5.2.3. Gastric Cancer
5.2.4. Oral Squamous Cell Carcinoma
5.3. Incorporation of Perineural Invasion Reporting in CAP Protocols of Some Tumors
5.3.1. Cutaneous Squamous Cell Carcinoma
5.3.2. Prostate Cancer
5.3.3. Gastric Cancer
5.3.4. Colorectal Cancer
5.3.5. Gallbladder Cancer
5.3.6. Pancreatic Ductal Adenocarcinoma
5.4. Incorporation of Perineural Invasion Reporting in WHO Protocols of Some Tumors
5.4.1. Cutaneous Squamous Cell Carcinoma
5.4.2. Laryngeal Squamous Cell Carcinoma
5.4.3. Prostate Cancer
5.4.4. Colorectal Cancer
5.4.5. Pancreatic Ductal Adenocarcinoma
6. Benign Neoplasms Demonstrating Perineural Invasion
6.1. Congenital Melanocytic Nevus
6.2. Blue Nevus
6.3. Granular Cell Tumor
6.4. Infiltrating Syringomatous Adenoma
6.5. Trichofolliculoma
6.6. Epithelial Sheath Neuroma
6.7. Benign Proliferative Breast Diseases
6.8. Adenomas of the Parotid Gland
6.9. Chronic Pancreatitis
6.10. Gallbladder and Extrahepatic Bile Duct Hyperplasia
6.11. Endometriosis
6.12. Vasitis Nodosa
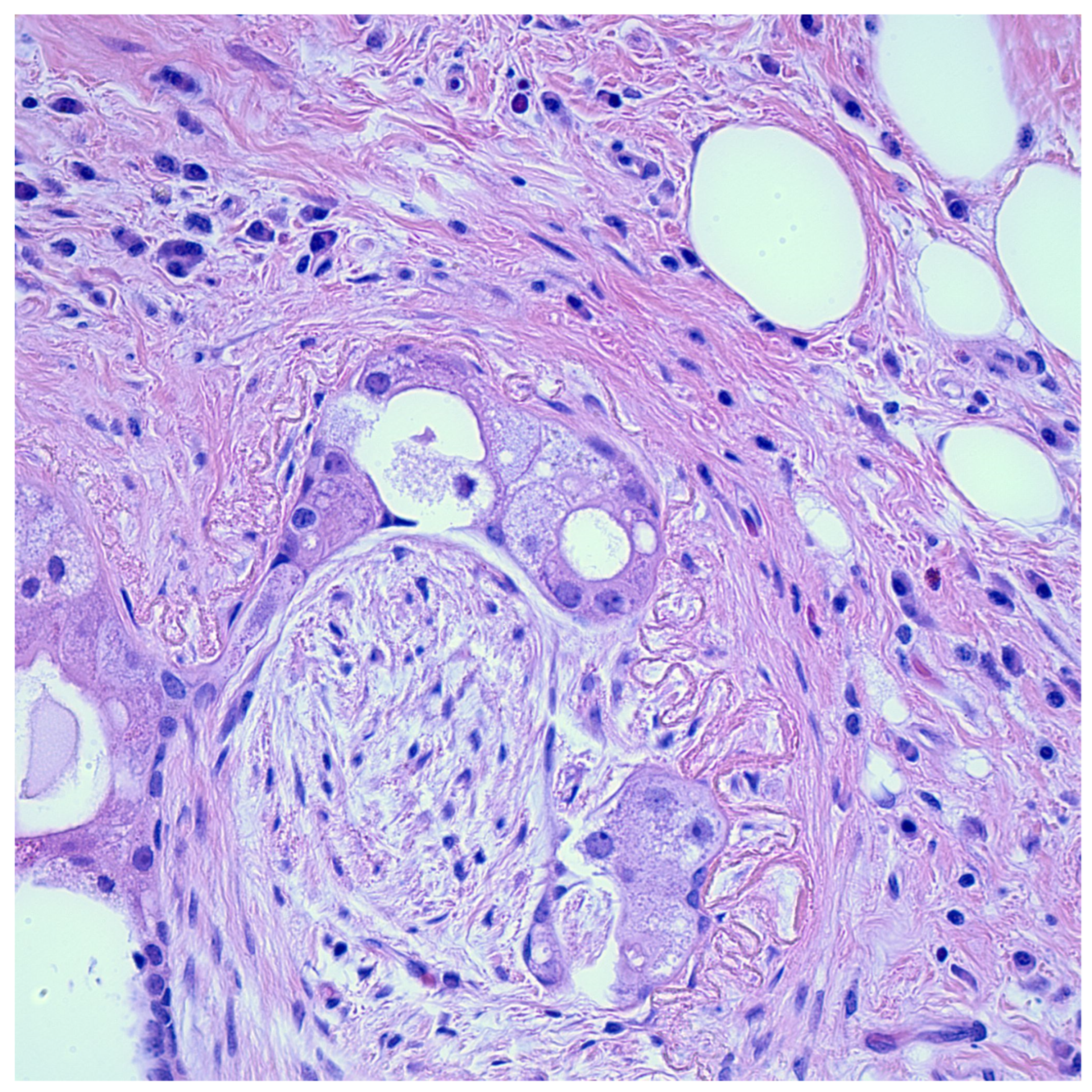
7. Histologic Mimics of Perineural Invasion
7.1. Peritumoral Fibrosis
7.2. Epithelial Sheath Neuroma
7.3. Re-Excision Perineural Invasion
7.4. Reactive Neuroepithelial Aggregates (RNEA) of the Skin
7.5. Reparative Perineural Proliferation or Reparative Perineural Hyperplasia
7.6. Renaut Bodies
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panizza, B.; Warren, T. Perineural invasion of head and neck skin cancer: Diagnostic and therapeutic implications. Curr. Oncol. Rep. 2013, 15, 128–133. [Google Scholar] [CrossRef]
- Chen, S.H.; Zhang, B.Y.; Zhou, B.; Zhu, C.Z.; Sun, L.Q.; Feng, Y.J. Perineural invasion of cancer: A complex crosstalk between cells and molecules in the perineural niche. Am. J. Cancer Res. 2019, 9, 1–21. [Google Scholar] [PubMed]
- Gadducci, A.; Pistolesi, S.; Cosio, S.; Naccarato, A.G. Is Perineural Invasion a Novel Prognostic Factor Useful to Tailor Adjuvant Treatment in Patients Treated With Primary Surgery for Cervical and Vulvar Carcinoma? Anticancer. Res. 2020, 40, 3031–3037. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, X.; Ding, Z.; Zhu, N.; Song, Y.; Zhang, X.; Jing, Y.; Yu, Y.; Huang, X.; Zhang, L.; et al. Worst Pattern of Perineural Invasion Redefines the Spatial Localization of Nerves in Oral Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 766902. [Google Scholar] [CrossRef] [PubMed]
- Casazza, G.C.; Monroe, M.M. Chapter 36—Imaging Cutaneous Squamous Cell Carcinoma of the Head and Neck. In Imaging in Dermatology; Hamblin, M.R., Avci, P., Gupta, G.K., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 491–504. [Google Scholar]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Mol. Biol. Rep. 2020, 47, 4587–4629. [Google Scholar] [CrossRef]
- Alkhadar, H.; Macluskey, M.; White, S.; Ellis, I. Nerve growth factor-induced migration in oral and salivary gland tumour cells utilises the PI3K/Akt signalling pathway: Is there a link to perineural invasion? J. Oral. Pathol. Med. 2020, 49, 227–234. [Google Scholar] [CrossRef]
- Jiang, J.; Bai, J.; Qin, T.; Wang, Z.; Han, L. NGF from pancreatic stellate cells induces pancreatic cancer proliferation and invasion by PI3K/AKT/GSK signal pathway. J. Cell Mol. Med. 2020, 24, 5901–5910. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, L.; Sun, Y.; Li, C.; Zhang, L.; Wang, X.; Chen, H. CORO1C is Associated With Poor Prognosis and Promotes Metastasis Through PI3K/AKT Pathway in Colorectal Cancer. Front. Mol. Biosci. 2021, 8, 682594. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Marais, R.; Wynne, J.; Treisman, R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell 1993, 73, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Sears, R.; Nuckolls, F.; Haura, E.; Taya, Y.; Tamai, K.; Nevins, J.R. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes. Dev. 2000, 14, 2501–2514. [Google Scholar] [CrossRef] [PubMed]
- Felton-Edkins, Z.A.; Fairley, J.A.; Graham, E.L.; Johnston, I.M.; White, R.J.; Scott, P.H. The mitogen-activated protein (MAP) kinase ERK induces tRNA synthesis by phosphorylating TFIIIB. EMBO. J. 2003, 22, 2422–2432. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Huang, C.; Li, Y.; Guo, Y.; Zhang, Z.; Lian, G.; Chen, Y.; Li, J.; Su, Y.; Li, J.; Yang, K.; et al. MMP1/PAR1/SP/NK1R paracrine loop modulates early perineural invasion of pancreatic cancer cells. Theranostics 2018, 8, 3074–3086. [Google Scholar] [CrossRef] [PubMed]
- Veit, C.; Genze, F.; Menke, A.; Hoeffert, S.; Gress, T.M.; Gierschik, P.; Giehl, K. Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer Res. 2004, 64, 5291–5300. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Peng, C.; Liang, B.; Shahbaz, M.; Liu, S.; Wang, B.; Sun, Q.; Niu, Z.; Niu, W.; Liu, E.; et al. β6 integrin induces the expression of metalloproteinase-3 and metalloproteinase-9 in colon cancer cells via ERK-ETS1 pathway. Cancer Lett. 2014, 354, 427–437. [Google Scholar] [CrossRef]
