The Evidence Surrounding Non-Alcoholic Fatty Liver Disease in Individuals with Cancer: A Systematic Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Quality Assessment and Risk of Bias
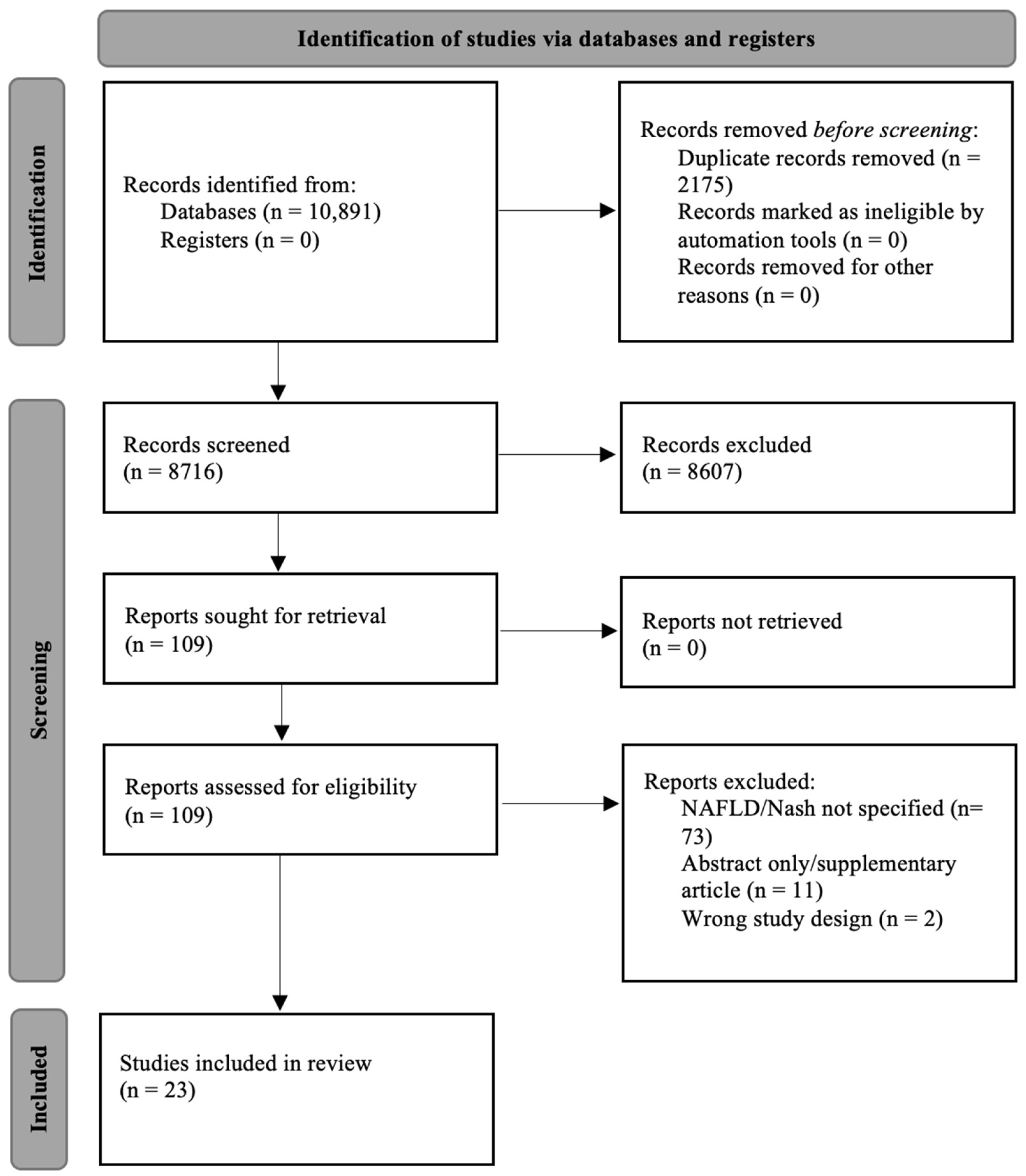
3. Results
3.1. Study Characteristics and Qualitative Assessment
3.2. The Effect of Cancer Treatment on NAFLD
- Chemotherapy and NAFLD
- ii
- Hormone therapy and NAFLD
- iii
- Surgery and NAFLD
3.3. Survival and Mortality
3.4. Metabolic Co-Morbidities
3.5. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
Search Strategy
- ‘survivor’ OR ‘survivors’ OR ‘patient’ OR ‘rehabilitation’ AND
- ‘cancer’ OR ‘cancer[MESH terms]’ OR ‘neoplasms[MESH terms]’) OR ‘breast cancer’ OR ‘colorectal cancer’ OR ‘prostate cancer’ OR ‘hepatocellular cancer’ OR ‘liver cancer’ OR ‘gastrointestinal cancer’ OR ‘gastric cancer’ OR ‘endometrial cancer’ OR ‘ovarian cancer’ OR ‘renal cancer’ OR ‘kidney cancer’ OR ‘hepatic cancer’ OR ‘genitourinary cancer’ OR ‘gynaecologic cancer’ OR ‘lung cancer’ OR ‘hematologic cancer’ OR ‘bladder cancer’ OR ‘rectal cancer’ OR ‘leukemia’ OR ‘lung cancer’ OR ‘pancreatic cancer’ OR ‘thyroid cancer’ OR ‘osteosarcoma’ OR ‘nasopharyngeal cancer’ OR ‘cervical cancer’ OR ‘nasopharyngeal cancer’ OR ‘skin cancer’ AND
- ‘nafld’ OR ‘nash’ OR ‘fatty liver’ OR ‘liver disease’ OR ‘non-alcoholic fatty liver’ OR ‘nonalcoholic fatty liver’ OR ‘non-alcoholic steatosis’ OR ‘nonalcoholic steatosis’ OR ‘steatosis’ OR ‘mafld’ OR ‘metabolic associated fatty liver’ OR ‘metabolic-associated fatty liver’.
