Clinical Lymph Node Involvement as a Predictor for Cancer-Specific Survival in Patients with Penile Squamous Cell Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Endpoints and Statistical Analysis
3. Results
3.1. Patient Characteristics
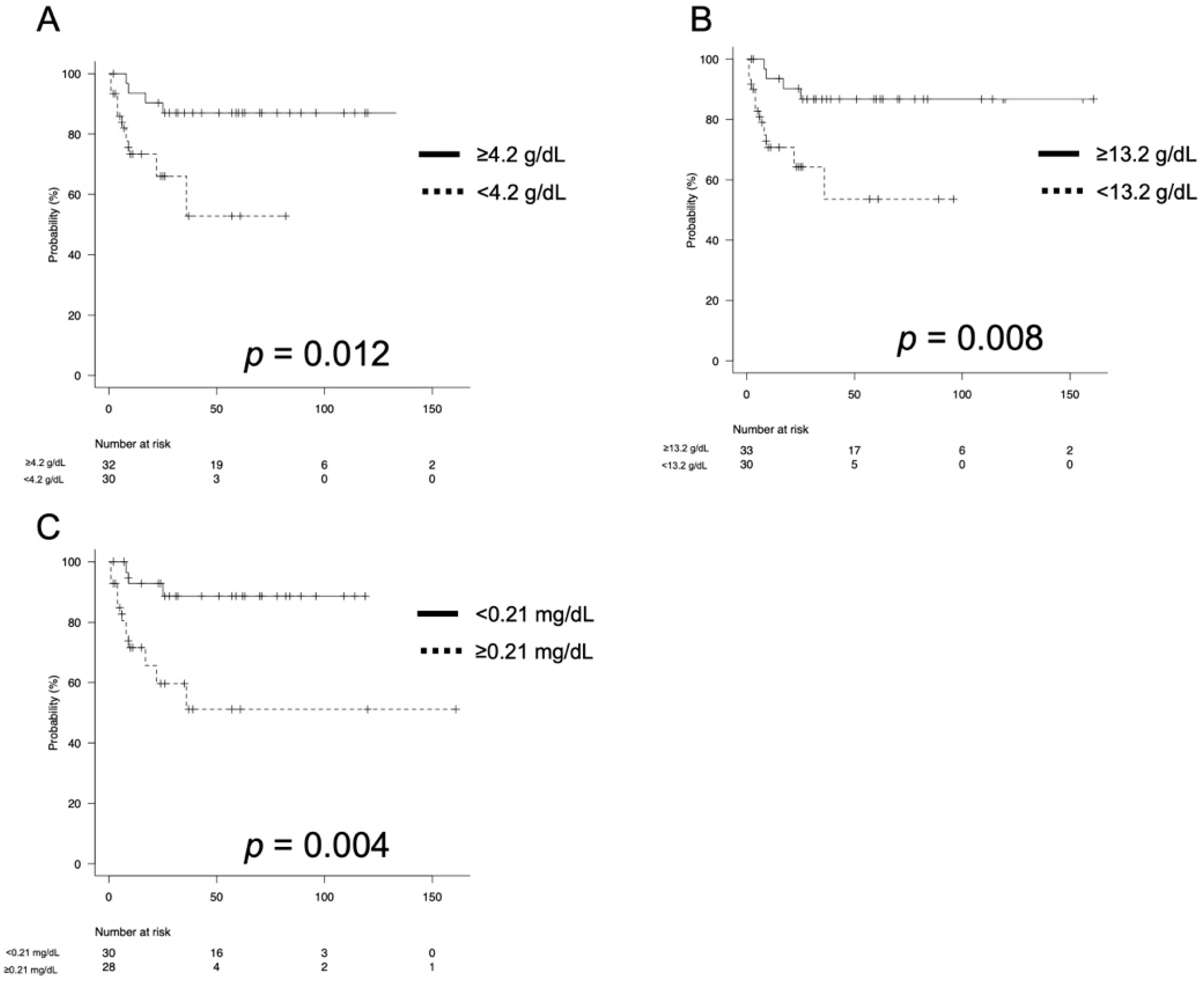
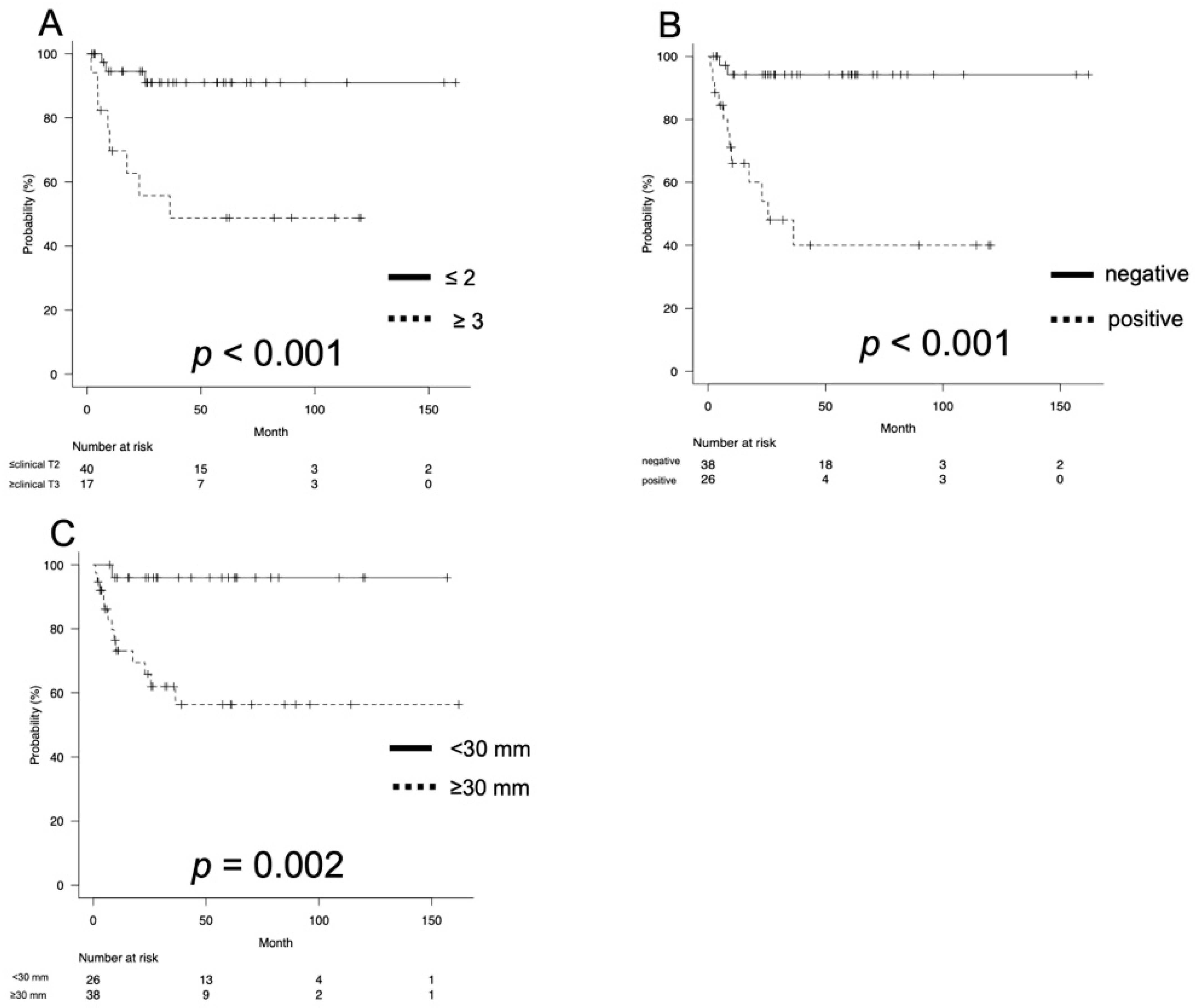
3.2. Oncological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [Green Version]
- Chavarriaga, J.; Pardo, J.; Suso-Palau, D.; Becerra, L.; Camacho, D.; Godoy, D.; Forero, J.; Cabrera, M.; Lopez-de-Mesa, B.; Ramirez, A.; et al. Real World Data of Penile Cancer Treatment at a High-Volume Center in South America: Insights and Survival Trends. Urology 2021, 156, 199–204. [Google Scholar] [CrossRef]
- Anderson, S.; Breen, K.J.; Davis, N.F.; Deady, S.; Sweeney, P. Penile cancer in Ireland–A national review. Surgeon 2021, 20, 187–193. [Google Scholar] [CrossRef]
- Marugame, T.; Katanoda, K.; Matsuda, T.; Hirabayashi, Y.; Kamo, K.; Ajiki, W.; Sobue, T. The Japan cancer surveillance report: Incidence of childhood, bone, penis and testis cancers. Jpn. J. Clin. Oncol. 2007, 37, 319–323. [Google Scholar] [CrossRef]
- Chipollini, J.; Pollock, G.; Hsu, C.H.; Batai, K.; Recio-Boiles, A.; Lee, B.R. National trends and survival outcomes of penile squamous cell carcinoma based on human papillomavirus status. Cancer Med. 2021, 10, 7466–7474. [Google Scholar] [CrossRef] [PubMed]
- Beech, B.; Izawa, J.; Pautler, S.; Chin, J.; Power, N. Penile cancer: Perspective from a Canadian tertiary care centre. Can. Urol. Assoc. J. 2015, 9, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Chakiryan, N.H.; Dahmen, A.; Bandini, M.; Pederzoli, F.; Marandino, L.; Albersen, M.; Roussel, E.; Zhu, Y.; Ye, D.; Ornellas, A.; et al. Risk factors and survival outcomes for upstaging after inguinal lymph node dissection for cN1 penile squamous cell carcinoma. Urol. Oncol. 2021, 39, 838.e7–838.e13. [Google Scholar] [CrossRef]
- Lughezzani, G.; Catanzaro, M.; Torelli, T.; Piva, L.; Biasoni, D.; Stagni, S.; Necchi, A.; Giannatempo, P.; Raggi, D.; Fare, E.; et al. Relationship between lymph node ratio and cancer-specific survival in a contemporary series of patients with penile cancer and lymph node metastases. BJU. Int. 2015, 116, 727–733. [Google Scholar] [CrossRef]