- Chuang, J.Y.; Tsai, C.F.; Chang, S.W.; Chiang, I.P.; Huang, S.M.; Lin, H.Y.; Yeh, W.L.; Lu, D.Y. Glial cell line-derived neurotrophic factor induces cell migration in human oral squamous cell carcinoma. Oral. Oncol. 2013, 49, 1103–1112. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Thomas, S.J.; Snowden, J.A.; Zeidler, M.P.; Danson, S.J. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef]
- Guo, K.; Ma, Q.; Li, J.; Wang, Z.; Shan, T.; Li, W.; Xu, Q.; Xie, K. Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling. Mol. Cancer Ther. 2013, 12, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Bressy, C.; Lac, S.; Nigri, J.; Leca, J.; Roques, J.; Lavaut, M.N.; Secq, V.; Guillaumond, F.; Bui, T.T.; Pietrasz, D.; et al. LIF Drives Neural Remodeling in Pancreatic Cancer and Offers a New Candidate Biomarker. Cancer Res. 2018, 78, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Zhao, Y.; Ma, P.; Cao, Y.; Liu, C.; Zhang, X.; Wang, W.; Chen, L.; Li, Y. Cancer-associated fibroblasts promote the migration and invasion of gastric cancer cells via activating IL-17a/JAK2/STAT3 signaling. Ann. Transl. Med. 2020, 8, 877. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Ma, Q.; Xu, Q.; Liu, H.; Duan, W.; Lei, J.; Ma, J.; Wang, X.; Lv, S.; et al. Sonic hedgehog paracrine signaling activates stromal cells to promote perineural invasion in pancreatic cancer. Clin. Cancer Res. 2014, 20, 4326–4338. [Google Scholar] [CrossRef]
- Qin, T.; Li, J.; Xiao, Y.; Wang, X.; Gong, M.; Wang, Q.; Zhu, Z.; Zhang, S.; Zhang, W.; Cao, F.; et al. Honokiol Suppresses Perineural Invasion of Pancreatic Cancer by Inhibiting SMAD2/3 Signaling. Front. Oncol. 2021, 11, 728583. [Google Scholar] [CrossRef]
- Hassounah, N.B.; Nagle, R.; Saboda, K.; Roe, D.J.; Dalkin, B.L.; McDermott, K.M. Primary cilia are lost in preinvasive and invasive prostate cancer. PLoS ONE 2013, 8, e68521. [Google Scholar] [CrossRef]
- Gasparini, G.; Pellegatta, M.; Crippa, S.; Lena, M.S.; Belfiori, G.; Doglioni, C.; Taveggia, C.; Falconi, M. Nerves and Pancreatic Cancer: New Insights into a Dangerous Relationship. Cancers 2019, 11, 893. [Google Scholar] [CrossRef]
- Bakst, R.L.; Wong, R.J. Mechanisms of Perineural Invasion. J. Neurol. Surg. B Skull Base 2016, 77, 96–106. [Google Scholar] [CrossRef]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer: A review of the literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef]
- Batsakis, J.G. Nerves and neurotropic carcinomas. Ann. Otol. Rhinol. Laryngol. 1985, 94, 426–427. [Google Scholar] [CrossRef]
- Veness, M.J. Perineural spread in head and neck skin cancer. Australas. J. Dermatol. 2000, 41, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.O.; Maksem, J. The prostatic perineural space and its relation to tumor spread: An ultrastructural study. Am. J. Surg. Pathol. 1980, 4, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.H.; Zhang, S.; Wang, H.; Xue, J.L.; Zhang, Z.G. Emerging experimental models for assessing perineural invasion in human cancers. Cancer Lett. 2022, 535, 215610. [Google Scholar] [CrossRef] [PubMed]
- Akert, K.; Sandri, C.; Weibel, E.R.; Peper, K.; Moor, H. The fine structure of the perineural endothelium. Cell Tissue Res. 1976, 165, 281–295. [Google Scholar] [CrossRef]
- Huyett, P.; Gilbert, M.; Liu, L.; Ferris, R.L.; Kim, S. A Model for Perineural Invasion in Head and Neck Squamous Cell Carcinoma. J. Vis. Exp. 2017, 55043. [Google Scholar] [CrossRef]
- Abiatari, I.; DeOliveira, T.; Kerkadze, V.; Schwager, C.; Esposito, I.; Giese, N.A.; Huber, P.; Bergman, F.; Abdollahi, A.; Friess, H.; et al. Consensus transcriptome signature of perineural invasion in pancreatic carcinoma. Mol. Cancer Ther. 2009, 8, 1494–1504. [Google Scholar] [CrossRef]
- Nigri, J.; Gironella, M.; Bressy, C.; Vila-Navarro, E.; Roques, J.; Lac, S.; Bontemps, C.; Kozaczyk, C.; Cros, J.; Pietrasz, D.; et al. PAP/REG3A favors perineural invasion in pancreatic adenocarcinoma and serves as a prognostic marker. Cell Mol. Life Sci. 2017, 74, 4231–4243. [Google Scholar] [CrossRef]
- Sinha, S.; Fu, Y.Y.; Grimont, A.; Ketcham, M.; Lafaro, K.; Saglimbeni, J.A.; Askan, G.; Bailey, J.M.; Melchor, J.P.; Zhong, Y.; et al. PanIN Neuroendocrine Cells Promote Tumorigenesis via Neuronal Cross-talk. Cancer Res. 2017, 77, 1868–1879. [Google Scholar] [CrossRef]
- Bakst, R.L.; Xiong, H.; Chen, C.H.; Deborde, S.; Lyubchik, A.; Zhou, Y.; He, S.; McNamara, W.; Lee, S.Y.; Olson, O.C.; et al. Inflammatory Monocytes Promote Perineural Invasion via CCL2-Mediated Recruitment and Cathepsin B Expression. Cancer Res. 2017, 77, 6400–6414. [Google Scholar] [CrossRef]