References
- Perumpail, B.J.; Khan, M.A.; Yoo, E.R.; Cholankeril, G.; Kim, D.; Ahmed, A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 8263. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Roberts, S.K.; Strasser, S.I.; Mahady, S.E.; Powell, E.; Estes, C.; Razavi, H.; George, J. Nonalcoholic fatty liver disease burden: Australia, 2019–2030. J. Gastroenterol. Hepatol. 2020, 35, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zheng, L.; Wang, M.; Du, Y.; Jiang, J. Prevalence trends in non-alcoholic fatty liver disease at the global, regional and national levels, 1990–2017: A population-based observational study. BMJ Open 2020, 10, e036663. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y. Recent advances in nonalcoholic fatty liver disease metabolomics. Clin. Mol. Hepatol. 2021, 27, 553. [Google Scholar] [CrossRef]
- Almeda-Valdés, P.; Cuevas-Ramos, D.; Aguilar-Salinas, C.A. Metabolic syndrome and non-alcoholic fatty liver disease. Ann. Hepatol. 2009, 8, 18–24. [Google Scholar] [CrossRef]
- Kang, S.H.; Lee, H.W.; Yoo, J.-J.; Cho, Y.; Kim, S.U.; Lee, T.H.; Jang, B.K.; Kim, S.G.; Ahn, S.B.; Kim, H. KASL clinical practice guidelines: Management of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2021, 27, 363. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Sanna, C.; Rosso, C.; Marietti, M.; Bugianesi, E. Non-alcoholic fatty liver disease and extra-hepatic cancers. Int. J. Mol. Sci. 2016, 17, 717. [Google Scholar] [CrossRef]
- AIHW. Cancer in Australia 2019; Australian Institute of Health and Welfare: Sydney, NSW, Australia, 2021; 174p. [CrossRef]
- 5-Year Relative Survival. Available online: https://ncci.canceraustralia.gov.au/outcomes/relative-survival-rate/5-year-relative-survival (accessed on 16 May 2022).
- Matthews, A.A.; Hinton, S.P.; Stanway, S.; Lyon, A.R.; Smeeth, L.; Lund, J.L.; Bhaskaran, K. Endocrine therapy use and cardiovascular risk in postmenopausal breast cancer survivors. Heart 2021, 107, 1327–1335. [Google Scholar] [CrossRef]
- Allen, A.M.; Hicks, S.B.; Mara, K.C.; Larson, J.J.; Therneau, T.M. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity—A longitudinal cohort study. J. Hepatol. 2019, 71, 1229–1236. [Google Scholar] [CrossRef]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared risk factors in cardiovascular disease and cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef]
- Maor, Y.; Malnick, S. Liver injury induced by anticancer chemotherapy and radiation therapy. Int. J. Hepatol. 2013, 2013, 815105. [Google Scholar] [CrossRef]
- Nuver, J.; Smit, A.J.; Postma, A.; Sleijfer, D.T.; Gietema, J.A. The metabolic syndrome in long-term cancer survivors, and important target for secondary preventive measures. Cancer Treat. Rev. 2002, 28, 195–214. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Cancer Types. Available online: https://www.cancer.gov/types/common-cancers (accessed on 7 June 2022).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA: A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Academy of Nutrition and Dietetics. Academy of Nutrition and Dietetics Evidence Analysis Library Quality Criteria Checklist. Available online: https://www.andeal.org/evidence-analysis-manual (accessed on 6 May 2022).
- Sterne, J.; Page, M.; Elbers, R.; Blencowe, N.; Boutron, I.; Cates, C.; Cheng, H.-Y.; Corbett, M.; Eldridge, S.; Hernán, M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. Nonalcoholic fatty liver disease and mortality among cancer survivors. Cancer Epidemiol. 2017, 48, 104–109. [Google Scholar] [CrossRef]
- Molla, N.W.; Hassanain, M.M.; Fadel, Z.; Boucher, L.M.; Madkhali, A.; Altahan, R.M.; Alrijraji, E.A.; Simoneau, E.B.; Alamri, H.; Salman, A.; et al. Effect of non-alcoholic liver disease on recurrence rate and liver regeneration after liver resection for colorectal liver metastases. Curr. Oncol. 2017, 24, e233–e243. [Google Scholar] [CrossRef][Green Version]
- Wu, K.; Zhai, M.Z.; Weltzien, E.K.; Cespedes Feliciano, E.M.; Meyerhardt, J.A.; Giovannucci, E.; Caan, B.J. Non-alcoholic fatty liver disease and colorectal cancer survival. Cancer Causes Control 2019, 30, 165–168. [Google Scholar] [CrossRef]
- Ariizumi, S.-i.; Kotera, Y.; Katagiri, S.; Nakano, M.; Nakanuma, Y.; Saito, A.; Yamamoto, M. Long-term survival of patients with cholangiolocellular carcinoma after curative hepatectomy. Ann. Surg. Oncol. 2014, 21, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-T.; Pan, H.-J.; Lee, C.-H. Prevention of tamoxifen-related nonalcoholic fatty liver disease in breast cancer patients. Clin. Breast Cancer 2018, 18, e677–e685. [Google Scholar] [CrossRef] [PubMed]
- Montomoli, J.; Erichsen, R.; Christiansen, C.F.; Ulrichsen, S.P.; Pedersen, L.; Nilsson, T.; Sørensen, H.T. Liver disease and 30-day mortality after colorectal cancer surgery: A Danish population-based cohort study. BMC Gastroenterol. 2013, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Gild, P.; Cole, A.P.; Krasnova, A.; Dickerman, B.A.; von Landenberg, N.; Sun, M.; Mucci, L.A.; Lipsitz, S.R.; Chun, F.K.-H.; Nguyen, P.L. Liver disease in men undergoing androgen deprivation therapy for prostate cancer. J. Urol. 2018, 200, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Rei, J.; Jamias, J.D. Prognostic features, treatment outcomes and survival of hepatocellular carcinoma patients in National Kidney and Transplant Institute. Phillippine J. Intern. Med. 2016, 31, 425. [Google Scholar]
- Mehta, N.; Fidelman, N.; Sarkar, M.; Yao, F.Y. Factors associated with outcomes and response to therapy in patients with infiltrative hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2013, 11, 572–578. [Google Scholar] [CrossRef]
- Pan, H.-J.; Chang, H.-T.; Lee, C.-H. Association between tamoxifen treatment and the development of different stages of nonalcoholic fatty liver disease among breast cancer patients. J. Formos. Med. Assoc. 2016, 115, 411–417. [Google Scholar] [CrossRef]
- Lee, J.I.; Yu, J.H.; Anh, S.G.; Lee, H.W.; Jeong, J.; Lee, K.S. Aromatase Inhibitors and Newly Developed Nonalcoholic Fatty Liver Disease in Postmenopausal Patients with Early Breast Cancer: A Propensity Score-Matched Cohort Study. Oncologist 2019, 24, e653. [Google Scholar] [CrossRef]