- Draeger, D.L.; Groh, S.; Buchholz, T.; Woehl, M.; Nolting, J.; Hakenberg, O.W. Prediction of treatment response and survival with chemotherapy for metastatic penile cancer by the modified Glasgow prognostic score. Urol. Int. 2021, 1–7. [Google Scholar] [CrossRef]
- Boehm, W.U.; Piontek, D.; Latarius, S.; Schoffer, O.; Borkowetz, A.; Klug, S.J.; Wirth, M.P. The Clinical Complexity of Penile Cancer: Current Clinical-Epidemiological Data from the Database of the Free State of Saxony/Germany. Urol. Int. 2022, 106, 706–715. [Google Scholar] [CrossRef]
- Azizi, M.; Peyton, C.C.; Boulware, D.C.; Chipollini, J.; Juwono, T.; Pow-Sang, J.M.; Spiess, P.E. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Penile Squamous Cell Carcinoma Patients Undergoing Inguinal Lymph Node Dissection. Eur. Urol. Focus. 2019, 5, 1085–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gondo, T.; Nakashima, J.; Ohno, Y.; Choichiro, O.; Horiguchi, Y.; Namiki, K.; Yoshioka, K.; Ohori, T.; Hatano, T.; Tachibana, M. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology 2012, 79, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, K.; Enomoto, T.; Kawada, K.; Fujimoto, S.; Ishida, T.; Takagi, K.; Nagai, S.; Ito, H.; Kawase, M.; Nakai, C.; et al. Utility of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune inflammation index as prognostic, predictive biomarkers in patients with metastatic renal cell carcinoma treated with nivolumab and ipilimumab. J. Clin. Med. 2021, 10, 5325. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.W.; Chia, S.J.; Chong, K.T. Management of penile cancer in a Singapore tertiary hospital. Arab. J. Urol. 2017, 15, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clinical. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Sanchez, D.F.; Fernandez-Nestosa, M.J.; Canete-Portillo, S.; Rodriguez, I.; Cubilla, A.L. What is new in the pathologic staging of penile carcinoma in the 8th edition of AJCC TNM model: Rationale for changes with practical stage-by-stage category diagnostic considerations. Adv. Anat. Pathol. 2021, 28, 209–227. [Google Scholar] [CrossRef]
- Perkins, N.J.; Schisterman, E.F. The inconsistency of ‘optimal’ cutpoints obtained using two criteria based on the receiver operating characteristics curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef] [Green Version]
