Bortezomib, Lenalidomide and Dexamethasone Combination Induced Complete Remission in Relapsed/Refractory Plasmablastic Lymphoma: Case Report of a Potential Novel Treatment Approach
Abstract
:1. Introduction
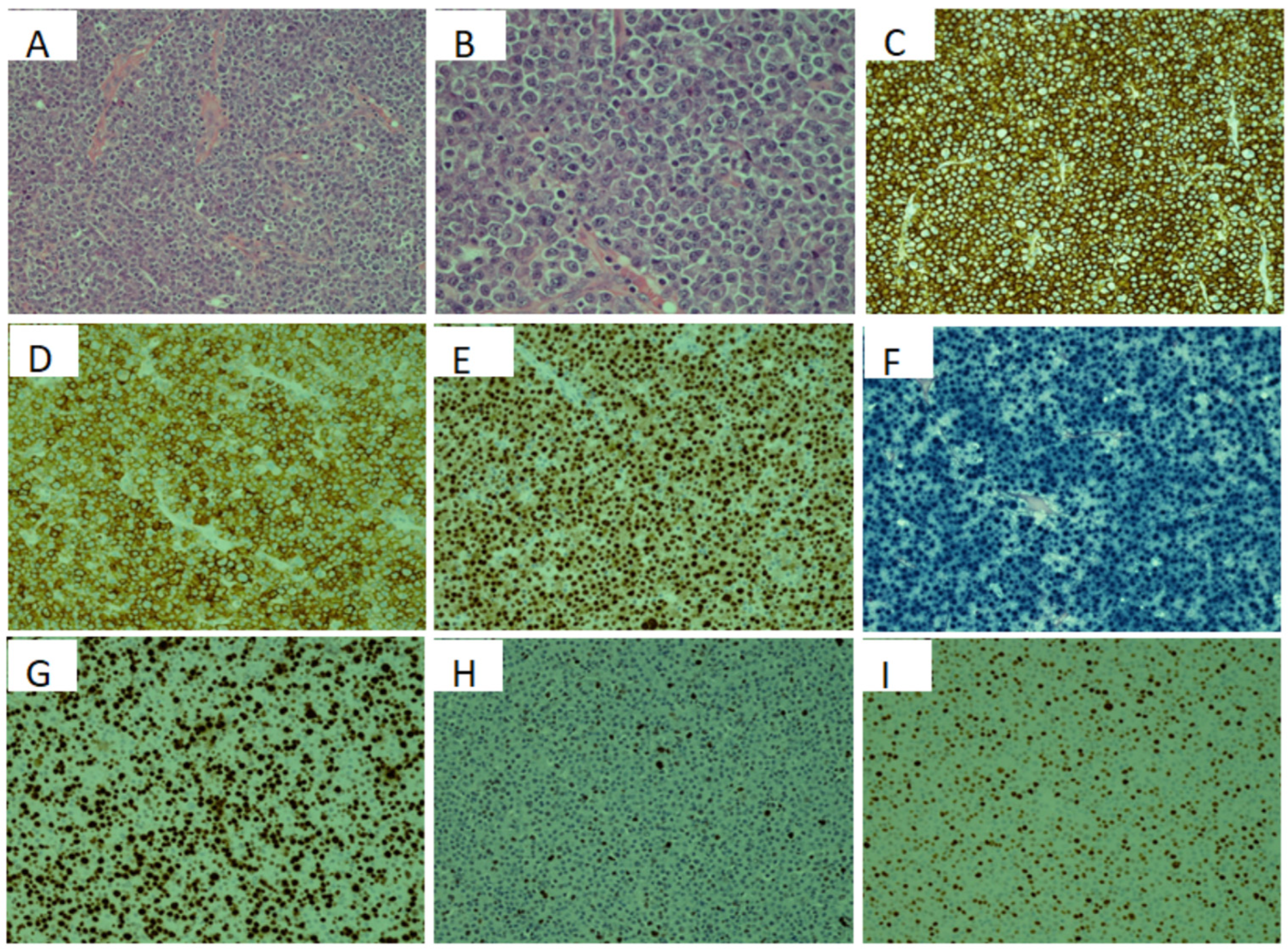
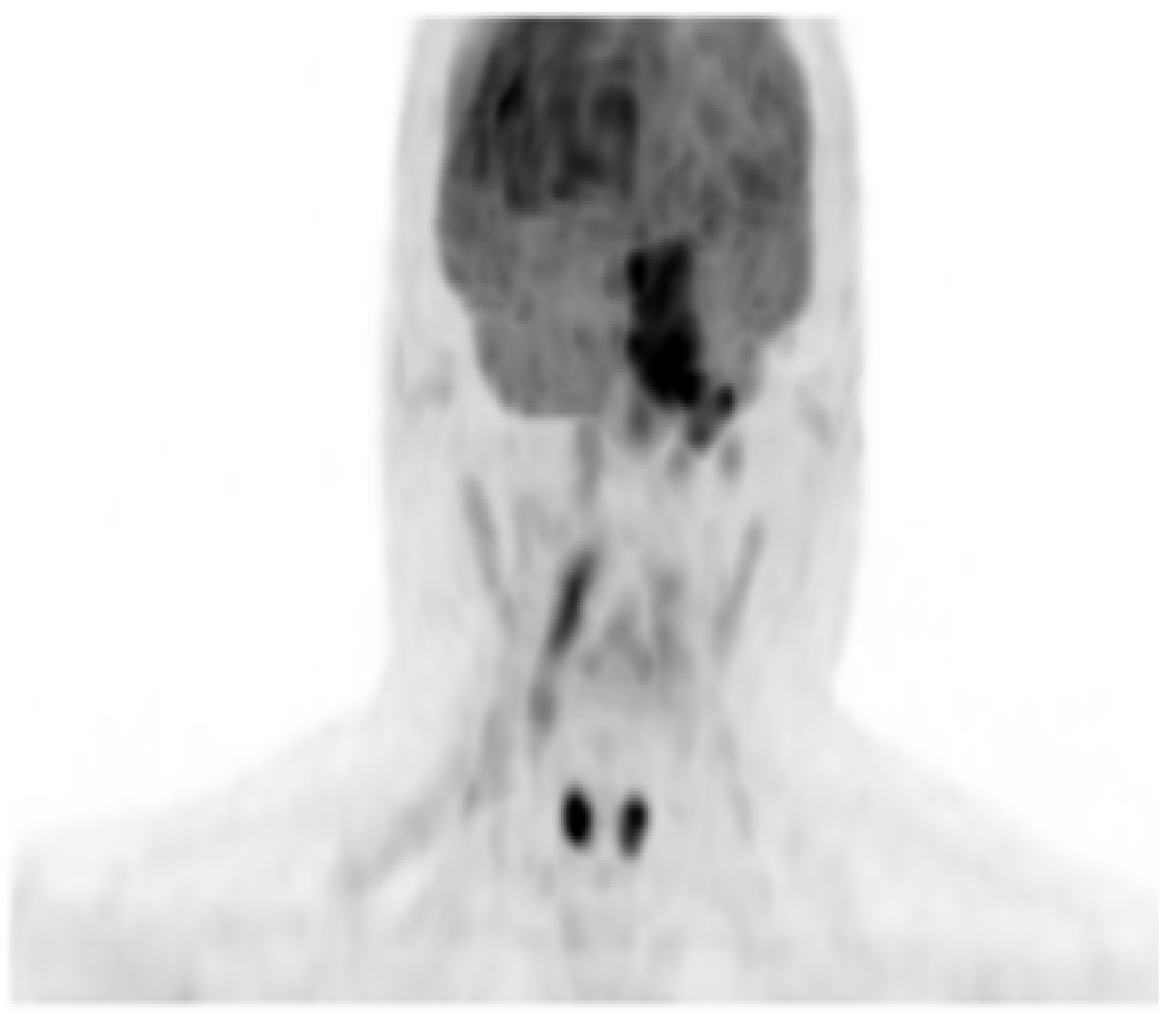
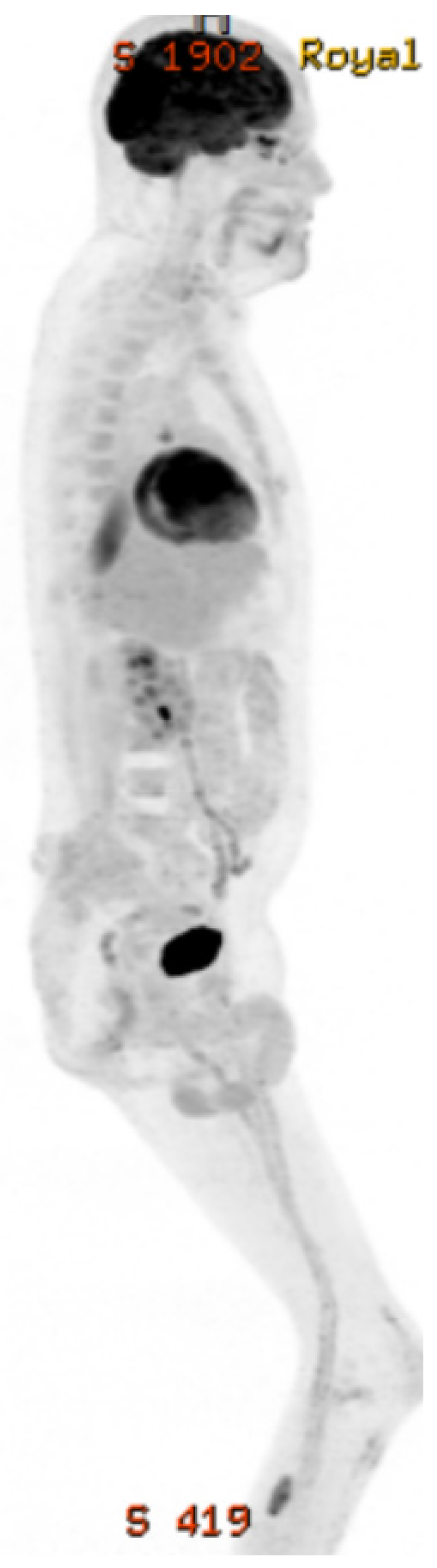
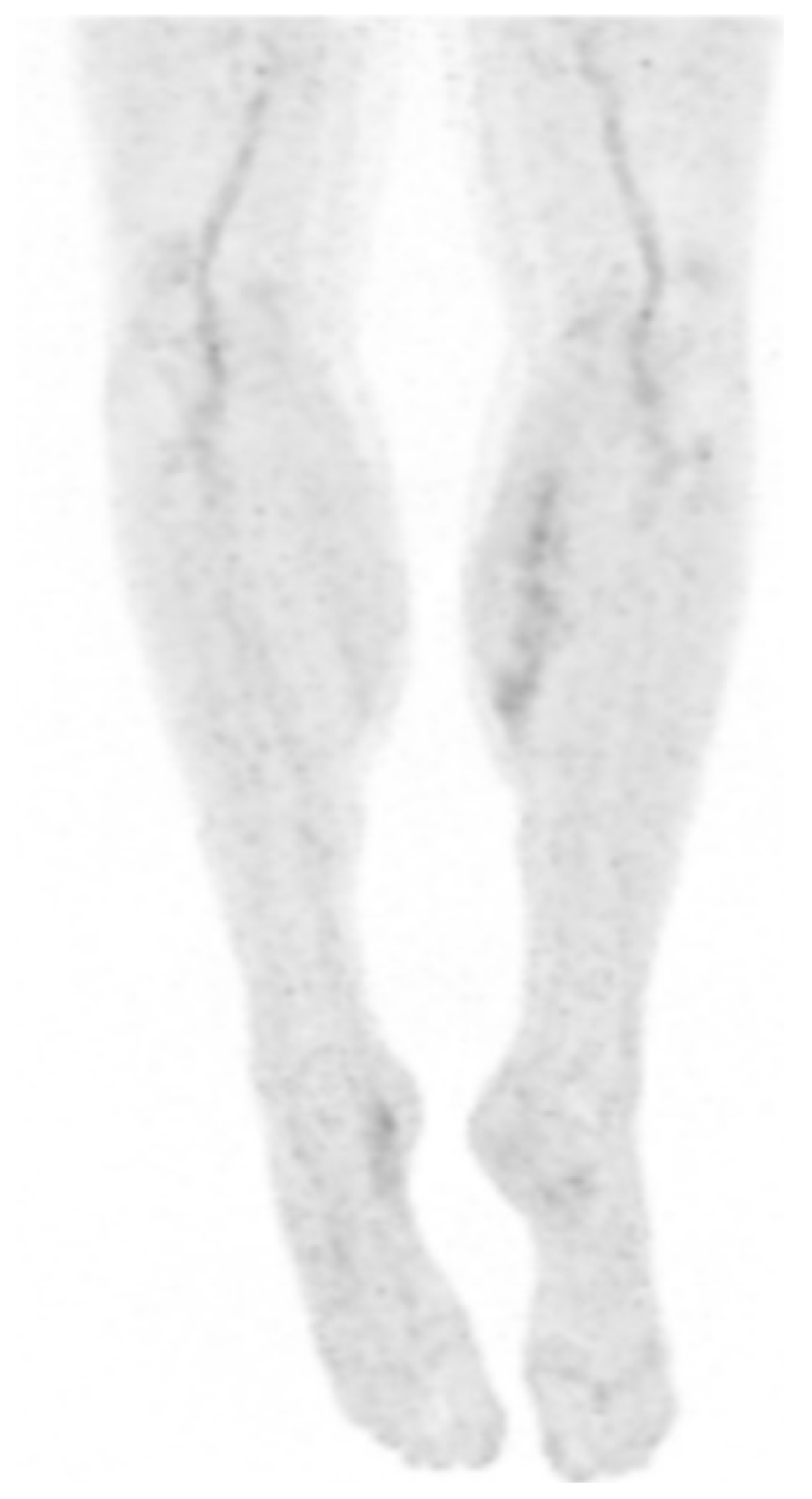
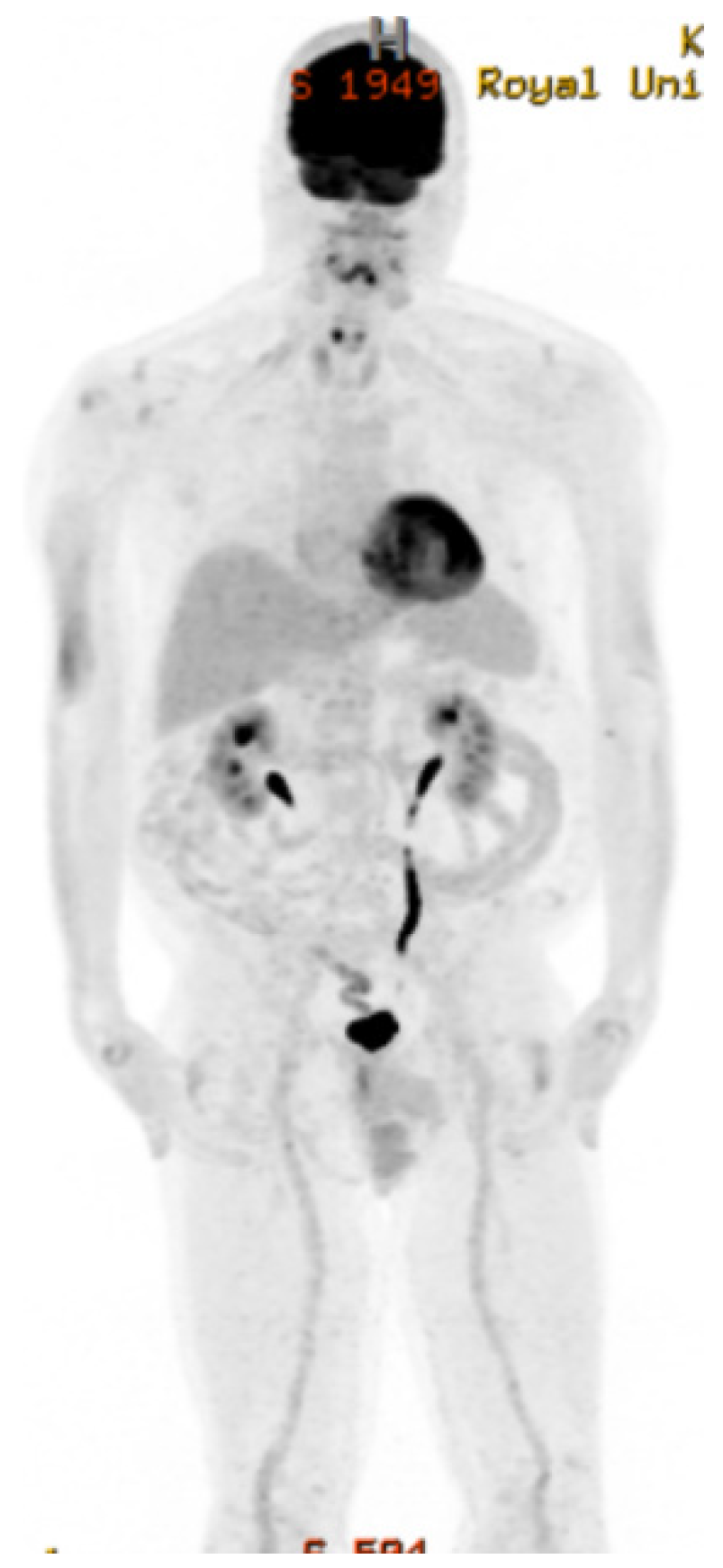
2. Case Presentation
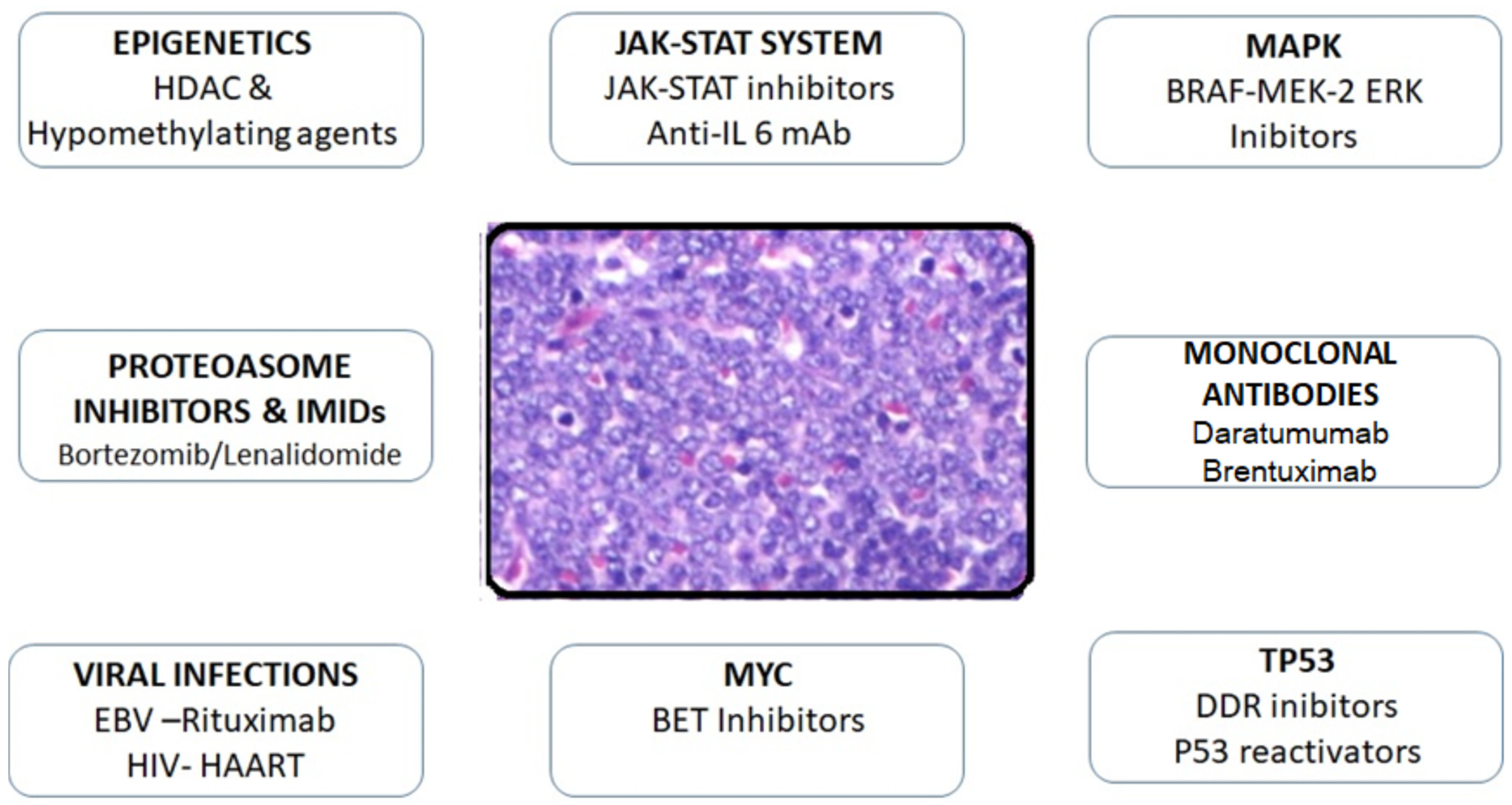
3. Discussion
3.1. Plasma Cell-Targeted Treatment Success
3.1.1. Bortezomib
3.1.2. Lenalidomide
3.1.3. Brentuximab
3.1.4. Daratumumab
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgment
Conflicts of Interest
References
- Carbone, A. AIDS-related non-Hodgkin’s lymphomas: From pathology and molecular pathogenesis to treatment. Hum. Pathol. 2002, 33, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Abrisqueta, P. Plasmablastic lymphoma: Current perspectives. Blood Lymphat. Cancer Targets Ther. 2018, 8, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega, F.; Chang, C.C.; Medeiros, L.J.; Udden, M.M.; Cho-Vega, J.H.; Lau, C.C.; Finch, C.J.; Vilchez, R.A.; McGregor, D.; Jorgensen, J.L. Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profiles. Mod. Pathol. 2005, 18, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Harmon, C.M.; Smith, L.B. Plasmablastic Lymphoma: A Review of Clinicopathologic Features and Differential Diagnosis. Arch. Pathol. Lab. Med. 2016, 140, 1074–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelenetz, A.D.; Gordon, L.I.; Wierda, W.G.; Abramson, J.S.; Advani, R.H.; Andreadis, C.B.; Bartlett, N.; Byrd, J.C.; Fayad, L.E.; Fisher, R.I.; et al. Diffuse large b-cell lymphoma version 1.2016: Clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2016, 14, 196–231. [Google Scholar] [CrossRef] [PubMed]