- Lee, S.; Jung, Y.; Bae, Y.; Yun, S.P.; Kim, S.; Jo, H.; Seo, H.I. Prevalence and risk factors of nonalcoholic fatty liver disease in breast cancer patients. Tumori 2017, 103, 187–192. [Google Scholar] [CrossRef]
- Hoffmann, A.; Bootsveld, K.; Gebhardt, U.; Daubenbüchel, A.M.; Sterkenburg, A.S.; Müller, H.L. Nonalcoholic fatty liver disease and fatigue in long-term survivors of childhood-onset craniopharyngioma. Eur. J. Endocrinol. 2015, 173, 389–397. [Google Scholar] [CrossRef]
- Izadpanahi, P.; Mohammadifard, M.; Tavakoli, T.; Abbasi, N.; Javadinia, S.A. Effect of chemotherapy on fatty liver occurrence in breast and gastrointestinal cancer patients: A case-controlled study. Hepat. Mon. 2020, 20, e97986. [Google Scholar] [CrossRef]
- Golabi, P.; Fazel, S.; Otgonsuren, M.; Sayiner, M.; Locklear, C.T.; Younossi, Z.M. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine 2017, 96, e5904. [Google Scholar] [CrossRef]
- Nseir, W.; Abu-Rahmeh, Z.; Tsipis, A.; Mograbi, J.; Mahamid, M. Relationship between Non-Alcoholic Fatty Liver Disease and Breast Cancer. Isr. Med. Assoc. J. IMAJ 2017, 19, 242–245. [Google Scholar]
- Bilici, A.; Ozguroglu, M.; Mihmanli, I.; Turna, H.; Adaletli, I. A case-control study of non-alcoholic fatty liver disease in breast cancer. Med. Oncol. 2007, 24, 367–371. [Google Scholar] [CrossRef]
- Aktas, E.; Uzman, M.; Yildirim, O.; Sahin, B.; Buyukcam, F.; Aktas, B.; Yilmaz, B.; Yildirim, A.M.; Basyigit, S.; Yeniova, O. Assessment of hepatic steatosis on contrast enhanced computed tomography in patients with colorectal cancer. Int. J. Clin. Exp. Med. 2014, 7, 4342. [Google Scholar]
- Kouzu, K.; Tsujimoto, H.; Nishikawa, M.; Harada, M.; Sugihara, T.; Nagata, H.; Hiraki, S.; Yaguchi, Y.; Takahata, R.; Nomura, S.; et al. Risk factors for nonalcoholic fatty liver disease after gastrectomy for gastric cancer. Gastric Cancer 2020, 23, 356–362. [Google Scholar] [CrossRef]
- Moeini, A.; Machida, H.; Takiuchi, T.; Blake, E.A.; Hom, M.S.; Miki, T.; Matsuo, O.; Matsuo, K. Association of Nonalcoholic Fatty Liver Disease and Venous Thromboembolism in Women with Endometrial Cancer. Clin. Appl. Thromb./Hemost. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Matsuo, K.; Gualtieri, M.R.; Cahoon, S.S.; Jung, C.E.; Paulson, R.J.; Shoupe, D.; Muderspach, L.I.; Wakatsuki, A.; Wright, J.D.; Roman, L.D. Surgical menopause and increased risk of nonalcoholic fatty liver disease in endometrial cancer. Menopause 2016, 23, 189. [Google Scholar] [CrossRef]
- Nemoto, Y.; Saibara, T.; Ogawa, Y.; Zhang, T.; Xu, N.; Ono, M.; Akisawa, N.; Iwasaki, S.; Maeda, T.; Onishi, S. Tamoxifen-induced nonalcoholic steatohepatitis in breast cancer patients treated with adjuvant tamoxifen. Intern. Med. 2002, 41, 345–350. [Google Scholar] [CrossRef]
- Bruno, S.; Maisonneuve, P.; Castellana, P.; Rotmensz, N.; Rossi, S.; Maggioni, M.; Persico, M.; Colombo, A.; Monasterolo, F.; Casadei-Giunchi, D. Incidence and risk factors for non-alcoholic steatohepatitis: Prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ 2005, 330, 932. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Loomba, R.; Anstee, Q.M.; Rinella, M.E.; Bugianesi, E.; Marchesini, G.; Neuschwander-Tetri, B.A.; Serfaty, L.; Negro, F.; Caldwell, S.H. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology 2018, 68, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Chitturi, S.; Farrell, G.C. Etiopathogenesis of nonalcoholic steatohepatitis. Semin. Liver Dis. 2001, 21, 027–042. [Google Scholar] [CrossRef] [PubMed]
- Peppercorn, P.; Reznek, R.; Wilson, P.; Slevin, M.; Gupta, R. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil-based therapy for advanced colorectal cancer. Br. J. Cancer 1998, 77, 2008–2011. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Maisonneuve, P.; Costa, A.; Sacchini, V.; Maltoni, C.; Robertson, C.; Rotmensz, N.; Boyle, P. Prevention of breast cancer with tamoxifen: Preliminary findings from the Italian randomised trial among hysterectomised women. Lancet 1998, 352, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U. Prevention of breast cancer with tamoxifen: The Italian study in hysterectomized women. Breast 1995, 4, 267–272. [Google Scholar] [CrossRef]
- Uzunlulu, M.; Caklili, O.T.; Oguz, A. Association between metabolic syndrome and cancer. Ann. Nutr. Metab. 2016, 68, 173–179. [Google Scholar] [CrossRef]
- Lei, L.; EI Mourabit, H.; Housset, C.; Cadoret, A.; Lemoinne, S. Role of Angiogenesis in the Pathogenesis of NAFLD. J. Clin. Med. 2021, 10, 1338. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef]
- Casco, S.; Soto-Vega, E. Development of metabolic syndrome associated to cancer therapy. Horm. Cancer 2016, 7, 289–295. [Google Scholar] [CrossRef]
- Bellastella, G.; Scappaticcio, L.; Esposito, K.; Giugliano, D.; Maiorino, M.I. Metabolic syndrome and cancer: “The common soil hypothesis”. Diabetes Res. Clin. Pract. 2018, 143, 389–397. [Google Scholar] [CrossRef]
- Makari-Judson, G.; Braun, B.; Jerry, D.J.; Mertens, W.C. Weight gain following breast cancer diagnosis: Implication and proposed mechanisms. World J. Clin. Oncol. 2014, 5, 272. [Google Scholar] [CrossRef]
- Gadea, E.; Thivat, E.; Planchat, E.; Morio, B.; Durando, X. Importance of metabolic changes induced by chemotherapy on prognosis of early-stage breast cancer patients: A review of potential mechanisms. Obes. Rev. 2012, 13, 368–380. [Google Scholar] [CrossRef]
- Sharma, A.; Houshyar, R.; Bhosale, P.; Choi, J.-I.; Gulati, R.; Lall, C. Chemotherapy induced liver abnormalities: An imaging perspective. Clin. Mol. Hepatol. 2014, 20, 317. [Google Scholar] [CrossRef]
- Senior, J.R. Unintended hepatic adverse events associated with cancer chemotherapy. Toxicol. Pathol. 2010, 38, 142–147. [Google Scholar] [CrossRef]
- Grigorian, A.; O’Brien, C.B. Hepatotoxicity secondary to chemotherapy. J. Clin. Transl. Hepatol. 2014, 2, 95. [Google Scholar]
- Yoo, J.-J.; Lim, Y.S.; Kim, M.S.; Lee, B.; Kim, B.-Y.; Kim, Z.; Lee, J.E.; Lee, M.H.; Kim, S.G.; Kim, Y.S. Risk of fatty liver after long-term use of tamoxifen in patients with breast cancer. PLoS ONE 2020, 15, e0236506. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Wong, L.; Waliany, S.; Mortimer, J.E. Metabolic syndrome and breast cancer survivors: A follow-up analysis after completion of chemotherapy. Diabetol. Metab. Syndr. 2022, 14, 36. [Google Scholar] [CrossRef]