- Moses, K.A.; Winer, A.; Sfakianos, J.P.; Poon, S.A.; Kent, M.; Bernstein, M.; Russo, P.; Dalbagni, G. Contemporary management of penile cancer: Greater than 15 year MSKCC experience. Can. J. Urol. 2014, 21, 7201–7206. [Google Scholar]
- Chavarriaga, J.; Camacho, D.; Suso-Palau, D.; Godoy, F.; Cabrera, M.; Forero, J.; Lopez-de-Mesa, B.; Varela, R. Inguinal lymph node density as a powerful predictor of cancer specific survival in patients with node-positive penile cancer. Urol. Oncol. 2021, 39, 839.e1–839.e8. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Long, Q.; Zhang, Z.; Liao, S.; Zheng, F. The prognostic value of lymph node ratio in comparison to positive lymph node count in penile squamous cell carcinoma. Int. Urol. Nephrol. 2021, 53, 2527–2540. [Google Scholar] [CrossRef]
- Liu, J.Y.; Li, Y.H.; Zhang, Z.L.; Yao, K.; Ye, Y.; Xie, D.; Han, H.; Liu, Z.; Qin, Z.; Zhou, F. The risk factors for the presence of pelvic lymph node metastasis in penile squamous cell carcinoma patients with inguinal lymph node dissection. World J. Urol. 2013, 31, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Lughezzani, G.; Catanzaro, M.; Torelli, T.; Piva, L.; Biasoni, D.; Stagni, S.; Crestani, A.; Guttilla, A.; Raggi, D.; Giannatempo, P.; et al. The relationship between characteristics of inguinal lymph nodes and pelvic lymph node involvement in penile squamous cell carcinoma: A single institution experience. J. Urol. 2014, 191, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Jeong, M.J.; Cha, J.; Lee, J.S.; Yoo, J.G.; Song, M.J.; Kim, J.H.; Lee, S.J.; Lee, H.N.; Yoon, J.H.; et al. Preoperative neutrophil-to-lymphocyte, platelet-to-lymphocyte and monocyte-to-lymphocyte ratio as a prognostic factor in non-endometrioid endometrial cancer. Int. J. Med. Sci. 2021, 18, 3712–3717. [Google Scholar] [CrossRef] [PubMed]
- Menon, H.; Patel, R.R.; Ludmir, E.B.; Muralidhar, V.; Cushman, T.R.; Amini, A.; Seyedin, S.N.; Nguyen, P.L.; Verma, V. Local management of preinvasive and clinical T1-3 penile cancer: Utilization of diverse treatment modalities. Future Oncol. 2020, 16, 955–960. [Google Scholar] [CrossRef]
