Diagnostic Value of Diffusion-Weighted Imaging with Background Body Signal Suppression (DWIBS) for the Pre-Therapeutic Loco-Regional Staging of Cervical Cancer: A Feasibility and Interobserver Reliability Study
Abstract
1. Introduction
2. Materials and Methods
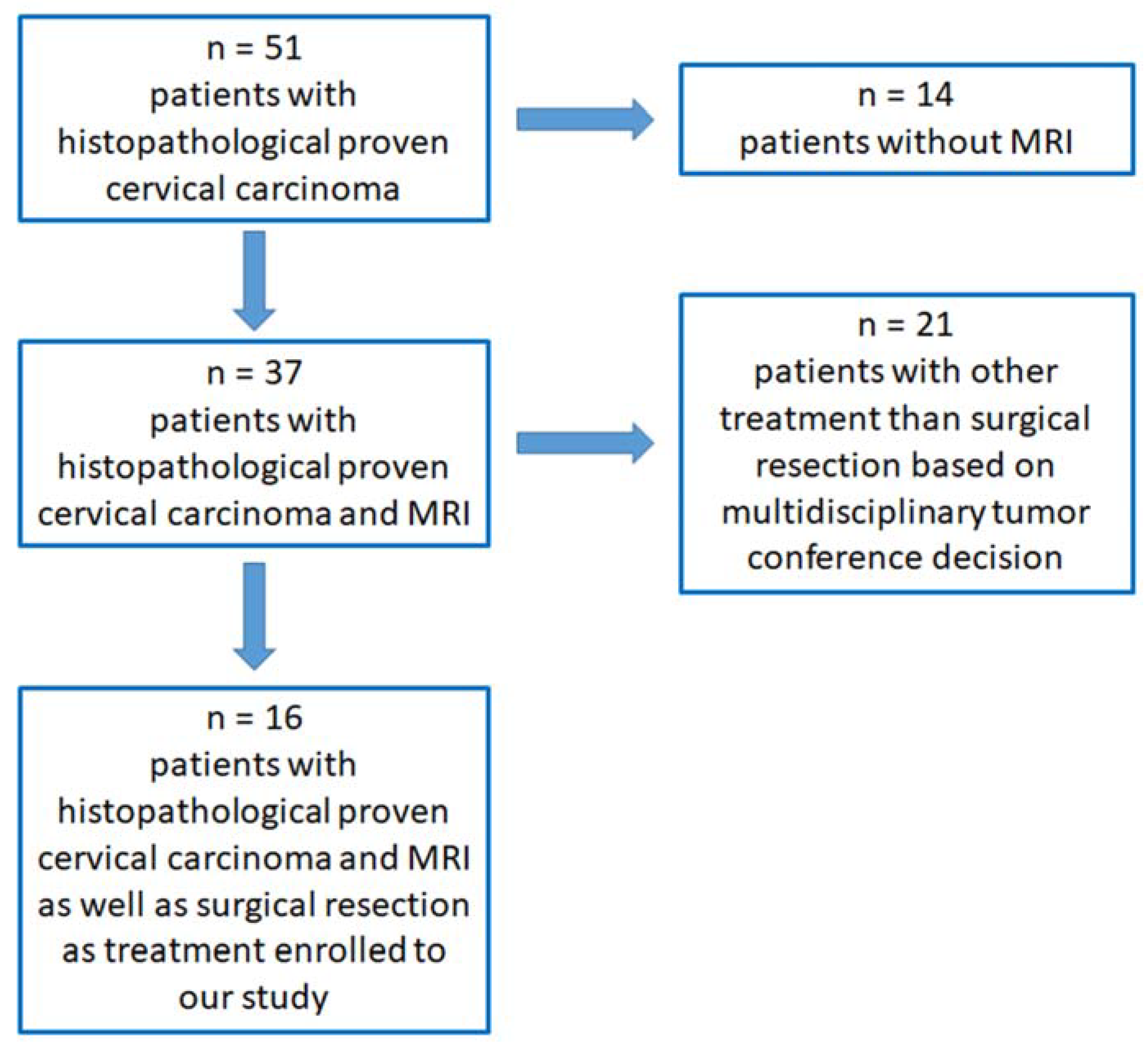
2.1. Study Design, Subjects and Variables
2.2. Patient Selection, Inclusion and Exclusion Criteria
2.3. MRI Examination
- (1)
- Coronal STIR (short tau inversion recovery; repetition time (TR) 5328, echo time (TE) 50 ms and flip angle (FA) 90°) with a slice thickness of 4 mm, section gap of 1 mm, field of view (FOV) of 300 × 431 × 189 mm, voxel size (VS) 1.00 × 1.48 × 1.00 mm and a scan time of approximately 4 min 26 s.