- Zhang, J.F.; Tao, L.Y.; Yang, M.W.; Xu, D.P.; Jiang, S.H.; Fu, X.L.; Liu, D.J.; Huo, Y.M.; Liu, W.; Yang, J.Y.; et al. CD74 promotes perineural invasion of cancer cells and mediates neuroplasticity via the AKT/EGR-1/GDNF axis in pancreatic ductal adenocarcinoma. Cancer Lett. 2021, 508, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Koide, N.; Yamada, T.; Shibata, R.; Mori, T.; Fukuma, M.; Yamazaki, K.; Aiura, K.; Shimazu, M.; Hirohashi, S.; Nimura, Y.; et al. Establishment of perineural invasion models and analysis of gene expression revealed an invariant chain (CD74) as a possible molecule involved in perineural invasion in pancreatic cancer. Clin. Cancer Res. 2006, 12, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, J.; Wang, J.; Pu, T.; Wei, J.; Li, Q.; Wu, B.J. MAOA promotes prostate cancer cell perineural invasion through SEMA3C/PlexinA2/NRP1-cMET signaling. Oncogene 2021, 40, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Le Magnen, C.; Dutta, A.; Abate-Shen, C. Optimizing mouse models for precision cancer prevention. Nat. Rev. Cancer 2016, 16, 187–196. [Google Scholar] [CrossRef]
- Jurcak, N.R.; Rucki, A.A.; Muth, S.; Thompson, E.; Sharma, R.; Ding, D.; Zhu, Q.; Eshleman, J.R.; Anders, R.A.; Jaffee, E.M.; et al. Axon Guidance Molecules Promote Perineural Invasion and Metastasis of Orthotopic Pancreatic Tumors in Mice. Gastroenterology 2019, 157, 838–850.e6. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM) assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef]
- Schmitd, L.B.; Liu, M.; Scanlon, C.S.; Banerjee, R.; D’Silva, N.J. The Chick Chorioallantoic Membrane In Vivo Model to Assess Perineural Invasion in Head and Neck Cancer. J. Vis. Exp. 2019, 59296. [Google Scholar] [CrossRef]
- Tassone, P.; Caruso, C.; White, M.; Dos Santos, H.T.; Galloway, T.; Dooley, L.; Zitsch III, R.; Layfield, J.L.; Baker, O. The role of matrixmetalloproteinase-2 expression by fibroblasts in perineural invasion by oral cavity squamous cell carcinoma. Oral. Oncol. 2022, 132, 106002. [Google Scholar] [CrossRef]
- Zhang, M.; Xian, H.C.; Dai, L.; Tang, Y.L.; Liang, X.H. MicroRNAs: Emerging driver of cancer perineural invasion. Cell Biosci. 2021, 11, 117. [Google Scholar] [CrossRef]
- Amit, M.; Na’ara, S.; Gil, Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. Cancer 2016, 16, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Kraus, R.D.; Barsky, A.; Ji, L.; Santos, P.M.G.; Cheng, N.; Groshen, S.; Vapiwala, N.; Ballas, L.K. The perineural invasion paradox: Is perineural invasion an independent prognostic indicator of biochemical recurrence risk in patients with pT2N0R0 prostate cancer? A multi-institutional study. Adv. Radiat. Oncol. 2019, 4, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Zareba, P.; Flavin, R.; Isikbay, M.; Rider, J.R.; Gerke, T.A.; Finn, S.; Pettersson, A.; Giunchi, F.; Unger, R.H.; Tinianow, A.M.; et al. Perineural Invasion and Risk of Lethal Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2017, 26, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-J.; Wu, B.; Zha, Z.-L.; Qu, W.; Zhao, H.; Yuan, J.; Feng, Y.-J. Perineural invasion as an independent predictor of biochemical recurrence in prostate cancer following radical prostatectomy or radiotherapy: A systematic review and meta-analysis. BMC Urol. 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Harnden, P.; Shelley, M.D.; Clements, H.; Coles, B.; Tyndale-Biscoe, R.S.; Naylor, B.; Mason, M.D. The prognostic significance of perineural invasion in prostatic cancer biopsies: A systematic review. Cancer 2007, 109, 13–24. [Google Scholar] [CrossRef]
- Merrilees, A.D.; Bethwaite, P.B.; Russell, G.L.; Robinson, R.G.; Delahunt, B. Parameters of perineural invasion in radical prostatectomy specimens lack prognostic significance. Mod. Pathol. 2008, 21, 1095–1100. [Google Scholar] [CrossRef][Green Version]
- Ng, J.C.; Koch, M.O.; Daggy, J.K.; Cheng, L. Perineural Invasion in Radical Prostatectomy Specimens: Lack of Prognostic Significance. J. Urol. 2004, 172, 2249–2251. [Google Scholar] [CrossRef]
- Lubig, S.; Thiesler, T.; Müller, S.; Vorreuther, R.; Leipner, N.; Kristiansen, G. Quantitative perineural invasion is a prognostic marker in prostate cancer. Pathology 2018, 50, 298–304. [Google Scholar] [CrossRef]
- Bakst, R.L.; Glastonbury, C.M.; Parvathaneni, U.; Katabi, N.; Hu, K.S.; Yom, S.S. Perineural Invasion and Perineural Tumor Spread in Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1109–1124. [Google Scholar] [CrossRef]
- Ko, H.C.; Gupta, V.; Mourad, W.F.; Hu, K.S.; Harrison, L.B.; Som, P.M.; Bakst, R.L. A contouring guide for head and neck cancers with perineural invasion. Pract. Radiat. Oncol. 2014, 4, e247–e258. [Google Scholar] [CrossRef]
- Deng, J.; You, Q.; Gao, Y.; Yu, Q.; Zhao, P.; Zheng, Y.; Fang, W.; Xu, N.; Teng, L. Prognostic value of perineural invasion in gastric cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e88907. [Google Scholar] [CrossRef] [PubMed]
