Changes in Inflammatory Markers Predict the Prognosis of Resected Hepatocellular Carcinoma with Child–Pugh A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Collection
2.2. Clinicopathologic Variables
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
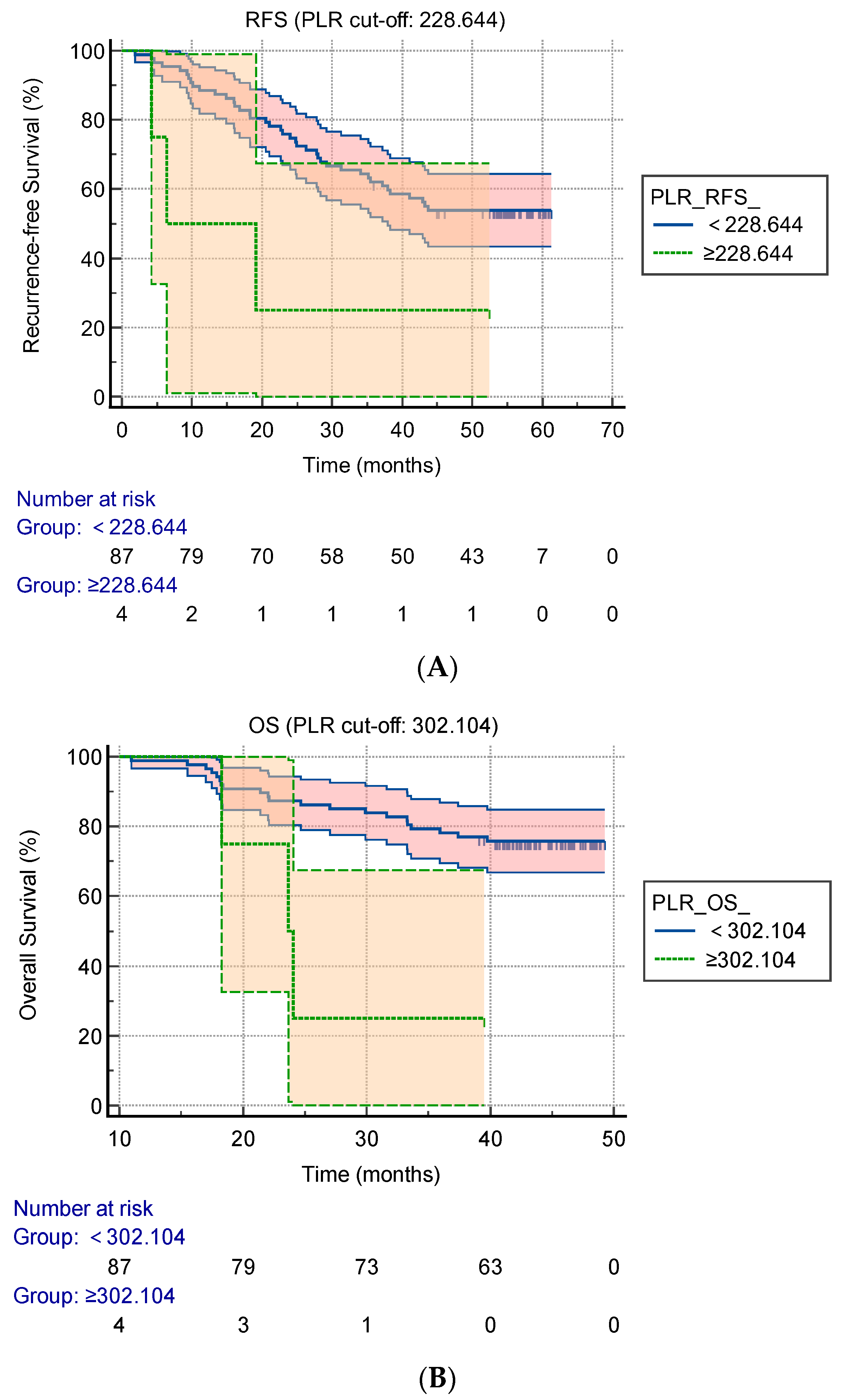
3.2. Cut-Off Values of the Preoperative AFP, PLR, NLR, LMR, OPNI, RDW-CV, and RDW-SD for Predicting the RFS and OS
3.3. Prognostic Factors of the RFS and OS
3.4. The Changes in the Pre-1-Week Operative and Post-6-Month Operative PLR and Their Impact on the OS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-Inflammatory Drugs as Anticancer Agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Hou, J.; Karin, M.; Sun, B. Targeting cancer-promoting inflammation-have anti-inflammatory therapies come of age? Nat. Rev. Clin. Oncol. 2021, 18, 261–279. [Google Scholar] [CrossRef]
- Rossi, J.F.; Lu, Z.Y.; Massart, C.; Levon, K. Dynamic Immune/Inflammation Precision Medicine: The Good and the Bad Inflammation in Infection and Cancer. Front. Immunol. 2021, 12, 595722. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Kurebayashi, Y.; Ojima, H.; Tsujikawa, H.; Kubota, N.; Maehara, J.; Abe, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y.; Sakamoto, M. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology 2018, 68, 1025–1041. [Google Scholar] [CrossRef]
- Ma, W.; Mao, S.; Bao, M.; Wu, Y.; Guo, Y.; Liu, J.; Wang, R.; Li, C.; Zhang, J.; Zhang, W.; et al. Prognostic significance of red cell distribution width in bladder cancer. Transl. Androl. Urol. 2020, 9, 295–302. [Google Scholar] [CrossRef]
- Herraez, I.; Bento, L.; Del Campo, R.; Sas, A.; Ramos, R.; Ibarra, J.; Mestre, F.; Alemany, R.; Bargay, J.; Sampol, A.; et al. Prognostic Role of the Red Blood Cell Distribution Width (RDW) in Hodgkin Lymphoma. Cancers 2020, 12, 3262. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef]
- Dharmapuri, S.; Özbek, U.; Lin, J.Y.; Sung, M.; Schwartz, M.; Branch, A.D.; Ang, C. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti-PD-1 therapy. Cancer Med. 2020, 9, 4962–4970. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Li, Z.; Zhang, X.; Deng, R.; Ma, Y. Inflammation-Based Prognostic Scores in Patients with Hepatitis B Virus-Related Hepatocellular Carcinoma After Liver Transplantation. J. Hepatocell. Carcinoma 2020, 7, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Mandaliya, H.; Jones, M.; Oldmeadow, C.; Nordman, I.I. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): Neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl. Lung Cancer Res. 2019, 8, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Deng, Y.; Yu, X.; Zhang, X.W.; Huo, C.L.; Sun, Z.G.; Chang, H. Prognostic significance of preoperative systemic inflammatory biomarkers in patients with hepatocellular carcinoma after microwave ablation and establishment of a nomogram. Sci. Rep. 2021, 11, 13814. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Templeton, A.J.; Ace, O.; McNamara, M.G.; Al-Mubarak, M.; Vera-Badillo, F.E.; Hermanns, T.; Seruga, B.; Ocaña, A.; Tannock, I.F.; Amir, E. Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1204–1212. [Google Scholar] [CrossRef]
- Gu, X.B.; Tian, T.; Tian, X.J.; Zhang, X.J. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: A meta-analysis. Sci. Rep. 2015, 5, 12493. [Google Scholar] [CrossRef]
- Okadome, K.; Baba, Y.; Yagi, T.; Kiyozumi, Y.; Ishimoto, T.; Iwatsuki, M.; Miyamoto, Y.; Yoshida, N.; Watanabe, M.; Baba, H. Prognostic Nutritional Index, Tumor-infiltrating Lymphocytes, and Prognosis in Patients with Esophageal Cancer. Ann. Surg. 2020, 271, 693–700. [Google Scholar] [CrossRef]
- Wang, D.; Bai, N.; Hu, X.; Ouyang, X.W.; Yao, L.; Tao, Y.; Wang, Z. Preoperative inflammatory markers of NLR and PLR as indicators of poor prognosis in resectable HCC. PeerJ 2019, 7, e7132. [Google Scholar] [CrossRef]
- Caputo, F.; Dadduzio, V.; Tovoli, F.; Bertolini, G.; Cabibbo, G.; Cerma, K.; Vivaldi, C.; Faloppi, L.; Rizzato, M.D.; Piscaglia, F.; et al. The role of PNI to predict survival in advanced hepatocellular carcinoma treated with Sorafenib. PLoS ONE 2020, 15, e0232449. [Google Scholar] [CrossRef]
- Hsiang, C.W.; Huang, W.Y.; Yang, J.F.; Shen, P.C.; Dai, Y.H.; Wang, Y.F.; Lin, C.S.; Chang, W.C.; Lo, C.H. Dynamic Changes in Neutrophil-to-Lymphocyte Ratio are Associated with Survival and Liver Toxicity Following Stereotactic Body Radiotherapy for Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Goto, T.; Hirotsu, Y.; Moriyama, M.; Omata, M. Molecular Mechanisms Driving Progression of Liver Cirrhosis towards Hepatocellular Carcinoma in Chronic Hepatitis B and C Infections: A Review. Int. J. Mol. Sci. 2019, 20, 1358. [Google Scholar] [CrossRef]