- (2)
- Sagittal T2-weighted scans without fat saturation (turbo spin echo; TR 3744 ms, TE 90 ms and FA 90°) and with a slice thickness of 4 mm, section gap of 1 mm, FOV of 300 × 300 × 180 mm, VS 0.9 × 1.21 × 4.00 mm and a scan time of approximately 2 min 55 s.
- (3)
- Transversal T1-weighted scans with fat saturation (turbo spin echo spectral pre-saturation with inversion recovery; TR 704 ms, TE 6 ms and FA 90°) with a slice thickness of 4 mm, section gap of 1 mm, FOV of 285 × 461 × 220 mm, VS 0.94 × 1.19 × 4.00 mm and a scan time of approximately 8 min 30 s.
- (4)
- Transversal T2-weighted scans with fat saturation (turbo spin echo spectral attenuated inversion recovery; TR 3744 ms, TE 90 ms and FA 90°) with a slice thickness of 4 mm, section gap of 1 mm, FOV of 255 × 396 × 219 mm, VS 1.14 × 1.44 × 4.00 mm and a scan time of approximately 6 min 55 s.
- (5)
- DWIBS in the axial plane with the following parameters: TR 9795 ms, TE 180 ms, FA 90°, FOV 280 mm × 400 × 276 mm, VS 3.04 × 3.00 × 4.00 mm, slice thickness 4 mm, no section gap, b value 0 and 1000 s/mm2 and a scan time of approximately 3 min 25 s.
- (6)
- Contrast enhanced transversal T1-weighted scans with fat saturation (turbo spin echo spectral pre-saturation with inversion recovery; TR 704 ms, TE 6 ms and FA 90°) with a slice thickness of 4 mm, section gap of 1 mm, FOV of 285 × 461 × 220 mm, VS 0.94 × 1.19 × 4.00 mm and a scan time of approximately 8 min 30 s.
- (7)
- Contrast enhanced sagittal T1-weighted scans with fat saturation (turbo spin echo spectral pre-saturation with inversion recovery; TR 692 ms, TE 8 ms and FA 90°) with a slice thickness of 4 mm, section gap of 1 mm, FOV of 289 × 406 × 257 mm, VS 1.13 × 1.36 × 5.00 mm and a scan time of approximately 2 min 30 s.
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
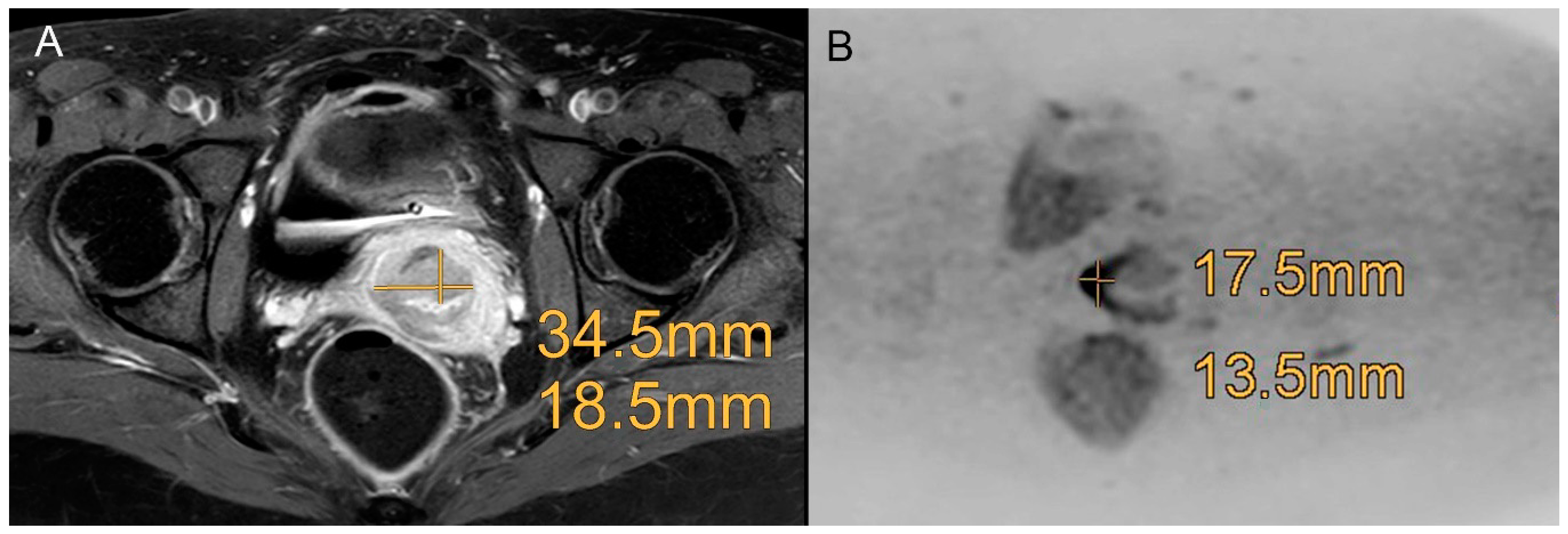
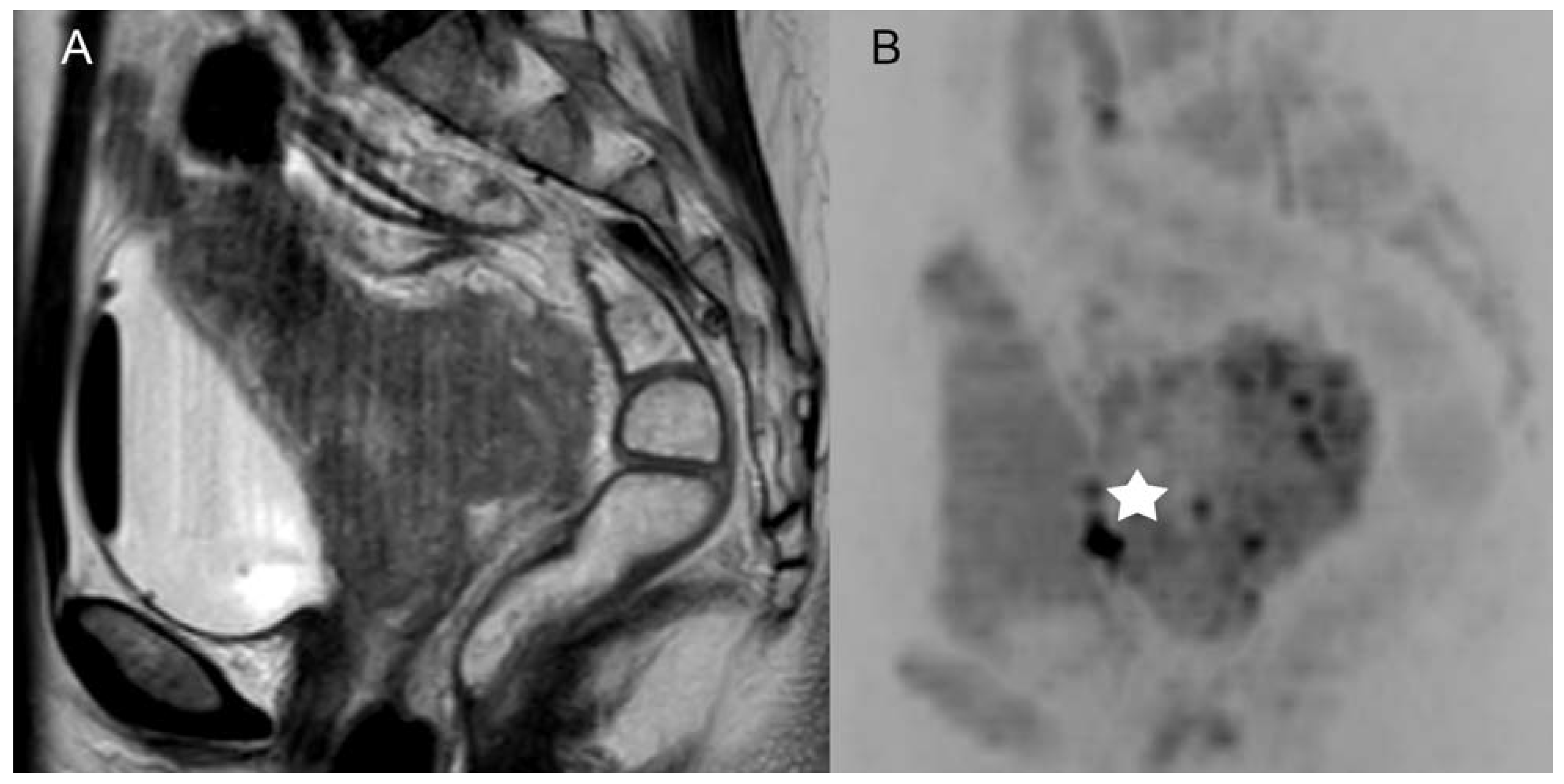
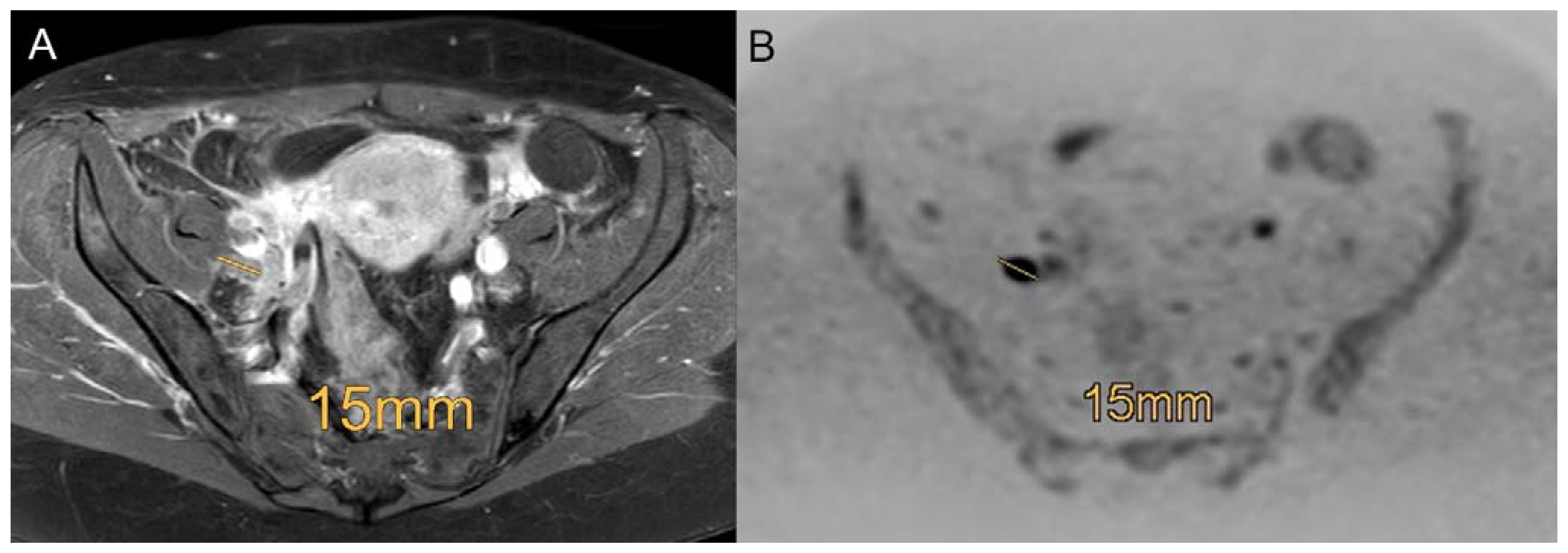
3.1. Diagnostic Efficacy of Standard MRI and MRI + DWIBS