- Muduly, D.K.; Kar, M.; Sultania, M.; Shahin, M.; Patra, S.; Singh, V.; Imaduddin, M.; Mohakud, S.; Nayak, H.K.; Panigraphi, M.K.; et al. Inclusion of Perineural Invasion with AJCC-TNM Staging: Outcomes from a South Asian Cohort of Curatively Treated Gastric Cancer Patients. J. Gastrointest. Cancer 2022, 54, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, F.; Melchiorre, E.; Casari, I.; Cinalli, S.; Cinalli, M.; Aceto, G.M.; Cotellese, R.; Garajova, I.; Falasca, M. Perineural Invasion in Pancreatic Ductal Adenocarcinoma: From Molecules towards Drugs of Clinical Relevance. Cancers 2022, 14, 5793. [Google Scholar] [CrossRef]
- Guo, X.; Gao, S.; Yu, J.; Zhou, Y.; Gao, C.; Hao, J. The imaging features of extrapancreatic perineural invasion (EPNI) in pancreatic Cancer: A comparative retrospective study. Pancreatology 2021, 21, 1516–1523. [Google Scholar] [CrossRef]
- Chatterjee, D.; Katz, M.H.; Rashid, A.; Wang, H.; Iuga, A.C.; Varadhachary, G.R.; Wolff, R.A.; Lee, J.E.; Pisters, P.W.; Crane, C.H.; et al. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am. J. Surg. Pathol. 2012, 36, 409–417. [Google Scholar] [CrossRef]
- Mitsunaga, S.; Hasebe, T.; Kinoshita, T.; Konishi, M.; Takahashi, S.; Gotohda, N.; Nakagohri, T.; Ochiai, A. Detail Histologic Analysis of Nerve Plexus Invasion in Invasive Ductal Carcinoma of the Pancreas and Its Prognostic Impact. Am. J. Surg. Pathol. 2007, 31, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.N.; Olcott, E.; Jeffrey, R.B. Extrapancreatic perineural invasion in pancreatic adenocarcinoma. Abdom. Radiol. 2018, 43, 323–331. [Google Scholar] [CrossRef]
- Ozaki, H.; Hiraoka, T.; Mizumoto, R.; Matsuno, S.; Matsumoto, Y.; Nakayama, T.; Tsunoda, T.; Suzuki, T.; Monden, M.; Saitoh, Y.; et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg. Today 1999, 29, 16–22. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Hua, R.; Sun, Y.-W.; Liu, W.; Huo, Y.-M.; Liu, D.-J.; Li, J. Influence of perineural invasion on survival and recurrence in patients with resected pancreatic cancer. Asian Pac. J. Cancer Prev. 2013, 14, 5133–5139. [Google Scholar] [CrossRef]
- Shah, M.M.; NeMoyer, R.E.; Greco, S.H.; Chen, C.; Moore, D.F.; Grandhi, M.S.; Langan, R.C.; Kennedy, T.J.; Javidian, P.; Jabbour, S.K.; et al. Subcategorizing T1 Staging in Pancreatic Adenocarcinoma Predicts Survival in Patients Undergoing Resection: An Analysis of the National Cancer Database. J. Pancreat. Cancer 2020, 6, 64–72. [Google Scholar] [CrossRef]
- Wakiya, T.; Ishido, K.; Yoshizawa, T.; Kanda, T.; Hakamada, K. Roles of the nervous system in pancreatic cancer. Ann. Gastroenterol. Surg. 2021, 5, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, H.; de Savornin Lohman, E.A.J.; van Dooren, M.; Braat, A.E.; Daams, F.; van Dam, R.; Erdmann, J.I.; Hagendoorn, J.; Hoogwater, F.J.H.; Groot Koerkamp, B.; et al. Extended Resections for Advanced Gallbladder Cancer: Results from a Nationwide Cohort Study. Ann. Surg. Oncol. 2020, 28, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Nagino, M.; Oda, K.; Kamiya, J.; Uesaka, K.; Nimura, Y. Perineural invasion has a negative impact on survival of patients with gallbladder carcinoma. Br. J. Surg. 2002, 89, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Asmita, C.; Alessandro, P. Gallbladder Cancer: Diagnosis and Surgical Management. In Biliary Tract—Review and Recent Progress; Qiang, Y., Zhiping, P., Eds.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Knijn, N.; Mogk, S.C.; Teerenstra, S.; Simmer, F.; Nagtegaal, I.D. Perineural invasion is a strong prognostic factor in colorectal cancer. Am. J. Surg. Pathol. 2016, 40, 103–112. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, X.; Sun, J.; Gao, P.; Song, Y.; Chen, X.; Zhao, J.; Wang, Z. Prognostic value of perineural invasion in colorectal cancer: A meta-analysis. J. Gastrointest. Surg. 2015, 19, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Fordice, J.; Kershaw, C.; El-Naggar, A.; Goepfert, H. Adenoid cystic carcinoma of the head and neck: Predictors of morbidity and mortality. Arch. Otolaryngol. Head Neck Surg. 1999, 125, 149–152. [Google Scholar] [CrossRef]
- Sezer, A.; Celik, M.; Bulbul, B.Y.; Can, N.; Tastekin, E.; Ayturk, S.; Ustun, F.; Guldiken, S.; Sut, N. Relationship between lymphovascular invasion and clinicopathological features of papillary thyroid carcinoma. Bosn. J. Basic Med. Sci. 2017, 17, 144–151. [Google Scholar] [CrossRef]
- Karak, S.G.; Quatrano, N.; Buckley, J.; Ricci, A., Jr. Prevalence and significance of perineural invasion in invasive breast carcinoma. Conn. Med. 2010, 74, 17–21. [Google Scholar]
- Zhang, J.; Wang, Y.; Wijaya, W.A.; Liang, Z.; Chen, J. Efficacy and prognostic factors of adjuvant radiotherapy for cutaneous squamous cell carcinoma: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1777–1787. [Google Scholar] [CrossRef]
- Liebig, C.; Ayala, G.; Wilks, J.; Verstovsek, G.; Liu, H.; Agarwal, N.; Berger, D.H.; Albo, D. Perineural invasion is an independent predictor of outcome in colorectal cancer. J. Clin. Oncol. 2009, 27, 5131–5137. [Google Scholar] [CrossRef]