- Li, K.; Wu, G.; Fan, C.; Yuan, H. The prognostic significance of primary tumor size in squamous cell carcinoma of the penis. Discov. Oncol. 2021, 12, 22. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Goddard, J.C.; Terry, T.R.; Summerton, D.J. The development of a supraregional network for the management of penile cancer. Ann. R Coll. Surg. Engl. 2012, 94, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Zhang, Y.; Wang, X.; Wang, P.; Geng, X.; Zhu, L.; Li, M. The prognostic significance of metastatic nodal size in non-surgical patients with esophageal squamous cell carcinoma. Front. Oncol. 2020, 10, 523. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Tan, X.; Wang, Y.; Zou, Y.; Chen, D.; Wu, Z.; Liu, Z.; Li, Z.; Qin, Z.; Han, H.; et al. Deep inguinal lymph node metastases can predict pelvic lymph node metastases and prognosis in penile squamous cell carcinoma. Front. Oncol. 2021, 11, 715–799. [Google Scholar] [CrossRef]
- Chechlinska, M.; Kowalewska, M.; Nowak, R. Systemic inflammation as a confounding factor in cancer biomarker discovery and validation. Nat. Rev. Cancer. 2010, 10, 2–3. [Google Scholar] [CrossRef]
- Roxburgh, C.S.; McMillan, D.C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010, 6, 149–163. [Google Scholar] [CrossRef]
- Patel, S.H.; Derweesh, I.H.; Saito, K.; Patil, D.; Meagher, M.F.; Bindayi, A.; Eldefrawy, A.; Patel, D.N.; Nasseri, R.; Yasuda, Y.; et al. Preoperative elevation of c-reactive protein Is a predictor for adverse oncologic survival outcomes for renal cell carcinoma: Analysis from the International Marker Consortium Renal Cancer (INMARC). Clin. Genitourin. Cancer. 2021, 198, e205–e215. [Google Scholar] [CrossRef] [PubMed]
- Steffens, S.; Ghazal, A.A.; Steinestel, J.; Lehmann, R.; Wegener, G.; Schnoeller, T.J.; Cronauer, M.V.; Jentzmik, F.; Schrader, M.; Kuczyk, M.A.; et al. High CRP value predict poor survival in patients with penile cancer. BMC Cancer 2013, 13, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | |
|---|---|
| Age (year, median, IQR) | 74 (63–82) |
| ECOG Performance Status (number, %) | |
| 0 | 31 (48.4) |
| 1 | 18 (28.1) |
| 2 | 8 (12.5) |
| 3 | 6 (9.4) |
| 4 | 1 (1.6) |
| Clinical T stage (number, %) | |
| Tis | 1 (1.6) |
| T1 | 26 (40.6) |
| T2 | 20 (31.3) |
| T3 | 14 (21.9) |
| T4 | 2 (3.1) |
| Clinical N stage (number, %) | |
| 0 | 38 (59.4) |
| 1 | 9 (14.1) |
| 2 | 8 (12.5) |
| 3 | 9 (14.1) |
| Clinical stage (number, %) | |
| Is | 1 (1.6) |
| 1 | 21 (32.8) |
| 2 | 14 (21.9) |
| 3 | 17 (26.6) |
| 4 | 11 (17.6) |
| Treatment modality (number, %) | |
| Surgery only | 51 (79.7) |
| Surgery and chemotherapy | 6 (9.4) |
| Radiation only | 1 (1.6) |
| Best supportive care | 6 (15.6) |