3.2. Interobserver Reliability Using Standard MRI and MRI + DWIBS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagner-Larsen, K.S.; Lura, N.; Salvesen, Ø.; Halle, M.K.; Forsse, D.; Trovik, J.; Smit, N.; Krakstad, C.; Haldorsen, I.S. Interobserver agreement and prognostic impact for MRI–based 2018 FIGO staging parameters in uterine cervical cancer. Eur. Radiol. 2022, 32, 6444–6455. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; .Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. S2), 22–36. [Google Scholar] [CrossRef]
- Wright, J.D.; Matsuo, K.; Huang, Y.; Tergas, A.I.; Hou, J.Y.; Khoury-Collado, F.; Clair, C.M.S.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L. Prognostic Performance of the 2018 International Federation of Gynecology and Obstetrics Cervical Cancer Staging Guidelines. Obstet. Gynecol. 2019, 134, 49–57. [Google Scholar] [CrossRef]
- de Gregorio, A.; Widschwendter, P.; Ebner, F.; Friedl, T.W.P.; Huober, J.; Janni, W.; de Gregorio, N. Influence of the New FIGO Classification for Cervical Cancer on Patient Survival: A Retrospective Analysis of 265 Histologically Confirmed Cases with FIGO Stages IA to IIB. Oncology 2020, 98, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Machida, H.; Mandelbaum, R.S.; Konishi, I.; Mikami, M. Validation of the 2018 FIGO cervical cancer staging system. Gynecol. Oncol. 2019, 152, 87–93. [Google Scholar] [CrossRef]
- Wagner, A.E.; Pappas, L.; Ghia, A.J.; Gaffney, D.K. Impact of tumor size on survival in cancer of the cervix and validation of stage IIA1 and IIA2 subdivisions. Gynecol. Oncol. 2013, 129, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, S.W.; Kim, J.R.; Kim, Y.S.; Yoon, M.S.; Jeong, S.; Kim, J.H.; Lee, J.Y.; Eom, K.Y.; Jeong, B.K.; et al. Tumour size, volume, and marker expression during radiation therapy can predict survival of cervical cancer patients: A multi-institutional retrospective analysis of KROG 16-01. Gynecol. Oncol. 2017, 147, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Sun, C.C.; Schmeler, K.M.; Deavers, M.T.; dos Reis, R.; Levenback, C.F.; Ramirez, P.T. Parametrial involvement in radical hysterectomy specimens for women with early-stage cervical cancer. Obstet. Gynecol. 