- Nair, D.; Mair, M.; Singhvi, H.; Mishra, A.; Nair, S.; Agrawal, J.; Chaturvedi, P. Perineural invasion: Independent prognostic factor in oral cancer that warrants adjuvant treatment. Head Neck 2018, 40, 1780–1787. [Google Scholar] [CrossRef]
- Haug, K.; Breuninger, H.; Metzler, G.; Eigentler, T.; Eichner, M.; Haefner, H.-M.; Schnabl, S.M. Prognostic impact of perineural invasion in cutaneous squamous cell carcinoma: Results of a prospective study of 1399 tumors. J. Investig. Dermatol. 2020, 140, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Seethala, R.; Shon, W.; Balzer, B.; Duvvuri, U.; Gharavi, N.; Lydiatt, W. Protocol for the Examination of Specimens from Patients with Cutaneous Squamous Cell Carcinoma of the Head and Neck; College of American Pathologists: Northfield, MN, USA, 2022. [Google Scholar]
- Paner, G.; Srigley, J.; Pettus, J.; Giannico, G.; Sirintrapun, J.; Harik, L. Protocol for the Examination of Prostate Needle Biopsies from Patients with Carcinoma of the Prostate Gland: Specimen Level Reporting; College of American Pathologists: Northfield, MN, USA, 2021. [Google Scholar]
- Burgart, L.; Chopp, W.; Jain, D. Protocol for the Examination of Specimens from Patients with Carcinoma of the Stomach; College of American Pathologists: Northfield, MN, USA, 2023. [Google Scholar]
- Burgart, L.; Chopp, W.; Jain, D. Protocol for the Examination of Excisional Biopsy Specimens from Patients with Primary Carcinoma of the Colon and Rectum; College of American Pathologists: Northfield, MN, USA, 2021. [Google Scholar]
- Burgart, L.; Chopp, W.; Jain, D. Protocol for the Examination of Specimens from Patients with Carcinoma of the Gallbladder; College of American Pathologists: Northfield, MN, USA, 2021. [Google Scholar]
- Burgart, L.; Chopp, W.; Jain, D. Protocol for the Examination of Specimens from Patients with Carcinoma of the Pancreas; College of American Pathologists: Northfield, MN, USA, 2021. [Google Scholar]
- Messina, J.; Calonje, J.; Zalaudek, I.; Fernandez-Figueras, M. Squamous cell carcinomas. In Skin Tumours, 5th ed.; WHO Classification of Tumours Editorial Board: Geneva, Switzerland, 2023. [Google Scholar]
- Nadal, A.; Bishop, J.; Brandwein-Weber, M.; Stenman, G.; Zidar, N. Conventional squamous cell carcinoma. In Head and Neck Tumours, 5th ed.; WHO Classification of Tumours Editorial Board: Geneva, Switzerland, 2023. [Google Scholar]
- Kench, J.; Kristiansen, G.; Berney, D.; Netto, G.; Marzo, A.; Litjens, G.; Magi-Galluzzi, C.; Yang, X.; Egevad, L. Prostatic acinar adenocarcinoma. In Urinary and Male Genital Tumours, 5th ed.; WHO Classification of Tumours Editorial Board: Geneva, Switzerland, 2022. [Google Scholar]
- Baig, F.A.; Hamid, A.; Mirza, T.; Syed, S. Ductal and Acinar Adenocarcinoma of Prostate: Morphological and Immunohistochemical Characterization. Oman. Med. J. 2015, 30, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.; Arends, M.; Salto-Tellez, M. Colorectal Adenocarcinoma. In Digestive System Tumours; IARC Press: Lyon, France, 2019. [Google Scholar]
- Hruban, R.; Adsay, N.; Esposito, I.; Klöppel, G.; Zamboni, G.; Furukawa, T.; Fukushima, N.; Offerhaus, G.; Pitman, M.; Notohara, K.; et al. Pancreatic Ductal Adenocarcinoma. In Digestive System Tumours; IARC Press: Lyon, France, 2019. [Google Scholar]
- Brown, I.S. Pathology of Perineural Spread. J. Neurol. Surgery. Part B Skull Base 2016, 77, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Ronaghy, A.; Yaar, R.; Goldberg, L.J.; Mahalingam, M.; Bhawan, J. Perineural Involvement: What Does it Mean? Am. J. Dermatopathol. 2010, 32, 469–476. [Google Scholar] [CrossRef]
- Alikhan, A.; Ibrahimi, O.A.; Eisen, D.B. Congenital melanocytic nevi: Where are we now? Part I. Clinical presentation, epidemiology, pathogenesis, histology, malignant transformation, and neurocutaneous melanosis. J. Am. Acad. Dermatol. 2012, 67, 495.e1–495.e17. [Google Scholar] [CrossRef]
- Jahnke, M.N.; O’Haver, J.; Gupta, D.; Hawryluk, E.B.; Finelt, N.; Kruse, L.; Jen, M.; Horii, K.A.; Frieden, I.J.; Price, H.; et al. Care of Congenital Melanocytic Nevi in Newborns and Infants: Review and Management Recommendations. Pediatrics 2021, 148, e2021051536. [Google Scholar] [CrossRef]
- Mark, G.J.; Mihm, M.C.; Liteplo, M.G.; Reed, R.J.; Clark, W.H. Congenital melanocytic nevi of the small and garment type. Clinical, histologic, and ultrastructural studies. Hum. Pathol. 1973, 4, 395–418. [Google Scholar] [CrossRef]
- Wang, L.; Wang, G.; Gao, T. Congenital melanocytic nevus with features of hybrid schwannoma/perineurioma. J. Cutan Pathol. 2013, 40, 497–502. [Google Scholar] [CrossRef]
- Dailey, V.L.; Hameed, O. Blue nevus of the prostate. Arch. Pathol. Lab. Med. 2011, 135, 799–802. [Google Scholar] [CrossRef]