- Valenti, A.C.; Vitolo, M.; Imberti, J.F.; Malavasi, V.L.; Boriani, G. Red Cell Distribution Width: A Routinely Available Biomarker with Important Clinical Implications in Patients with Atrial Fibrillation. Curr. Pharm. Des. 2021, 27, 3901–3912. [Google Scholar] [CrossRef] [PubMed]
- Ellahony, D.M.; El-Mekkawy, M.S.; Farag, M.M. A Study of Red Cell Distribution Width in Neonatal Sepsis. Pediatr. Emerg. Care 2020, 36, 378–383. [Google Scholar] [CrossRef]
- Marvisi, M.; Mancini, C.; Balzarini, L.; Ramponi, S. Red cell distribution width: A new parameter for predicting the risk of exacerbation in COPD patients. Int. J. Clin. Pract. 2021, 75, e14468. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Zhou, K.; Li, J.; Che, G. Prognostic value of pre-treatment red blood cell distribution width in lung cancer: A meta-analysis. Biomarkers 2020, 25, 241–247. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, M.C.; Tian, T.; Huang, J.; Yuan, S.X.; Liu, L.; Zhu, P.; Gu, F.M.; Fu, S.Y.; Jiang, B.G.; et al. A High Preoperative Platelet-Lymphocyte Ratio Is a Negative Predictor of Survival After Liver Resection for Hepatitis B Virus-Related Hepatocellular Carcinoma: A Retrospective Study. Front. Oncol. 2020, 10, 576205. [Google Scholar] [CrossRef]
- Wang, C.; He, W.; Yuan, Y.; Zhang, Y.; Li, K.; Zou, R.; Liao, Y.; Liu, W.; Yang, Z.; Zuo, D.; et al. Comparison of the prognostic value of inflammation-based scores in early recurrent hepatocellular carcinoma after hepatectomy. Liver Int. 2020, 40, 229–239. [Google Scholar] [CrossRef]
- Jing, J.S.; Fu, X.L.; Zhao, W.; Kong, L.B. Red Cell Distribution Width as a Prognostic Factor in Patients with Hepatocellular Carcinoma. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Wang, D.; Hu, X.; Xiao, L.; Long, G.; Yao, L.; Wang, Z.; Zhou, L. Prognostic Nutritional Index and Systemic Immune-Inflammation Index Predict the Prognosis of Patients with HCC. J. Gastrointest. Surg. 2021, 25, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell 2018, 33, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Roweth, H.G.; Battinelli, E.M. Lessons to learn from tumor-educated platelets. Blood 2021, 137, 3174–3180. [Google Scholar] [CrossRef] [PubMed]
- Mammadova-Bach, E.; Gil-Pulido, J.; Sarukhanyan, E.; Burkard, P.; Shityakov, S.; Schonhart, C.; Stegner, D.; Remer, K.; Nurden, P.; Nurden, A.T.; et al. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived galectin-3. Blood 2020, 135, 1146–1160. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.L.; Tassi Yunga, S.; Williams, C.D.; McCarty, O.J.T. Aspirin and antiplatelet treatments in cancer. Blood 2021, 137, 3201–3211. [Google Scholar] [CrossRef]