- Valera, A.; Balagué, O.; Colomo, L. IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas. Am. J. Surg. Pathol. 2011, 34, 1686–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelenetz, A.D.; Abramson, J.S.; Advani, R.H.; Andreadis, C.B.; Byrd, J.C.; Czuczman, M.S.; Fayad, L.; Forero, A.; Glenn, M.J.; Gockerman, J.P.; et al. NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin’s lymphomas. J. Natl. Compr. Canc. Netw. 2010, 8, 288–334. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, Y.; Uehira, T.; Ota, Y.; Ogawa, Y.; Yajima, K.; Tanuma, J.; Yotsumoto, M.; Hagiwara, S.; Ikegaya, S.; Watanabe, D.; et al. Clinical and pathological aspects of human immunodeficiency virus-associated plasmablastic lymphoma: Analysis of 24 cases. Int. J. Hematol. 2016, 104, 669–681. [Google Scholar] [CrossRef]
- Sparano, J.A.; Lee, J.Y.; Kaplan, L.D.; Levine, A.M.; Ramos, J.C.; Ambinder, R.F.; Wachsman, W.; Aboulafia, D.; Noy, A.; Henry, D.H.; et al. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood 2010, 115, 3008–3016. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, R.; Dhaval; Desai, S. Plasmablastic Lymphoma; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Plummer, R.M.; Linden, M.A.; Beckman, A.K. Update on B-cell lymphoproliferative disorders of the gastrointestinal tract. Semin. Diagn. Pathol. 2021, 38, 14–20. [Google Scholar] [CrossRef]
- Lurain, K.; Ramaswami, R.; Mangusan, R.; Widell, A.; Ekwede, I.; George, J.; Ambinder, R.; Cheever, M.; Gulley, J.L.; Goncalves, P.H.; et al. Use of pembrolizumab with or without pomalidomide in HIV-associated non-Hodgkin’s lymphoma. J. Immunother. Cancer 2021, 9, e002097. [Google Scholar] [CrossRef] [PubMed]