2009, 114, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Canaz, E.; Ozyurek, E.S.; Erdem, B.; Talmac, M.A.; Ozaydin, I.Y.; Akbayir, O.; Numanoglu, C.; Ulker, V. Preoperatively Assessable Clinical and Pathological Risk Factors for Parametrial Involvement in Surgically Treated FIGO Stage IB-IIA Cervical Cancer. Int. J. Gynecol. Cancer 2017, 27, 1722–1728. [Google Scholar] [CrossRef]
- Koyama, T.; Tamai, K.; Togashi, K. Staging of carcinoma of the uterine cervix and endometrium. Eur. Radiol. 2007, 17, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E. Cancer statistics, 2006. CA Cancer J. Clin. 2006, 56, 106–130. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, L.; Lakhman, Y.; Bharwani, N.; Gui, B.; Gigli, S.; Vinci, V.; Rizzo, S.; Kido, A.; Cunha, T.M.; Sala, E.; et al. Staging, recurrence and follow-up of uterine cervical cancer using MRI: Updated Guidelines of the European Society of Urogenital Radiology after revised FIGO staging 2018. Eur. Radiol. 2021, 31, 7802–7816. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother. Oncol. 2018, 127, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Thomeer, M.G.; Gerestein, C.; Spronk, S.; van Doorn, H.C.; van der Ham, E.; Hunink, M.G. Clinical examination versus magnetic resonance imaging in the pretreatment staging of cervical carcinoma: Systematic review and meta-analysis. Eur. Radiol. 2013, 23, 2005–2018. [Google Scholar] [CrossRef]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Magnetic resonance imaging for detection of parametrial invasion in cervical cancer: An updated systematic review and meta-analysis of the literature between 2012 and 2016. Eur. Radiol. 2017, 28, 530–541. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Yang, J.; Xue, H.; Cao, D.; Huang, H.; Wu, M.; Cui, Q.; Chen, J.; Lang, J.; et al. The role of magnetic resonance imaging in pretreatment evaluation of early-stage cervical cancer. Int. J. Gynecol. Cancer 2014, 24, 1292–1298. [Google Scholar] [CrossRef]
- Balcacer, P.; Shergill, A.; Litkouhi, B. MRI of cervical cancer with a surgical perspective: Staging, prognostic implications and pitfalls. Abdom. Radiol. 2019, 44, 2557–2571. [Google Scholar] [CrossRef]