- Scheiner, B.; Kirstein, M.; Popp, S.; Hucke, F.; Bota, S.; Rohr-Udilova, N.; Reiberger, T.; Müller, C.; Trauner, M.; Peck-Radosavljevic, M.; et al. Association of Platelet Count and Mean Platelet Volume with Overall Survival in Patients with Cirrhosis and Unresectable Hepatocellular Carcinoma. Liver Cancer 2019, 8, 203–217. [Google Scholar] [CrossRef]
- Wang, W.C.; Zhang, Z.Q.; Li, P.P.; Ma, J.Y.; Chen, L.; Qian, H.H.; Shi, L.H.; Yin, Z.F.; Sun, B.; Zhang, X.F. Anti-tumor activity and mechanism of oligoclonal hepatocellular carcinoma tumor-infiltrating lymphocytes in vivo and in vitro. Cancer Biol. Ther. 2019, 20, 1187–1194. [Google Scholar] [CrossRef]

| Variables | All (n = 91) | Preoperative Parameter | Value |
|---|---|---|---|
| Gender Male Female | 81 (89.0%) 10 (11.0%) | Neutrophil (×109/L) | 2.81 (2.05–3.77) |
| Age (years) <45 ≥45 | 27 (29.7%) 64 (70.3%) | Lymphocyte (×109/L) | 1.41 (1.09–1.72) |
| HBsAg a Positive Negative | 77 (84.6%) 14 (15.4%) | Monocytes (×109/L) | 0.42 (0.32–0.57) |
| Cirrhosis Present Absent | 53 (58.2%) 38 (41.8%) | Platelet (×109/L) | 154 (107–207) |
| Tumor number 1 ≥2 | 79 (86.8%) 12 (13.2%) | ALT c (U/L) | 30 (21–45) |
| Tumor diameter ≤5 cm >5 cm | 63 (69.2%) 28 (30.8%) | AST d (U/L) | 28 (22–47) |
| Differentiation grade High–medium Low | 58 (63.7%) 33 (36.3%) | TBIL e (umol/L) | 12.40 (9.50–17.00) |
| Vascular invasion Yes No | 23 (25.3%) 68 (74.7%) | γ-GT f (U/L) | 46 (27–80) |
| TNM b stage I–II III–IV | 73 (80.2%) 18 (19.8%) | Albumin (g/L) | 40.98 ± 4.72 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Gender | 0.953 (0.340–2.670) | 0.927 | ||
| Age (≥45 years) | 1.095 (0.562–2.133) | 0.789 | ||
| HBsAg a (yes) | 0.930 (0.413–2.091) | 0.860 | ||
| Cirrhosis (yes) | 0.796 (0.431–1.467) | 0.464 | ||
| Tumor number (=1) | 1.271 (0.564–2.863) | 0.562 | ||
| Tumor diameter (>5 cm) | 2.104 (1.145–3.866) | 0.017 | 1.737 (0.897–3.362) | 0.101 |
| Differentiation grade (I) | 0.644 (0.343–1.208) | 0.170 | ||
| Vascular invasion (yes) | 0.523 (0.279–0.981) | 0.043 | 0.685 (0.342–1.372) | 0.286 |
| TNM b stage (I–II) | 1.660 (0.836–3.295) | 0.148 | ||
| Albumin (<35 g/L) | 0.392 (0.173–0.884) | 0.024 | 0.889 (0.340–2.323) | 0.810 |
| AFP c (≥10.130 ng/mL) | 2.392 (1.204–4.751) | 0.013 | 1.986 (0.943–4.180) | 0.071 |