- Ishida, M.; Kagotani, A.; Yoshida, K.; Iwai, M.; Okabe, H. Endometrioid adenocarcinoma concurrent with a blue nevus of the endometrium and uterine cervix: A case report. Oncol. Lett. 2013, 6, 1219–1221. [Google Scholar] [CrossRef] [PubMed]
- Castelli, E.; Morello, V.; Tomasino, R.M. Perineural pattern of aggregation of cellular blue nevus: Probable histoarchitectural reminiscence of histogenesis. Am. J. Dermatopathol. 2008, 30, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Habougit, C.; Michiels-Marzais, D.; Wang, Q.; Pissaloux, D.; de la Fouchardiere, A. Linear variant of large plaque-type blue naevus with subcutaneous cellular nodules. Pathology 2017, 49, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Misago, N. The relationship between melanocytes and peripheral nerve sheath cells (Part II): Blue nevus with peripheral nerve sheath differentiation. Am. J. Dermatopathol. 2000, 22, 230–236. [Google Scholar] [CrossRef]
- Temple-Camp, C.R.; Saxe, N.; King, H. Benign and malignant cellular blue nevus. A clinicopathological study of 30 cases. Am. J. Dermatopathol. 1988, 10, 289–296. [Google Scholar] [CrossRef]
- Lee, M.Y.; Jin, S.; Lee, K.H.; Park, M.H.; Jung, S.T.; Rubin, A.; Yun, S.J. A cellular blue nevus with pigmented epithelioid melanocytoma-like pattern on the ipsilateral upper arm associated with a congenital plaque-type blue nevus on the hand. J. Cutan Pathol. 2019, 46, 383–388. [Google Scholar] [CrossRef]
- Ordonez, N.G.; Mackay, B. Granular cell tumor: A review of the pathology and histogenesis. Ultrastruct. Pathol. 1999, 23, 207–222. [Google Scholar] [CrossRef]
- Lack, E.E.; Worsham, G.F.; Callihan, M.D.; Crawford, B.E.; Klappenbach, S.; Rowden, G.; Chun, B. Granular cell tumor: A clinicopathologic study of 110 patients. J. Surg. Oncol. 1980, 13, 301–316. [Google Scholar] [CrossRef]
- Mobarki, M.; Dumollard, J.M.; Dal Col, P.; Camy, F.; Peoc’h, M.; Karpathiou, G. Granular cell tumor a study of 42 cases and systemic review of the literature. Pathol. Res. Pract. 2020, 216, 152865. [Google Scholar] [CrossRef]
- Stemm, M.; Suster, D.; Wakely, P.E., Jr.; Suster, S. Typical and Atypical Granular Cell Tumors of Soft Tissue: A Clinicopathologic Study of 50 Patients. Am. J. Clin. Pathol. 2017, 148, 161–166. [Google Scholar] [CrossRef]
- Chow, L.T.C.; Chow, M. Intraneural granular cell tumor: Histologic spectrum and histogenetic implication. J. Cutan. Pathol. 2020, 47, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Favre, N.M.; Sabih, Q.; L’Huillier, J.C.; Takabe, K.; Cappuccino, H. Syringomatous Tumor of the Nipple. World J. Oncol. 2022, 13, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.W.; Norris, H.J.; Snyder, R.C. Infiltrating syringomatous adenoma of the nipple. A clinical and pathological study of 11 cases. Am. J. Surg Pathol. 1989, 13, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Oo, K.Z.; Xiao, P.Q. Infiltrating syringomatous adenoma of the nipple: Clinical presentation and literature review. Arch. Pathol. Lab. Med. 2009, 133, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Massara, B.; Sellami, K.; Graja, S.; Boudaouara, O.; Miladi, S.; Hammami, F.; Bahloul, E.; Boudaouara, T.; Turki, H. Trichofolliculoma: A Case Series. J. Clin. Aesthet. Dermatol. 2023, 16, 41–43. [Google Scholar] [PubMed]
- Stern, J.B.; Stout, D.A. Trichofolliculoma showing perineural invasion. Trichofolliculocarcinoma? Arch. Dermatol. 1979, 115, 1003–1004. [Google Scholar] [CrossRef]
- Lin, T.Y.; Zhang, A.Y.; Bayer-Garner, I.B.; Krell, J.M.; Acker, S.M. Epithelial sheath neuroma: A case report and discussion of the literature. Am. J. Dermatopathol. 2006, 28, 216–219. [Google Scholar] [CrossRef]
- Requena, L.; Grosshans, E.; Kutzner, H.; Ryckaert, C.; Cribier, B.; Resnik, K.S.; LeBoit, P.E. Epithelial sheath neuroma: A new entity. Am. J. Surg Pathol. 2000, 24, 190–196. [Google Scholar] [CrossRef]
- Hirano-Ali, S.A.; Bryant, E.A.; Warren, S.J. Epithelial sheath neuroma: Evidence supporting a hyperplastic etiology and epidermal origin. J. Cutan. Pathol. 2016, 43, 531–534. [Google Scholar] [CrossRef]
- Husain, E.A.; Al-Daraji, W.I. Epithelial sheath neuroma: Be aware of benign perineural invasion! J. Cutan. Pathol. 2009, 36, 570–572. [Google Scholar] [CrossRef]
- Wang, J.Y.; Nuovo, G.; Kline, M.; Magro, C.M. Reexcision Perineural Invasion and Epithelial Sheath Neuroma Possibly on a Spectrum of Postinjury Reactive Hyperplasia Mediated by IL-6. Am. J. Dermatopathol. 2017, 39, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Cerilli, L.A.; Fechner, R.E. Benign Intraneural Epithelium in the Breast. Arch. Pathol. Lab. Med. 2000, 124, 465. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.J.; Chen, S.L. Nerve invasion by epithelial cells in benign breast diseases. J. Chin. Med. Assoc. 2009, 72, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Elfituri, O.; Emmadi, R. Perineural and intraneural involvement in ductal carcinoma in-situ of breast: Case report. Pathol. Res. Pract. 2019, 215, 152624. [Google Scholar] [CrossRef] [PubMed]