- Van Vierzen, P.B.; Massuger, L.F.; Ruys, S.H.; Barentsz, J.O. Fast dynamic contrast enhanced MR imaging of cervical carcinoma. Clin. Radiol. 1998, 53, 183–192. [Google Scholar] [CrossRef]
- Becker, M.; Monnier, Y.; de Vito, C. MR Imaging of Laryngeal and Hypopharyngeal Cancer. Magn. Reson. Imaging Clin. N. Am. 2022, 30, 53–72. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, D.; Ye, W.; Long, L.; Lu, Y.; Wei, Y. Diffusion-weighted imaging with background body signal suppression (DWIBS) distinguishes benign lesions from malignant pulmonary solitary lesions. Am. J. Transl. Res. 2021, 13, 88–101. [Google Scholar] [PubMed]
- Takahara, T.; Imai, Y.; Yamashita, T.; Yasuda, S.; Nasu, S.; Van Cauteren, M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): Technical improvement using free breathing, STIR and high resolution 3D display. Radiat. Med. 2004, 22, 275–282. [Google Scholar] [PubMed]
- Sun, M.; Cheng, J.; Zhang, Y.; Bai, J.; Wang, F.; Meng, Y.; Li, Z. Application of DWIBS in malignant lymphoma: Correlation between ADC values and Ki-67 index. Eur. Radiol. 2017, 28, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Balaji, R.; Devi, R.; Stumpf, J. Diffusion-Weighted Whole-Body Imaging with Background Body Signal Suppression (DWIBS)—Application in Planning for Cyberknife Therapy in Patients With Gliomas. Pract. Radiat. Oncol. 2013, 3 (Suppl S1), S35. [Google Scholar] [CrossRef] [PubMed]
- Kumasaka, S.; Motegi, S.; Kumasaka, Y.; Nishikata, T.; Otomo, M.; Tsushima, Y. Whole-body magnetic resonance imaging (WB-MRI) with diffusion-weighted whole-body imaging with background body signal suppression (DWIBS) in prostate cancer: Prevalence and clinical significance of incidental findings. Br. J. Radiol. 2022, 95, 20210459. [Google Scholar] [CrossRef] [PubMed]
- Ishiguchi, H.; Ito, S.; Kato, K.; Sakurai, Y.; Kawai, H.; Fujita, N.; Abe, S.; Narita, A.; Nishio, N.; Muramatsu, H.; et al. Diagnostic performance of 18F-FDG PET/CT and whole-body diffusion-weighted imaging with background body suppression (DWIBS) in detection of lymph node and bone metastases from pediatric neuroblastoma. Ann. Nucl. Med. 2018, 32, 348–362. [Google Scholar] [CrossRef]