- Rong, C.; Sheng, L.; Wu, A.; Sun, Y.; Ouyang, G. Allogeneic hematopoietic stem cell transplantation in a patient with HIV-negative recurrent plasmablastic lymphoma: A case report. Medicine 2021, 100, e24498. [Google Scholar] [CrossRef] [PubMed]
- Tchernonog, E.; Faurie, P.; Coppo, P.; Monjanel, H.; Bonnet, A.; Algarte Génin, M.; Mercier, M.; Dupuis, J.; Bijou, F.; Herbaux, C.; et al. Clinical characteristics and prognostic factors of plasmablastic lymphoma patients: Analysis of 135 patients from the LYSA group. Ann. Oncol. 2017, 28, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Furman, M.; Beltrán, B.E.; Bibas, M.; Bower, M.; Chen, W.; Díez-Martín, J.L.; Liu, J.J.; Miranda, R.N.; Montoto, S.; et al. Human immunodeficiency virus-associated plasmablastic lymphoma: Poor prognosis in the era of highly active antiretroviral therapy. Cancer 2012, 118, 5270–5277. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, C.; Re, A.; Ungari, M.; Peli, A.; Casari, S.; Castelnuovo, F.; Fisogni, S.; Lonardi, S.; Pellegrini, V.; Petullà, M.; et al. Plasmablastic lymphoma among human immunodeficiency virus-positive patients: Results of a single center’s experience. Leuk. Lymphoma 2015, 56, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Re, A.; Cattaneo, C.; Michieli, M.; Casari, S.; Spina, M.; Rupolo, M.; Allione, B.; Nosari, A.; Schiantarelli, C.; Vigano, M.; et al. High-dose therapy and autologous peripheral-blood stem-cell transplantation as salvage treatment for HIV-associated lymphoma in patients receiving highly active antiretroviral therapy. J. Clin. Oncol. 2003, 21, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, B.; Liu, B.; Wang, Q.; Ding, L.; Xia, C.; Dong, L. Human immunodeficiency virus-negative plasmablastic lymphoma: A comprehensive analysis of 114 cases. Oncol. Rep. 2015, 33, 1615–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamadani, M.; Devine, S.M. Reduced-intensity conditioning allogeneic stem cell transplantation in HIV patients with hematologic malignancies: Yes, we can. Blood 2009, 114, 2564–2566. [Google Scholar] [CrossRef]