- Westerink, N.; Nuver, J.; Lefrandt, J.; Vrieling, A.; Gietema, J.; Walenkamp, A. Cancer treatment induced metabolic syndrome: Improving outcome with lifestyle. Crit. Rev. Oncol./Hematol. 2016, 108, 128–136. [Google Scholar] [CrossRef]
- Golabi, P.; Gerber, L.; Paik, J.M.; Deshpande, R.; de Avila, L.; Younossi, Z.M. Contribution of sarcopenia and physical inactivity to mortality in people with non-alcoholic fatty liver disease. JHEP Rep. 2020, 2, 100171. [Google Scholar] [CrossRef]
- Tapper, E.B.; Parikh, N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: Observational study. BMJ 2018, 362, k2817. [Google Scholar] [CrossRef]
- Lawler, S.; Spathonis, K.; Masters, J.; Adams, J.; Eakin, E. Follow-up care after breast cancer treatment: Experiences and perceptions of service provision and provider interactions in rural Australian women. Support. Care Cancer 2011, 19, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015, 149, 367–378.e365. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Criteria |
|---|---|
| Participants | Adults aged >18 years old, with a diagnosis of cancer. |
| Intervention | Treatment modalities, lifestyle factors and/or metabolic comorbidities. |
| Comparison | N/A |
| Outcomes | NAFLD and/or NASH measured by ultrasonography, abdominal computed tomography, liver magnetic resonance imaging, hepatic steatosis index, ICD-9 codes or histology |
| Study design | Randomized and non-randomized clinical trials, cohort, cross-sectional or case-control study design. |
| Author, Year, Country | Study Design, Length of Study, Median Length of Follow-Up | Participant Characteristics (n, Age, BMI, Sex) | Cancer Type and Treatment | NAFLD Diagnosis and Classification of Liver Disease | n(NAFLD), % | Primary Study Outcomes | BMI Outcomes | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Brown et al., 2017 United States [22] | Cohort study 17.9 years | n = 387 (cancer survivors) Age range: 20–74 years Mean age: 51.6 ± 1.11 BMI: 26.5 ± 0.4 F: 72%, M: 28% | Multiple cancer types Treatment: NR for each cancer type | Ultrasonography | n = 68 out of 387 (17.6%) Breast 19.2% Gastrointestinal 13.9% Genitourinary 17.0% Gynecologic 22.1% Lung 0% Hematologic 7.8% | The influence of NAFLD as an independent predictor of all-cause and cause-specific mortality among cancer survivors. | Subgroup analyses—overweight or obese (BMI > 25 kg/m2) cancer survivors with NAFLD were more likely to die than normal weight (BMI < 25 kg/m2) cancer survivors with NAFLD. | Among 387 cancer survivors, 17.6% had NAFLD. NAFLD was associated with an increased risk of all-cause mortality (HR = 2.52, 95% CI 1.47–4.34; p = 0.001) and cancer-specific mortality (HR = 3.21, 95% CI 1.46–7.07; p = 0.004). Younger cancer survivors (<60 years) with NAFLD were more likely to die than older cancer survivors with NAFLD (HR = 3.15, 95% CI 1.42–6.97; p = 0.005). Patients who had NAFLD had higher fasting insulin levels (117.0 vs. 62.2 pmol/L; p = 0.001), higher degrees of insulin resistance (5.9 vs. 2.8; p = 0.012), higher BMI (31.1 vs. 25.4 kg/m2; p < 0.001), wider WC (106.4 vs. 89.5 cm; p < 0.001) compared to those without NAFLD. |
| Nseir et al., 2017 Israel [37] | Case control study 4 years | n = 146 n (cases) = 73 Age (cases) = 54.8 ± 12 n (controls) = 73 Age (controls) = 57.5 ± 9.6 | Breast Cancer | Abdominal CT | n = 33 out of 73, (45.2%) | Exploring correlation between NAFLD and BC. | BMI = 29.7 | NAFLD was prevalent in 33 out of 73 women with BC and in 12 out of 73 controls (45.2% vs. 16.4%, respectively, p = 0.002). Multivariate analysis showed NAFLD (odds ratio 2.82, 95% confidence interval 1.2–5.5, p = 0.016) to be associated with BC. |
| Lee et al., 2017 Korea [33] | Cross sectional study | n = 104 Age (control): 49.29 ± 9.11 Age (NAFLD): 57.16 ± 11.51 BMI (control): 22.83 ± 2.92 BMI (NAFLD): 26.72 ± 5.17 F: 100% | Breast cancer | Liver MRI using Achieva 3.0 TX MRI scanner Fat signal percentage cut off of 5% to denote steatosis | n = 19 out of 104 (18.3%) | Evaluate the prevalence of NAFLD in breast cancer patients and compared it with the reported prevalence of NAFLD in general population. | Multivariate analysis—factors associated with NAFLD were high BMI OR = 1.403; 95% CI: 1.111–1.771; p = 0.05 30 obese patients with BMI > 25 kg/m2, 10 (33.3%) had NAFLD, whereas 74 patients with BMI < 25 kg/m2, only 9 (12.2%) had NAFLD. | 19 out of 104 breast cancer patients were diagnosed with NAFLD (18.3%) In multivariate analysis, factors associated with NAFLD were high BMI (OR = 1.403; 95% CI: 1.111–1.771; p = 0.005), type 2 diabetes (OR = 11.872, 95% CI: 1.065–132.373; p = 0.004), and elevated triglycerides (OR = 50.267; 95% CI: 4.409–573.03; p = 0.002). |
| Bilici et al., 2007 Turkey [38] | Case-control study 1 year | n = 165 Group 1: 40 newly diagnosed, previously untreated breast cancer Group 2: 45 cases of treated with systemic therapy. Group 3: 40 cases of ovarian cancer. Group 4: 40 healthy women Mean age: Group 1 = 47.5 ± 11.9 Group 2 = 48.5 ± 11.6 Group 3 = 49.6 ± 11.8 Group 4 = 43.4 ± 6.0 | Breast cancer, Ovarian cancer Treatment: Chemotherapy, Tamoxifen | Sonography | Group 1: n = 25 out of 40 (63%) Group 2: n = 33 out of 45 (72%) | Evaluated the influence of primary disease and treatment on steatosis in breast cancer. In addition, rate of steatosis in breast cancer cases was compared with a different solid cancer group and healthy population. | No BMI outcomes reported | Detected steatosis in 63%, 72%, 77%, and 48% of patients in groups 1, 2, 3, and 4, respectively. Steatosis was more frequent in breast cancer patients (group 1 and 2). Correlation was found between tamoxifen use and chemotherapy on development of non-alcoholic fatty liver disease. Detection of hepatic steatosis was seen in 83.3%, 84.3%, and 77.7% of cases with DM, obesity, and hypertriglyceridemia, respectively. |