| Albumin (g/dL, median, IQR) | 4.2 (3.7–4.4) |
| Hemoglobin (g/dL, median, IQR) | 13.2 (11.4–14.8) |
| C-reactive protein (mg/dL, median, IQR) | 0.21 (0.06–1.28) |
| Tumor size (mm, median, IQR) | 30 (20–40) |
| Number of LNM (number, median, IQR) | 2 (1–6) |
| NLR (median, IQR) | 3.05 (2.03–4.96) |
| PLR (median, IQR) | 148 (105–228) |
| Pretreatment SCC (ng/mL, median, IQR) | 1.65 (1.23–4.18) |
| Follow-up period (months, median, IQR) | 26.0 (9.0–61.3) |
| Variables | p Value | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|
| Age (year) | |||
| ≥74 | 0.967 | 1.027 | 0.298–3.537 |
| Tumor size (mm) | |||
| ≥30 | 0.056 | 8.532 | 0.942–77.25 |
| Clinical T stage | |||
| ≥3 | 0.348 | 0.526 | 0.137–2.014 |
| Clinical N stage | |||
| ≥1 | 0.014 | 14.56 | 1.726–122.8 |
| C-reactive protein | |||
| ≥0.21 | 0.080 | 3.536 | 0.861–14.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawase, M.; Takagi, K.; Kawada, K.; Ishida, T.; Tomioka, M.; Enomoto, T.; Fujimoto, S.; Taniguchi, T.; Ito, H.; Kameyama, K.; et al. Clinical Lymph Node Involvement as a Predictor for Cancer-Specific Survival in Patients with Penile Squamous Cell Cancer. Curr. Oncol. 2022, 29, 5466-5474. https://doi.org/10.3390/curroncol29080432
Kawase M, Takagi K, Kawada K, Ishida T, Tomioka M, Enomoto T, Fujimoto S, Taniguchi T, Ito H, Kameyama K, et al. Clinical Lymph Node Involvement as a Predictor for Cancer-Specific Survival in Patients with Penile Squamous Cell Cancer. Current Oncology. 2022; 29(8):5466-5474. https://doi.org/10.3390/curroncol29080432
Chicago/Turabian StyleKawase, Makoto, Kimiaki Takagi, Kei Kawada, Takashi Ishida, Masayuki Tomioka, Torai Enomoto, Shota Fujimoto, Tomoki Taniguchi, Hiroki Ito, Koji Kameyama, and et al. 2022. "Clinical Lymph Node Involvement as a Predictor for Cancer-Specific Survival in Patients with Penile Squamous Cell Cancer" Current Oncology 29, no. 8: 5466-5474. https://doi.org/10.3390/curroncol29080432
APA StyleKawase, M., Takagi, K., Kawada, K., Ishida, T., Tomioka, M., Enomoto, T., Fujimoto, S., Taniguchi, T., Ito, H., Kameyama, K., Yamada, T., Kawase, K., Kato, D., Takai, M., Iinuma, K., Nakane, K., & Koie, T. (2022). Clinical Lymph Node Involvement as a Predictor for Cancer-Specific Survival in Patients with Penile Squamous Cell Cancer. Current Oncology, 29(8), 5466-5474. https://doi.org/10.3390/curroncol29080432