- Al-Malki, M.M.; Castillo, J.J.; Sloan, J.M.; Re, A. Hematopoietic cell transplantation for plasmablastic lymphoma: A review. Biol. Blood Marrow Transplant. 2014, 20, 1877–1884. [Google Scholar] [CrossRef] [Green Version]
- Pinnix, C.C.; Shah, J.J.; Chuang, H.; Costelloe, C.M.; Medeiros, L.J.; Wogan, C.F.; Reed, V.; Smith, G.L.; Milgrom, S.; Patel, K.; et al. Doxorubicin-Based Chemotherapy and Radiation Therapy Produces Favorable Outcomes in Limited-Stage Plasmablastic Lymphoma: A Single-Institution Review. Clin. Lymphoma Myeloma Leuk. 2016, 16, 122–128. [Google Scholar] [CrossRef]
- Li, Y.J.; Li, J.W.; Chen, K.L.; Li, J.; Zhong, M.Z.; Liu, X.L.; Yi, P.Y.; Zhou, H. HIV-negative plasmablastic lymphoma: Report of 8 cases and a comprehensive review of 394 published cases. Blood Res. 2020, 55, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Phipps, C.; Yeoh, K.W.; Lee, Y.S.; Nagarajan, C.; Gopalakrishnan, S.; Ho, L.P.; Hwang, W.Y.K.; Goh, Y.T.; Grigoropoulos, N.F. Durable remission is achievable with localized treatment and reduction of immunosuppression in limited stage EBV-related plasmablastic lymphoma. Ann. Hematol. 2017, 96, 1959–1960. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.; Bradrick, J.; Liu, Y.C. Spontaneous regression of an HIV-associated plasmablastic lymphoma in the oral cavity: A case report. J. Oral Maxillofac. Surg. 2007, 65, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Nasta, S.D.; Carrum, G.M.; Shahab, I.; Hanania, N.A.; Udden, M.M. Regression of a plasmblastic lymphoma in a patient with HIV on highly active