| Aktas et al., 2014 Turkey [39] | Retrospective case control study 5 years | n (CRC) = 105 M = 65, F = 40 Mean age = 60.17 ± 12 years n (control) = 94 M = 48, F = 46 Mean age = 59.27 ± 16.4 years | Colorectal cancer | Abdominopelvic computed tomography images Serum transaminase | n = 21 out of 105 (20%) in CRC group | Retrospectively determine the relationship between non-alcoholic fatty liver disease and colorectal cancer by evaluating patients who underwent scanning or diagnosis colonoscopy | No BMI outcomes reported | The liver density measurement on contrast abdominopelvic computed tomography of CRC patients was low, indicating NAFLD. Although the ALT values were higher in the patient group, there was no statistical significance. In 21 patients (20%) of the CRC patient group, non-fatty areas were determined in the anterior of the portal vein (n = 15, 71.4%) and adjacent to the gallbladder (n = 6, 28.6%) |
| Molla et al., 2017 Canada [23] | Retrospective cohort study 27.5 months | n = 60 Age: 68.5 years BMI: 26.5 kg/m2 F: 40%, M: 60% | Colorectal cancer Treatment: liver resection, hepatectomy for colorectal cancer. 32 patients underwent right hepatectomy (53.33%), 9 underwent left hepatectomy (15%), 10 underwent left lateral hepatectomy (16.66%), and only 3 patients underwent right tri-segmentectomy. The rest either underwent wedge resection, single segmentectomy, or bi-segmentectomy. | The histologic features of NAFLD were scored using the NAFLD activity score, and the degree of fibrosis was determined. Preoperative and postoperative (at 12 weeks or more after surgery) CT imaging was retrieved for each patient. | n = 23 out of 60 (38.33%) | Examine the correlations between the degree of NAFLD, liver regeneration, and tumour recurrence after hepatectomy for colorectal cancer. | No BMI outcomes reported | The hepatic recurrence rate was 38.33%. Multivariate analysis revealed significant correlations of hepatic disease-free survival with hepatocyte ballooning (p = 0.0009), lesion diameter (p = 0.014), and synchronous disease (p = 0.006). Univariate and multivariate analyses did not reveal any correlation with degree of steatosis or recurrence rate. |
| Wu et al., 2019 United States [24] | Retrospective cohort study 10 years 6.6 years | n = 3262 Age range: 18–80 years | Colorectal cancer, stage I–III Treatment: surgical resection | Non-enhanced computed tomography scan | n = 83 out of 3262 (2.5%) | To examine the association between pre-existing NAFLD and CRC-specific mortality in stage I–III CRC patients utilizing data from the C- SCANS (Colorectal Cancer-Sarcopenia and Near-term Survival) project. | Associations were independent of BMI and were similar when the study restricted analysis to obese patients. Results for total mortality were similar when restricted analysis to CRC patients who were obese (BMI ≥ 30 kg/m2, 38 NAFLD cases, and 14 deaths; HR 1.79, 95% CI 1.03–3.11). Study did not examine associations separately for normal weight and overweight patients because of small number of deaths among CRC patients with NAFLD. | Cases diagnosed with NAFLD before and within 1 month after CRC diagnosis (pre-existing NAFLD; n = 83) had a HR of 1.64 (95% CI 1.06–2.54) for overall and a HR of 1.85 (95% CI 1.03–3.30) for CRC-specific mortality compared to those without NAFLD. According to the Kaplan–Meier survival function, patients with NAFLD had a shorter survival time than those without NAFLD. Findings did not differ significantly by sex, stage, tumor location, and smoking status, and were also similar when restricted to obese patients only. |
| Hoffmann et al., 2015 Germany [34] | Cross sectional study 5 years | n = 19 Age: without steatosis: 23.7 years; NAFLD: 25.2 years F: 53%, M: 47%, | Childhood-onset craniopharyngioma Treatment: Methylphenidate, modafinil to treat secondary narcolepsy and sever daytime sleepiness | Analyses of liver density were performed by non-contrasted CT and blood serum parameters | n = 10 out of 19 (52.6%) | To detect the risk for NAFLD in childhood-onset craniopharyngioma | No significant differences were detected in BMI. Signs of steatosis hepatis were not associated with BMI in study cohort. | NAFLD occurs in about 50% of childhood-onset craniopharyngioma patients with hypothalamic involvement and is associated with elevated liver enzymes. 10 out of 19 patients were identified with steatosis hepatis—three of them with severe steatosis hepatis (mean HU < 20) and seven with a moderate steatosis (mean HU 20–45). A significant association was found between steatosis hepatis and elevated liver enzymes; AST p = 0.041; Gamma-GT p = 0.016; GLDH p = 0.006) |
| Kouzu et al., 2020 Japan [40] | Retrospective case control 8 years | n = 721 Mean age: 68.4 years Preoperative BMI: 21.7 ± 2.9 Postoperative BMI: 19.3 ± 2.9 F: 23%, M: 77% | Gastric cancer | Plane abdominal CT. The average CT attenuation values of five arbitrary regions of the liver parenchyma without vessels were measured. | n = 35 out of 721 (4.9%) | Identify the risk factors for NAFLD after gastrectomy for gastric cancer. | NAFLD occurred at a high rate in patients with a high BMI. Univariate analysis identified the following factor as being significantly associated with the incidence of NAFLD: preoperative BMI ≥ 25 kg/m2 The NAFLD group had significantly higher preoperative and postoperative (1 year after) BMI (p = 0.001) than the non-NAFLD group. | The incidence of postoperative NAFLD was 4.9% (35/721). Following factors were significantly associated with the incidence of NAFLD: age (p = 0.003), preoperative BMI ≥ 25 kg/m2 (p = 0.005), tumor depth of pT3 ≤ (p = 0.016), lymph node metastasis grade of pN2 ≤ (p = 0.017), cholecystectomy (p = 0.005), D2 lymphadenectomy (p = 0.014), adjuvant chemotherapy (p < 0.001), high preoperative cholinesterase serum level (p= 0.029), and low grade of preoperative FIB-4 index (p < 0.001). Independent risk factors for NAFLD 1 year after gastrectomy were chemotherapy (p < 0.001) and high preoperative cholinesterase serum level (p = 0.021). |