- Larsen, S.K.A.; Løgager, V.; Bylov, C.; Nellemann, H.; Agerbæk, M.; Als, A.B.; Pedersen, E.M. Can whole-body MRI replace CT in management of metastatic testicular cancer? A prospective, non-inferiority study. J. Cancer Res. Clin. Oncol. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Schicho, A.; Habicher, W.; Wendl, C.; Stroszczynski, C.; Strotzer, Q.; Dollinger, M.; Schreyer, A.G.; Schleder, S. Clinical Value of Diffusion-Weighted Whole-Body Imaging with Background Body Signal Suppression (DWIBS) for Staging of Patients with Suspected Head and Neck Cancer. Tomography 2022, 8, 2522–2532. [Google Scholar] [CrossRef]
- Schleder, S.; May, M.; Habicher, W.; Dinkel, J.; Schreyer, A.G.; Gostian, A.-O.; Schicho, A. Additional Diffusion-Weighted Imaging with Background Body Signal Suppression (DWIBS) Improves Pre-Therapeutical Detection of Early-Stage (pT1a) Glottic Cancer: A Feasibility and Interobserver Reliability Study. Diagnostics 2022, 12, 3200. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, S.H.; Choi, H.J.; Park, B.K.; Lee, H.J. Preoperative magnetic resonance imaging staging of uterine cervical carcinoma: Results of prospective study. J. Comput. Assist. Tomogr. 2004, 28, 620–627. [Google Scholar] [CrossRef]
- Brennan, P.; Silman, A. Statistical methods for assessing observer variability in clinical measures. BMJ 1992, 304, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Pálsdóttir, K.; Fridsten, S.; Blomqvist, L.; Alagic, Z.; Fischerova, D.; Gaurilcikas, A.; Hasselrot, K.; Jäderling, F.; Testa, A.C.; Sundin, A.; et al. Interobserver agreement of transvaginal ultrasound and magnetic resonance imaging in local staging of cervical cancer. Ultrasound Obstet. Gynecol. 2021, 58, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Diagnostik, Therapie und Nachsorge der Patientin mit Zervixkarzinom, Langversion, 2.2, 2022, AWMF-Registernummer: 032/033OL. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/zervixkarzinom/ (accessed on 7 October 2022).