| NLR d (≥2.271) | 1.703 (0.923–3.139) | 0.088 | ||
| PLR e (≥228.644) | 3.757 (1.146–12.318) | 0.029 | 9.870 (2.573–37.861) | 0.001 |
| LMR f (≥4.633) | 1.361 (0.686–2.703) | 0.378 | ||
| OPNI g (≥51.925) | 1.247 (0.649–2.394) | 0.057 | ||
| RDW-CV h (≥13.700) | 3.126 (1.642–5.949) | 0.001 | 2.391 (1.101–5.193) | 0.028 |
| RDW-SD i (≥42.550) | 2.358 (1.160–4.794) | 0.018 | 2.305 (1.045–5.085) | 0.038 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Gender | 1.893 (0.647–5.542) | 0.244 | ||
| Age (≥45 years) | 0.873 (0.374–2.040) | 0.754 | ||
| HBsAg a (yes) | 0.766 (0.228–2.568) | 0.666 | ||
| Cirrhosis (yes) | 0.522 (0.217–1.260) | 0.148 | ||
| Tumor number (=1) | 0.763 (0.261–2.232) | 0.621 | ||
| Tumor diameter (>5 cm) | 0.459 (0.205–1.024) | 0.057 | ||
| Differentiation grade (I) | 2.131 (0.934–4.862) | 0.072 | ||
| Vascular invasion (yes) | 1.910 (0.836–4.365) | 0.125 | ||
| TNM b stage (I–II) | 0.655 (0.260–1.651) | 0.370 | ||
| Albumin (<35 g/L) | 0.385 (0.144–1.031) | 0.058 | ||
| AFP c (≥10.535 ng/ml) | 2.181 (0.865–5.496) | 0.098 | ||
| NLR d (≥4.191) | 4.712 (1.748–12.700) | 0.020 | 2.203 (0.721–6.728) | 0.166 |
| PLR e (≥302.104) | 4.894 (1.429–16.758) | 0.011 | 9.423 (1.922–46.208) | 0.006 |
| LMR f (≥3.785) | 1.542 (0.691–3.444) | 0.290 | ||
| OPNI g (≥56.200) | 2.583 (0.882–7.564) | 0.083 | ||
| RDW-CV h (≥13.250) | 2.451 (1.088–5.524) | 0.031 | 2.014 (0.847–4.787) | 0.113 |
| RDW-SD i (≥42.650) | 3.557 (1.215–10.410) | 0.021 | 3.949 (1.134–13.748) | 0.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Yang, D. Changes in Inflammatory Markers Predict the Prognosis of Resected Hepatocellular Carcinoma with Child–Pugh A. Curr. Oncol. 2022, 29, 5800-5809. https://doi.org/10.3390/curroncol29080457
Zhou J, Yang D. Changes in Inflammatory Markers Predict the Prognosis of Resected Hepatocellular Carcinoma with Child–Pugh A. Current Oncology. 2022; 29(8):5800-5809. https://doi.org/10.3390/curroncol29080457
Chicago/Turabian StyleZhou, Jing, and Daofeng Yang. 2022. "Changes in Inflammatory Markers Predict the Prognosis of Resected Hepatocellular Carcinoma with Child–Pugh A" Current Oncology 29, no. 8: 5800-5809. https://doi.org/10.3390/curroncol29080457
APA StyleZhou, J., & Yang, D. (2022). Changes in Inflammatory Markers Predict the Prognosis of Resected Hepatocellular Carcinoma with Child–Pugh A. Current Oncology, 29(8), 5800-5809. https://doi.org/10.3390/curroncol29080457