| Moeini et al., 2017 United States [41] | Retrospective Case control study 28.8 months | n = 714 Mean age: 53.1 years F: 100% | Endometrial cancer Treatment: Oophorectomy | Radiology reports to diagnose NAFLD. NAFLD was defined as abnormal liver function testing in addition to radiographic evidence of increased hepatic echogenicity on ultrasonography or attenuation of the liver on CT. | n = 181 out of 714 (25.4%) | The association between NAFLD and venous thromboembolism examined in patients with endometrial cancer. | BMI reported but no significant outcomes | NAFLD was seen in 181 (25.4%) cases. There was 1 (0.1%) case of cirrhosis related to NAFLD, and no NASH case was reported in this study cohort. |
| Ariizumi et al., 2014 Japan [25] | Retrospective Study 21 years | n (CoCC) = 29 n (ICC) = 130 Age = 65 years median | Cholangiocellular carcinoma (CoCC) Intrahepatic cholangiocarcinoma (ICC) Hepatic resection | CT scans or multidetector helical CT Ultrasonography | n = 2 out of 29 (6.9%) | Comparison of surgical outcomes was compared between patients with CoCC and ICC. | No BMI outcomes reported | The number of patients with chronic liver disease was significantly higher in the CoCC group than in the ICC group. |
| Chang et al., 2018 Taiwan [26] | Retrospective cohort study 1 year | n = 266 Age = 52.9 ± 8.1 BMI = 24.1 ± 4.1 | Breast cancer Tamoxifen | Ultrasound examination | n = 39 out of 266 (14.7%) | Assessed the potential risk and protective factors for tamoxifen-related NAFLD among BC patients. | BMI of > 22 kg/m2 is a risk factor for tamoxifen-related fatty liver | From 266 patients: 11 (4.1%) presented with alleviation of fatty liver 93 (35.0%) with normal and no change 39 (14.7%) with fatty liver and no change 65 (24.4%) with normal changing to fatty liver |
| Izadpanhai et al., 2020 Iran [35] | Cross sectional study | n = 152 patients n (BC) = 85 n (Gastrointestinal cancer) = 67 Median age = 45–54 years Body Mass Index = 23.17 ± 4.52 | Breast cancer Gastrointestinal cancer Chemotherapy | Sonography for fatty liver | n (BC) = 40 out of 85 (47.1%) n = (GIC) = 41 out of 67 (61.2%) | Determine the prevalence of fatty liver in breast and gastrointestinal cancer patients during and after chemotherapy treatment. | No significant relationship between chemotherapy-induced fatty liver and BMI (p = 0.17). | The frequency of fatty liver after chemotherapy was significantly higher in females than in males (52.4% and 34.7%, respectively, p = 0.04). No significant relationship between chemotherapy-induced fatty liver and age (p = 0.9), and the presence of metabolic syndrome (p = 0.4). The results indicate that chemotherapy was associated with a significantly increased risk of fatty liver, which was more in women than in men. The highest frequency of fatty liver was observed in patients treated with paclitaxel, FOLFOX, and ECF with 53.5%, 42.9%, and 29.2%, respectively (p = 0.09). |
| Lee et al., 2019 Korea [32] | Retrospective Cohort Study 8.4 years | n (breast cancer) = 253 Median age = 69 years BMI = 22.9 ± 2.4 n(controls) = 220 Median age = 69 years BMI = 24.3 ± 3.5 | Breast Cancer Aromatase inhibitors | Hepatic steatosis index (HIS) The cutoff value of HIS > 36 was used to detect NAFLD with specificity of 92.4% | n = 175 out 440 (39.8%) | Evaluate the role of aromatase inhibitors on the development of NAFLD and liver fibrosis in post- menopausal patients with early breast cancer | No BMI outcomes reported | Inhibition of estrogen synthesis in postmenopausal women undergoing treatment (aromatase inhibitors) could increase the risk of NAFLD. HIS was significantly higher in the aromatase inhibitor-treated group (33.15 ±4.35 vs. 38.08 ± 8.03; p = 0.001), and the proportion of patients with HIS > 36 who were considered to have high probability of NAFLD was significantly larger in the aromatase inhibitor-treated patients (25.9% vs. 53.6%; p = 0.001). |
| Pan et al., 2016 Taiwan [31] | Retrospective cohort study 26.7 months | n = 406 Tamoxifen group = 266 Control group = 140 mean age 53.2 ± 8.2 years BMI = 24.1 ± 3.9 | Breast cancer Tamoxifen treatment | Abdominal ultrasound | Control n (Initial) Normal = 87 (62.1%) Mild = 39 (27.9%) Moderate = 13 (9.3%) Severe = 1 (0.7%) n (Follow-up) Normal = 92(65.7%) Mild = 32(22.9%) Moderate = 16(11.4%) Severe = 0 Tamoxifen n (Initial) Normal = 158 (60.1%) Mild = 83 (31.6%) Moderate = 21 (8.0%) Severe = 1 (0.4%) n (Follow-up) Normal = 101 (38.0%) Mild = 68 (25.6%) Moderate = 76 (28.6%) Severe = 21 (7/9%) | Examine the effects of tamoxifen under pre-existing fatty liver conditions and evaluate the prevalence of tamoxifen-related impaired liver function. | No BMI outcomes reported | The tamoxifen group had a higher risk of newly developed fatty liver HR = 3.69; 95% confidence interval CI = 1.678.13), lower rate of improved fatty liver (HR = 0.33; 95% CI 0.15–0.75), and higher rate of worsened fatty liver (HR = 2.11; 95% CI 1.02–4.35). |
| Nemoto et al., 2001 Japan [43] | Case control study 5 years | n = 56 | Breast Cancer Tamoxifen—oral tamoxifen (40 mg/day for 2 to 3 years) as adjuvant endocrine therapy with systemic chemotherapy | CT of spleen and liver | n = 19 out of 56 (33.9%) | Representative clinical features of tamoxifen-induced NASH | No significant different in inverse values of body weight between patients with hepatic steatosis and patients without hepatic steatosis. | 19 out 56 patients developed hepatic steatosis within 2 years. |
| Golabi et al., 2017 United States [36] | Cross sectional study 4 months | n = 11,187 Age of diagnosis: 72 years F: 31%, M: 69% | HCC Treatment: liver transplantation, surgical resection, Trans arterial chemoembolization | 4 categories of chronic liver diseases were identified using the ICD-9 codes: (1) hepatic C virus, (2) Hepatitis B virus, (3) alcoholic liver disease with codes and (4) non-viral and nonalcoholic cryptogenic liver disease. | Non-viral and non-alcoholic cryptogenic liver disease =1277 out of 11,187 (18.6%) Decompensated hepatic cirrhosis = 3768 out of 11,187 (33.7%) | Assess mortality within 2 years postdiagnosis among participants with HCC according to treatment modalities. | No BMI outcomes reported | 34% HCC patients had decompensated cirrhosis and 9% had non-viral and nonalcoholic/cryptogenic liver disease. 17% of HCC patients treated with surgical resection had non-viral and nonalcoholic/cryptogenic liver disease. Presence of decompensated cirrhosis (HR: 1.84, 95% CI = 1.73–1.96) increased within 2 years mortality. HCC patients with NAFLD (1.11 times) were more likely to die within 2 years of diagnosis. |