antiretroviral therapy. Leuk. Lymphoma 2002, 43, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Bibas, M.; Miranda, R.N. The biology and treatment of plasmablastic lymphoma. Blood 2015, 125, 2323–2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, J.J.; Pantanowitz, L.; Dezube, B.J. HIV-associated plasmablastic lymphoma: Lessons learned from 112 published cases. Am. J. Hematol. 2008, 83, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Morscio, J.; Dierickx, D.; Nijs, J.; Verhoef, G.; Bittoun, E.; Vanoeteren, X.; Wlodarska, I.; Sagaert, X.; Tousseyn, T. Clinicopathologic comparison of plasmablastic lymphoma in HIV-positive, immunocompetent, and posttransplant patients: Single-center series of 25 cases and meta-analysis of 277 reported cases. Am. J. Surg. Pathol. 2014, 38, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Noy, A.; Chadburn, A.; Moore, S.Y.L. Plasmablastic Lymphoma Is Curable the HAART Era. A 10 Year Retrospective by the AIDS Malignancy Consortium (AMC). Blood 2013, 122, 1801. [Google Scholar] [CrossRef]
- Patel, K.; Feng, L.; Oki, Y.; Qazilbash, M.; Muzzafar, T.; Weber, D.; Fowler, N.; Poapat, U.; Thomas, S.; Fanale, M.; et al. Plasmablastic lymphoma: 28 patient single institution experience. Blood 2013, 122, 4310. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhang, L.; Ayala, E.; Field, T.; Ochoa-Bayona, J.L.; Perez, L.; Bello, C.M.; Chervenick, P.A.; Bruno, S.; Cultrera, J.L.; et al. Human immunodeficiency virus (HIV)-negative plasmablastic lymphoma: A single institutional experience and literature review. Leuk. Res. 2011, 35, 1571–1577. [Google Scholar] [CrossRef]
- Shipp, M.A.; Harrington, D.P.; Anderson, J.R.; Armitage, J.O.; Bonadonna, G.; Brittinger, G.; Cabanillas, F.; Canellos, G.P.; Coiffier, B.; Connors, J.M.; et al. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N. Engl. J. Med. 1993, 329, 987–994. [Google Scholar]