- Fellegara, G.; Kuhn, E. Perineural and intraneural “invasion” in benign proliferative breast disease. Int. J. Surg. Pathol. 2007, 15, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, H.; Jensen, R.A.; Simpson, J.F.; Olson, S.J.; Page, D.L. Atypical ductal hyperplasia and ductal carcinoma in situ of the breast associated with perineural invasion. Hum. Pathol. 2001, 32, 785–790. [Google Scholar] [CrossRef]
- Gould, V.E.; Rogers, D.R.; Sommers, S.C. Epithelial-nerve intermingling in benign breast lesions. Arch Pathol. 1975, 99, 596–598. [Google Scholar]
- Taylor, H.B.; Norris, H.J. Epithelial invasion of nerves in benign diseases of the breast. Cancer 1967, 20, 2245–2249. [Google Scholar] [CrossRef]
- Ackerman, L.V. Seminar on Lesions of the Breast; American Society of Clinical Pathologists: Chicago, IL, USA, 1957. [Google Scholar]
- Selesnick, S.H.; Burt, B.M. Regional spread of nonneurogenic tumors to the skull base via the facial nerve. Otol. Neurotol. 2003, 24, 326–333. [Google Scholar] [CrossRef]
- Roncati, L.; Maiorana, A. Benign Recurrent Pleomorphic Adenoma of the Parotid Gland with Perineural Space Invasion. Chonnam Med. J. 2017, 53, 236–237. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Elhammady, G.; Gass, J.M.; Paramo, J.C.; Poppiti, R.; Alexis, J. PIK3R1, HRAS and AR Gene Alterations Associated with Sclerosing Polycystic Adenoma of the Parotid Gland. Curr. Issues Mol. Biol. 2023, 45, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Klöppel, G.; Adsay, N.V. Chronic Pancreatitis and the Differential Diagnosis Versus Pancreatic Cancer. Arch. Pathol. Lab. Med. 2009, 133, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Costa, J. Benign epithelial inclusions in pancreatic nerves. Am. J. Clin. Pathol. 1977, 67, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.L. Benign neural invasion in chronic pancreatitis. J. Clin. Gastroenterol. 1984, 6, 41–44. [Google Scholar]
- Moghimi, M.; Joukar, F.; Salehi-Abargouei, A.; Mozayan, M.R.; Aryanfar, A. Perineural Pseudoinvasion: An Unusual Phenomenon in Nonmalignancies. Adv. Anat. Pathol. 2017, 24, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Albores-Saavedra, J.; Henson, D.E. Adenomyomatous hyperplasia of the gallbladder with perineural invasion. Arch. Pathol. Lab. Med. 1995, 119, 1173–1176. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Henson, D.E. Pyloric gland metaplasia with perineural invasion of the gallbladder: A lesion that can be confused with adenocarcinoma. Cancer 1999, 86, 2625–2631. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Keenportz, B.; Bejarano, P.A.; Alexander, A.A.; Henson, D.E. Adenomyomatous hyperplasia of the gallbladder with perineural invasion: Revisited. Am. J. Surg. Pathol. 2007, 31, 1598–1604. [Google Scholar] [CrossRef]
- Roth, L.M. Endometriosis with perineural involvement. Am. J. Clin. Pathol. 1973, 59, 807–809. [Google Scholar] [CrossRef]
- Lenz, J.; Chvatal, R.; Fiala, L.; Konecna, P.; Lenz, D. Comparative immunohistochemical study of deep infiltrating endometriosis, lymph node endometriosis and atypical ovarian endometriosis including description of a perineural invasion. Biomed. Pap. 2021, 165, 69–79. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, D.; Yang, F.; Pan, W.; Zeng, F.; Wu, J.; Xie, H.; Li, J.; Yao, S. Perineural invasion in endometriotic lesions contributes to endometriosis-associated pain. J. Pain Res. 2018, 11, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.; Hassan, M.; Choudhry, M.S.; Shahani, B.; Ali, M. Vasitis Nodosa: A Rare Diagnosis for Inguinal Swelling. Cureus 2021, 13, e13759. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.S. AMA Drug Evaluations. J. Am. Med. Assoc. 1974, 228, 1519. [Google Scholar] [CrossRef]
- Balogh, K.; Travis, W.D. The frequency of perineurial ductules in vasitis nodosa. Am. J. Clin. Pathol. 1984, 82, 710–713. [Google Scholar] [CrossRef]
- Dunn, M.; Morgan, M.B.; Beer, T.W.; Chen, K.T.; Acker, S.M. Histologic mimics of perineural invasion. J. Cutan. Pathol. 2009, 36, 937–942. [Google Scholar] [CrossRef] [PubMed]
- França, K.; Alqubaisy, Y.; Hassanein, A.; Nouri, K.; Lotti, T. Histopathologic pitfalls of Mohs micrographic surgery and a review of tumor histology. Wien. Med. Wochenschr. 2018, 168, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Flora, A.; Kim, R.H.; Lara Rivero, A.D.; Carr, U.; Isaacs, F. Epithelial sheath neuroma: A case series. JAAD Case Rep. 2020, 6, 240–242. [Google Scholar] [CrossRef]
- Ide, F.; Ito, Y.; Matsuoka, K.; Muramatsu, T.; Saito, I. Re-excision perineural invasion in oral squamous cell carcinoma. Oral Dis. 2014, 20, 219–220. [Google Scholar] [CrossRef]
- Beer, T.W. Reexcision perineural invasion: A mimic of malignancy. Am. J. Dermatopathol. 2006, 28, 423–425. [Google Scholar] [CrossRef]