| Mehta et al., 2013 USA [30] | Retrospective cohort study 8 years | n = 155 M = 122 F = 33 Median age = 60 years | Infiltrative hepatocellular carcinoma (iHCC) trans arterial chemoembolization | Contrast-enhanced computed tomography or magnetic resonance imaging | N = 15 out of 155 (9.7%) | Aim of the present study was to assess the outcomes, effect of treatment, and factors predicting prognosis in a large cohort of patients with iHCC. | No BMI outcomes reported | Nonalcoholic fatty liver disease (9.7%) Most of the patients had tumours of Barcelona Clinic Liver Cancer Stage C (70%) or D (22%). On multivariate analysis, predictors of 6-month mortality were Child–Pugh class B or C cirrhosis; lack of tumour-directed therapy with chemoembolization. The percentages of patients surviving 6 and 12 months were 17% and 2% for those who received no therapy (n = 109), 73% and 36% for those who received sorafenib (n = 11), and 45% and 17% for those who received trans arterial chemoembolization (n = 18) (all p values < 0.01). |
| Prieto et al., 2016 Philippines [29] | Retrospective cohort study 7 years | n = 346 Mean age: 61.47 + 13.08 F: 18.8%, M: 81.8% | Hepatocellular carcinoma Treatment: 44 patients (12.94%) underwent surgical treatment. 99 patients (29.12%) had TACE and/or RFA. 26 patients (7.65%) had systemic/oral chemotherapy. 171 (50.29%) patients had supportive care | Ultrasound, Dynamic CT scan, MRI using liver specific contrast, elevated AFP, and biopsy | n = 27 out of 346 (7.8%) | To investigate prognostic features, treatment outcomes and survival of hepatocellular carcinoma patients at the National Kidney and Transplant Institute. | No BMI outcomes reported | Median survival was 13.17 months (range, < one month—92 months). Those who had locoregional therapy had the longest median survival (30.33 months), followed by systemic chemotherapy (26.67 months) then surgery (13.17 months). Median survival time between those with and without liver cirrhosis was significantly different (9.43 months vs. 38.47 months, p < 0.001). |
| Bruno et al., 2005 Italy [44] | Prospective, randomized, double blind, placebo-controlled trial 8.7 years | n = 64 Median age = 51 years Median BMI = 27.0 | Endometrial cancer Hysterectomies Tamoxifen | Ultrasonography | n = 52 suspected of having developed NAFLD (81.25%) | Assess the risk of development of non-alcoholic fatty liver disease, including non-alcoholic steatohepatitis, in relation to tamoxifen in women | Women with high alanine aminotransferase at baseline were heavier, had a higher BMI, and more often had diabetes than women with normal concentrations at baseline and during follow-up (p < 0.0001). | Developed non-alcoholic fatty liver disease = 52 (34 tamoxifen, 18 placebo)—hazard ratio = 2.0 (95% confidence interval 1.1 to 3.5; p = 0.04). Factors associated with the development NAFLD include overweight (2.4, 1.2 to 4.8), obesity (3.6, 1.7 to 7.6), hypercholesterolaemia (3.4, 1.4 to 7.8), and arterial hypertension (2.0, 1.0 to 3.8). Twenty women had liver biopsies: 15 were diagnosed as having mild to moderate steatohepatitis (12 tamoxifen, 3 placebo), and five had fatty liver alone (1 tamoxifen, 4 placebo). |
| Matuso et al., 2016 Japan [42] | Retrospective cohort study 1, 2 and 5 years follow-up | n (total) = 875 Oophorectomy cases = 712 No oophorectomy cases = 163 Mean age = 50.8 years | Endometrial cancer Surgical treatment | Ultrasonography | n = 232 out of 875 cases (26.5%) | Examine factors associated with development of NAFLD among women with endometrial cancer who underwent surgical staging | Women who developed NAFLD were obese (p = 0.029). | NAFLD was diagnosed in 232 cases (26.5%) at the time of endometrial tumour diagnosis or during follow-up after surgical operation. Prevalence of NAFLD in 875 women with endometrial tumour was 14.1%, 20.5%, and 38.4% at 1, 2, and 5 years after surgical operation, respectively. Oophorectomy in women with endometrial cancer significantly increases the risk of NAFLD. NAFLD cases were diagnosed after surgical operation (n = 168; 72.4%) and were commonly diagnosed by ultrasonography (76.2%). |
| Gild et al., 2018 Germany [28] | Cohort study 6.1 years | n = 82,938 No Androgen-deprivation therapy n = 51,821 Age = 72.2 (68.8–76.5) Androgen-deprivation therapy = 31,117 Age = 75.3 (71.1–80.0) | Prostate cancer Androgen-deprivation therapy | CT scan | n (No Androgen-deprivation therapy) = 259 out of 51,821 (0.5%) n (Androgen-deprivation therapy) = 265 out of 31,117 (0.9%) | Association between androgen-deprivation therapy and prostate cancer. The primary study outcome was the diagnosis of nonalcoholic chronic liver disease, including NAFLD and NASH. | No BMI outcomes reported | Of the men who underwent androgen-deprivation therapy, they were most likely to be diagnosed with nonalcoholic fatty liver disease (HR 1.54, 95% CI 1.40–1.68), liver cirrhosis (HR 1.35, 95% CI 1.12–1.60), liver necrosis (HR 1.41, 95% CI 1.15–1.72) and any liver disease (HR 1.47, 95% CI 1.35–1.60). |
| Montomoli et al., 2013 Denmark [27] | Cohort study 14 years 30 days | n = 39,840 Non-cirrhotic liver disease = 369 F: 48.9%, M: 51.1% Liver cirrhosis = 158 F: 49.1%, M: 50.1% | Colorectal cancer Treatment: Colorectal surgery—radical resection, laparoscopic and open surgery. | Danish National Registry of Patients to identify patients with a diagnosis of liver disease. | n = 34 out of 369 (9.2%) | Examined 30-day mortality after CRC surgery in patients with liver disease compared to those without liver disease. | No BMI outcomes reported | Thirty-day mortality was 13.3% in patients with non-cirrhotic liver disease and 24.1% among patients with liver cirrhosis, compared to 8.7% in patients without liver disease. Patients with liver cirrhosis, mortality was 24.1%, Adjusted RR = 2.59, 95% CI: 1.86–3.61 CRC patients with liver disease, especially those with liver cirrhosis, were more likely to have comorbid conditions, including non-hepatic alcohol-related disease, than patients without liver disease. |