- Liu, B.; Gao, S.; Li, S. A Comprehensive Comparison of CT, MRI, Positron Emission Tomography or Positron Emission Tomography/CT, and Diffusion Weighted Imaging-MRI for Detecting the Lymph Nodes Metastases in Patients with Cervical Cancer: A Meta-Analysis Based on 67 Studies. Gynecol. Obstet. Investig. 2017, 82, 209–222. [Google Scholar] [CrossRef]
- Testa, A.C.; Di Legge, A.; De Blasis, I.; Moruzzi, M.C.; Bonatti, M.; Collarino, A.; Rufini, V.; Manfredi, R. Imaging techniques for the evaluation of cervical cancer. Best Pract Res. Clin. Obstet. Gynaecol. 2014, 28, 741–768. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Testa, A.; Gaurilcikas, A.; Di Legge, A.; Ameye, L.; Atstupenaite, V.; Valentini, A.L.; Gui, B.; Wallengren, N.-O.; Pudaric, S.; et al. Early-stage cervical cancer: Tumor delineation by magnetic resonance imaging and ultrasound—A European multicenter trial. Gynecol. Oncol. 2013, 128, 449–453. [Google Scholar] [CrossRef]
| Abbreviation Used | Histopathological Category Analyzed by Both Readers | Associated FIGO 3 2018 Category |
|---|---|---|
| TS 1 < 2 cm | invasive carcinoma ≤2 cm in greatest dimension (tumor size) | at least stage IB1 |
| TS 1 2–4 cm | invasive carcinoma >2 cm and ≤4 cm in greatest dimension (tumor size) | at least stage IB2 |
| TS 1 > 4 cm | invasive carcinoma >4 cm in greatest dimension (tumor size) | at least stage IB3 |
| parametrial infiltration | with parametrial involvement but not up to the pelvic wall | at least stage IIB |
| infiltration of vaginal lower third | the carcinoma involves the lower third of the vagina | at least stage III |
| infiltration of adjacent organs | the carcinoma has extended beyond the true pelvis or has involved (biopsy proven) the mucosa of the bladder or rectum. | stage IV |
| loco-regional LN 2+ | involvement of pelvic and/or para-aortic lymph nodes | at least stage IIIC1 |
| Patient Number | Age | TNM 1 | Histol. FIGO 4 | MRI 2 Reader 1-rFIGO 5 | MRI 2 + DWIBS 3 Reader 1–rFIGO 5 | MRI 2 Reader 2–rFIGO 5 | MRI2 + DWIBS 3 Reader 2–rFIGO 5 |
|---|---|---|---|---|---|---|---|
| 1 | 44 | pT2a2 pN1 cM0 | IIIC1 | IIIC1 | IIIC1 | IIIC1 | IIIC1 |
| 2 | 57 | pT1b1 pN1 cM0 | IIIC1 | IIIC1 | IIIC1 | IIIC1 | IIIC1 |
| 3 | 59 | pT2a pN0 cM0 | IIA2 | IIA2 | IIA2 | IIA2 | IIA2 |
| 4 | 33 | pT2b pN0 cM0 | IIB | IIIA | IIA2 | IIIA | IIA2 |
| 5 | 37 | pT2b pN0 cM0 | IIB | IIA2 | IIA2 | IIIA | IIA2 |
| 6 | 43 | pT1b1 pN0 cM0 | IB1 | IB1 | IB1 | IB1 | IB1 |
| 7 | 57 | pT4 pN1 cM0 | IVA | IVA | IVA | IVA | IVA |
| 8 | 62 | pT2a1 pN0 cM0 | IIA1 | IIA1 | IIA1 | IIA2 | IIA2 |
| 9 | 42 | pT1b1 pN1 cM0 | IB1 | IB1 | IB1 | IB1 | IB1 |
| 10 | 60 | pT1b1 pN0 cM0 | IB1 | no tumor | no tumor | no tumor | no tumor |
| 11 | 34 | pT1b2 pN0 cM0 | IB2 | IB2 | IB2 | IB2 | IB3 |