- Schommers, P.; Wyen, C.; Hentrich, M.; Gillor, D.; Zoufaly, A.; Jensen, B.; Bogner, J.R.; Thoden, J.; Wasmuth, J.C.; Fätkenheuer, G.; et al. Poor outcome of HIV-infected patients with plasmablastic lymphoma: Results from the German AIDS-related lymphoma cohort study. AIDS 2013, 27, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Loghavi, S.; Khoury, J.D.; Medeiros, L.J. Epstein-Barr Virus-Positive Plasmacytoma in Immunocompetent Patients. Histopathology 2015, 67, 225–234. [Google Scholar] [CrossRef]
- Castillo, J.J.; Winer, E.S.; Stachurski, D.; Perez, K.; Jabbour, M.; Milani, C.; Colvin, G.; Butera, J.N. Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphoma. Leuk. Lymphoma 2010, 51, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Winer, E.S.; Stachurski, D.; Perez, K.; Jabbour, M.; Milani, C.; Colvin, G.; Butera, J.N. Prognostic factors in chemotherapy-treated patients with HIV-associated Plasmablastic lymphoma. Oncologist 2010, 15, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Castillo, J.J.; Winer, E.S.; Stachurski, D.; Perez, K.; Jabbour, M.; Milani, C.; Colvin, G.; Butera, J.N. HIV-negative plasmablastic lymphoma: Not in the mouth. Clin. Lymphoma Myeloma Leuk. 2011, 11, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.P.; Kolibaba, K.; Reeves, J.A.; Tulpule, A.; Flinn, I.W.; Kolevska, T.; Robles, R.; Flowers, C.R.; Collins, R.; DiBella, N.J.; et al. Randomized phase 2 open-label study of R-CHOP ± bortezomib in patients (Pts) with untreated non-germinal center B-cell-like (Non-GCB) subtype diffuse large cell lymphoma (DLBCL): Results from the Pyramid trial (NCT00931918). Blood 2015, 126, 811. [Google Scholar] [CrossRef]