| Author, Year | Cancer Treatment: Chemotherapy | Cancer Treatment: Hormone Therapy | Cancer Treatment: Surgery | Survival and Mortality | Metabolic Co-Morbidities |
|---|---|---|---|---|---|
| Brown et al., 2017 [22] | − | − | − | + | + |
| Nseir et al., 2017 [37] | − | − | − | − | |
| Lee et al., 2017 [33] | − | − | − | − | + |
| Bilici et al., 2007 [38] | + | − | − | − | + |
| Aktas et al., 2014 [39] | − | − | − | − | − |
| Molla et al., 2017 [23] | − | − | − | + | − |
| Wu et al., 2019 [24] | − | − | − | − | + |
| Hoffmann et al., 2015 [34] | − | − | − | − | + |
| Kouzu et al., 2020 [40] | − | − | − | − | + |
| Moeini et al., 2017 [41] | − | − | + | − | − |
| Ariizumi et al., 2014 [25] | − | − | − | − | − |
| Chang et al., 2018 [26] | − | + | − | − | − |
| Izadpanhai et al., 2020 [35] | + | − | − | − | − |
| Lee et al., 2019 [32] | − | + | − | + | − |
| Pan et al., 2015 [31] | + | + | − | − | − |
| Nemoto et al., 2001 [43] | − | + | − | − | − |
| Golabi et al., 2017 [36] | + | − | + | + | − |
| Mehta et al., 2013 [30] | − | − | − | − | − |
| Prieto et al., 2016 [29] | − | − | − | − | − |
| Bruno et al., 2005 [44] | − | + | − | − | − |
| Matuso et al., 2016 [42] | − | − | + | − | + |
| Gild et al., 2018 [28] | − | + | − | − | − |
| Montomoli et al., 2013 [27] | − | − | − | + | + |
| Aktas et al., 2014 [39] | Ariizumi et al., 2014 [25] | Bilici et al., 2007 [38] | Brown et al., 2017 [22] | Chang et al., 2018 [26] | Gild et al., 2018 [28] | Golabi et al., 2017 [36] | Hoffmann et al., 2015 [34] | Izadpanahi et al., 2020 [35] | Kouzu et al., 2020 [40] | Lee et al., 2019 [32] | Lee et al., 2017 [33] | Moeini et al., 2017 [41] | Matuso et al., 2016 [42] | Molla et al., 2017 [23] | Mehta et al., 2013 [30] | Montomoli et al., 2013 [27] | Nemoto et al., 2002 [43] | Nseir et al., 2017 [37] | Pan et al., 2016 [31] | Prieto et al., 2016 [29] | Wu et al., 2019 [24] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relevance Questions | ||||||||||||||||||||||
| NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 1. Would implementing the studied intervention or procedure (if found successful) result in improved outcomes for the patients/clients/population group? | NA | NA | NA | NA | NA | NA |
| Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 2. Did the authors study an outcome (dependent variable) or topic that the patients/clients/population group would care about? | Y | Y | Y | Y | Y | Y |
| NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 3. Is the focus of the intervention or procedure (independent variable) or topic of study a common issue of concern to dietetics practice? | NA | NA | NA | NA | NA | NA |
| NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 4. Is the intervention or procedure feasible? | NA | Y | Y | Y | NA | NA |
| Validity Questions | ||||||||||||||||||||||
| Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 1. Was the research question clearly stated? | Y | Y | Y | Y | Y | Y |
| Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 2. Was the selection of study subjects/patients free from bias? | Y | U | Y | Y | Y | Y |
| Y | NA | NA | NA | Y | NA | NA | NA | NA | NA | Y | NA | NA | NA | NA | NA | 3. Were study groups comparable or was an appropriate reference standard used? | NA | NA | NA | NA | NA | NA |
| NA | NA | NA | NA | NA | U | NA | U | NA | NA | Y | NA | U | Y | NA | Y | 4. Were methods of handling losses from the original sample (withdrawals) described? | NA | NA | NA | NA | NA | U |
| NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 5. Was blinding used to prevent introduction of bias? | NA | NA | NA | NA | U | NA |
| NA | NA | NA | NA | NA | NA | NA | NA | Y | NA | Y | NA | NA | Y | NA | Y | 6. Was the intervention/treatment regimen/exposure factor, procedure, process or product of interest, and any comparison(s) described in detail? Were intervening factors described? | NA | Y | Y | Y | Y | Y |
| Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 7. Were outcomes or condition or status of interest clearly defined and the measurements validand reliable? | Y | N | Y | Y | Y | Y |
| Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 8. Was the statistical analysis appropriate for the study design and type of outcome indicators? | Y | N | Y | Y | Y | Y |
| Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 9. Are conclusions supported by results with biases and limitations taken into consideration? | Y | Y | Y | Y | Y | Y |
| Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 10. Is bias due to study’s funding or sponsorship unlikely? | Y | N | N | N | N | Y |
| + | Ø | Ø | Ø | + | Ø | Ø | Ø | + | Ø | + | Ø | Ø | + | Ø | + | Overall Rating | Ø | Ø | + | + | + | + |
| Author | Randomized Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall |
|---|---|---|---|---|---|---|
| Bruno et al., 2005 [44] | Some concerns | High risk | Low risk | Some concerns | Some concerns | Some concerns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
George, E.S.; Sood, S.; Kiss, N.; Daly, R.M.; Nicoll, A.J.; Roberts, S.K.; Baguley, B.J. The Evidence Surrounding Non-Alcoholic Fatty Liver Disease in Individuals with Cancer: A Systematic Literature Review. Curr. Oncol. 2023, 30, 48-74. https://doi.org/10.3390/curroncol30010005
George ES, Sood S, Kiss N, Daly RM, Nicoll AJ, Roberts SK, Baguley BJ. The Evidence Surrounding Non-Alcoholic Fatty Liver Disease in Individuals with Cancer: A Systematic Literature Review. Current Oncology. 2023; 30(1):48-74. https://doi.org/10.3390/curroncol30010005
Chicago/Turabian StyleGeorge, Elena S., Surbhi Sood, Nicole Kiss, Robin M. Daly, Amanda J. Nicoll, Stuart K. Roberts, and Brenton J. Baguley. 2023. "The Evidence Surrounding Non-Alcoholic Fatty Liver Disease in Individuals with Cancer: A Systematic Literature Review" Current Oncology 30, no. 1: 48-74. https://doi.org/10.3390/curroncol30010005
APA StyleGeorge, E. S., Sood, S., Kiss, N., Daly, R. M., Nicoll, A. J., Roberts, S. K., & Baguley, B. J. (2023). The Evidence Surrounding Non-Alcoholic Fatty Liver Disease in Individuals with Cancer: A Systematic Literature Review. Current Oncology, 30(1), 48-74. https://doi.org/10.3390/curroncol30010005