| 12 | 46 | pT2b pN0 cM0 | IIIA | IIIA | IIIA | IIA2 | IIIA |
| 13 | 37 | pT1b1 pN0 cM0 | IB1 | IB2 | IB1 | IB2 | IB1 |
| 14 | 37 | pT4 pN1 cM0 | IVA | IVA | IVA | IVA | IVA |
| 15 | 42 | pT1b1 pN0 cM0 | IB1 | no tumor | no tumor | no tumor | no tumor |
| 16 | 72 | pT1b1 pN0 cM0 | IB1 | IB1 | IB1 | IB1 | IB1 |
| Histopathological Category | Cohen´s Kappa (95%-CI 1) for Standard MRI 2 | Cohen´s Kappa (95%-CI 1) for MRI 2 with DWIBS 3 |
|---|---|---|
| TS 4 < 2 cm | k = 1.00 (1.00–1.00) | k = 1.00 (1.00–1.00) |
| TS 4 2–4 cm | k = 0.75 (0.44–1.00) | k = 0.59 (0.08–1.00) |
| TS 4 > 4 cm | k = 0.67 (0.27–1.00) | k = 0.71 (0.34–1.00) |
| parametrial infiltration | k = 1.00 (1.00–1.00) | k = 0.43 (0.08–1.00) |
| infiltration of vaginal lower third | k = 0.81 (0.61–1.00) | k = 1.00 (1.00–1.00) |
| infiltration of adjacent organs | k = 1.00 (1.00–1.00) | k = 1.00 (1.00–1.00) |
| loco-regional LN 5+ | k = 1.00 (1.00–1.00) | k = 1.00 (1.00–1.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schleder, S.; May, M.; Scholz, C.; Dinkel, J.; Strotzer, Q.; Einspieler, I.; Dollinger, M.; Schreyer, A.G.; Grassinger, J.; Schicho, A. Diagnostic Value of Diffusion-Weighted Imaging with Background Body Signal Suppression (DWIBS) for the Pre-Therapeutic Loco-Regional Staging of Cervical Cancer: A Feasibility and Interobserver Reliability Study. Curr. Oncol. 2023, 30, 1164-1173. https://doi.org/10.3390/curroncol30010089
Schleder S, May M, Scholz C, Dinkel J, Strotzer Q, Einspieler I, Dollinger M, Schreyer AG, Grassinger J, Schicho A. Diagnostic Value of Diffusion-Weighted Imaging with Background Body Signal Suppression (DWIBS) for the Pre-Therapeutic Loco-Regional Staging of Cervical Cancer: A Feasibility and Interobserver Reliability Study. Current Oncology. 2023; 30(1):1164-1173. https://doi.org/10.3390/curroncol30010089
Chicago/Turabian StyleSchleder, Stephan, Matthias May, Carsten Scholz, Johannes Dinkel, Quirin Strotzer, Ingo Einspieler, Marco Dollinger, Andreas G. Schreyer, Jochen Grassinger, and Andreas Schicho. 2023. "Diagnostic Value of Diffusion-Weighted Imaging with Background Body Signal Suppression (DWIBS) for the Pre-Therapeutic Loco-Regional Staging of Cervical Cancer: A Feasibility and Interobserver Reliability Study" Current Oncology 30, no. 1: 1164-1173. https://doi.org/10.3390/curroncol30010089
APA StyleSchleder, S., May, M., Scholz, C., Dinkel, J., Strotzer, Q., Einspieler, I., Dollinger, M., Schreyer, A. G., Grassinger, J., & Schicho, A. (2023). Diagnostic Value of Diffusion-Weighted Imaging with Background Body Signal Suppression (DWIBS) for the Pre-Therapeutic Loco-Regional Staging of Cervical Cancer: A Feasibility and Interobserver Reliability Study. Current Oncology, 30(1), 1164-1173. https://doi.org/10.3390/curroncol30010089