- Chen, K.T. Reactive neuroepithelial aggregates of the skin. Int. J. Surg. Pathol. 2003, 11, 205–210. [Google Scholar] [CrossRef]
- Beer, T.W. Reparative perineural hyperplasia: A series of 10 cases. Am. J. Dermatopathol. 2009, 31, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, D.; Neary, D.; Eames, R.A. Renaut body distribution at sites of human peripheral nerve entrapment. J. Neurol. Sci. 1981, 49, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Jedrych, J.; Leffell, D.; McNiff, J.M. Desmoplastic trichoepithelioma with perineural involvement: A series of seven cases. J. Cutan. Pathol. 2012, 39, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, R.A.; Woosley, J.T.; Tomsick, R.S. Renaut bodies. Benign disease process mimicking neurotropic tumor infiltration. Dermatol. Surg. 1996, 22, 969–971. [Google Scholar] [CrossRef]

| Type | Description | Benefits | Limitations |
|---|---|---|---|
| In Vitro Models | |||
| Transwell Model [35] | Two-dimensional culture using Transwell inserts, cancer cells placed in the apical chamber, chemotactic mechanisms studied | Straightforward, reproducible and cost-effective | Limited in reproducing the full range of cellular behavior observed in the extracellular domain |
| Dorsal Root Ganglia Co-Culture Model [35] | Three-dimensional culture with insertion of dorsal root ganglia in Matrigel, cancer cells placed nearby, and invasion observed via fluorescence | Enhanced visualization and cost-effective | Limited representation of extracellular behavior and does not capture the full range of intercellular interactions between neurons and cancer cells |
| Ex Vivo Models | |||
| Explanted Vagus Nerve Model [35] | Harvested vagus nerve placed in the chamber and then on culture media, cancer cells inserted, and invasion observed in culture medium | Valuable for determining the invasive potential of cancer cells of interest | Limited representation of neural microenvironment and lacks the full range of neural-cancer cell interactions |
| Explanted Sciatic Nerve Model [35] | Cancer cells propagated, sciatic nerves were placed on cultured cells, and sciatic nerves were removed for histologic analysis | Allows for investigation of the impact of tumor suppressors on PNI interactions between Schwann and cancer cells | Limited ability to replicate in vivo innervation |
| Organoid Model [35] | Co-culture of dorsal root ganglia with representative organoids | Allows for investigation of intercellular signaling, accurately replicates neural microenvironment | Current technology limits the extent to which intercellular signaling can be observed |
| In vivo Models | |||
| Heterotopic Xenograft Model [35] | Cancer cells are directly inserted into the target tissue or native organs, and PNI frequency is analyzed histologically | Diverse set of cell lines able to be studied, and the degree of PNI is readily quantifiable | Limited concurrent neural alterations may not fully mimic human PNI |
| Genetically Engineered Mouse Models | Genetically manipulated mice | Accurate replication of cancer as seen in humans | Restricted neuroplasticity and lack of significant neural invasion |
| CAM-Dorsal Root Ganglia Model [35] | Grafted dorsal root ganglia in chicken embryo chorionic epithelium; cancer cells grafted near neural cells | Mimics neural microenvironment and allows for investigation of roles of molecules or signaling pathways | Restricted observation period due to embryologic immune activation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahmad, H.F.; Gogola, S.; Rejzer, M.; Stoyanov, K.; Gomez, A.S.; Valencia, A.-K.; Cummings, A.; Skerry, T.; Alloush, F.; Aljamal, A.A.; et al. Unraveling the Mysteries of Perineural Invasion in Benign and Malignant Conditions. Curr. Oncol. 2023, 30, 8948-8972. https://doi.org/10.3390/curroncol30100647
Bahmad HF, Gogola S, Rejzer M, Stoyanov K, Gomez AS, Valencia A-K, Cummings A, Skerry T, Alloush F, Aljamal AA, et al. Unraveling the Mysteries of Perineural Invasion in Benign and Malignant Conditions. Current Oncology. 2023; 30(10):8948-8972. https://doi.org/10.3390/curroncol30100647
Chicago/Turabian StyleBahmad, Hisham F., Samantha Gogola, Michael Rejzer, Kalin Stoyanov, Aaron S. Gomez, Ann-Katrin Valencia, Adonicah Cummings, Timothy Skerry, Ferial Alloush, Abed A. Aljamal, and et al. 2023. "Unraveling the Mysteries of Perineural Invasion in Benign and Malignant Conditions" Current Oncology 30, no. 10: 8948-8972. https://doi.org/10.3390/curroncol30100647
APA StyleBahmad, H. F., Gogola, S., Rejzer, M., Stoyanov, K., Gomez, A. S., Valencia, A.-K., Cummings, A., Skerry, T., Alloush, F., Aljamal, A. A., Deb, A., Alghamdi, S., & Poppiti, R. (2023). Unraveling the Mysteries of Perineural Invasion in Benign and Malignant Conditions. Current Oncology, 30(10), 8948-8972. https://doi.org/10.3390/curroncol30100647