- Castillo, J.J.; Advani, R.H.; Branagan, A.R.; Buske, C.; Dimopoulos, M.A.; D’Sa, S.; Kersten, M.J.; Leblond, V.; Minnema, M.C.; Owen, R.G.; et al. Consensus treatment recommendations from the tenth International Workshop for Waldenström Macroglobulinaemia. Lancet Haematol. 2020, 7, e827–e837. [Google Scholar] [CrossRef]
- Robak, T.; Huang, H.; Jin, J.; Zhu, J.; Liu, T.; Samoilova, O.; Pylypenko, H.; Verhoef, G.; Siritanaratkul, N.; Osmanov, E.; et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N. Engl. J. Med. 2015, 372, 944–953. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Garcia, T.A.; Mogollon, R.J.; Castillo, J.J. Bortezomib in plasma-blastic lymphoma: A glimpse of hope for a hard-to-treat disease. Leuk. Res. 2017, 62, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Alvarez, R.; Gonzalez-Rodriguez, A.P.; Rubio-Castro, A.; Gonzalez, M.E.; Payer, A.R.; Alonso-Garcia, A.; Rodriguez-Villar, D.; Dominguez-Iglesias, F.; Sancho, J.M. Bortezomib plus CHOP for the treatment of HIV-associated plasmablas-tic lymphoma: Clinical experience in three patients. Leuk. Lymphoma 2016, 57, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Guerrero-Garcia, T.; Baldini, F.; Tchernonog, E.; Cartron, G.; Ninkovic, S.; Cwynarski, K.; Dierickx, D.; Tousseyn, T.; Lansigan, F.; et al. Bortezomib plus EPOCH is effective as frontline treatment in patients with plasmablastic lymphoma. Br. J. Haematol. 2019, 184, 679–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dittus, C.; Grover, N.; Ellsworth, S.; Tan, X.; Park, S.I. Bortezomib in combination with dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) induces long-term survival in patients with plasmablastic lymphoma: A retrospective analysis. Leuk Lymphoma 2018, 59, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhu, D.; Dong, H.; Ji, M.; Wu, J.; Xu, X.; Cheng, G.; Wu, C.; Jiang, J. Lenalidomide alone or in combination with chemotherapy treatment for subtypes of diffuse large B cell lymphoma: A systematic review and meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 10705–10713. [Google Scholar]
- Carras, S.; Regny, C.; Peoc’h, M.; Gervasoni, J.; Gressin, R.; Cahn, J.Y.; Molina, L. Dramatic efficacy of low dose lenalidomide as single agent in a patient with refractory gastric nonhuman immunodeficiency virus-associated plasmablastic lymphoma. Leuk. Lymphoma 2015, 56, 2986–2988. [Google Scholar] [CrossRef] [PubMed]
- Bibas, M.; Grisetti, S.; Alba, L. Patient with HIV-associated plasmablastic lymphoma responding to bortezomib alone and in combination with dexamethasone, gemcitabine, oxaliplatin, cytarabine, and pegfilgrastim chemotherapy and lenalidomide alone. J. Clin. Oncol. 2012, 30, 2012–2014. [Google Scholar] [CrossRef]
- Yanamandra, U.; Sahu, K.K.; Jain, N.; Prakash, G.; Saikia, U.; Malhotra, P. Plasmablastic lymphoma: Successful management with CHOP and lenalidomide in resource constraint settings. Ann. Hematol. 2016, 95, 1715–1717. [Google Scholar] [CrossRef]
- Schmit, J.M.; Delaune, J.; Norkin, M.; Grosbach, A. A case of plasmablastic lymphoma achieving complete response and durable remission after lenalidomide-based therapy. Oncol. Res. Treat. 2017, 40, 46–48. [Google Scholar] [CrossRef]
- Ando, K.; Imaizumi, Y.; Kobayashi, Y.; Niino, D.; Hourai, M.; Sato, S.; Sawayama, Y.; Hata, T.; Ohshima, K.; Miyazaki, Y. Bortezomib- and Lenalidomide-Based Treatment of Refractory Plasmablastic Lymphoma. Oncol. Res. Treat. 2020, 43, 112–116. [Google Scholar] [CrossRef]
- Cheng, L.; Song, Q.; Liu, M.; Wang, Y.; Yi, H.; Qian, Y.; Xu, P.; Cheng, S.; Wang, C.; Wang, L.; et al. Case Report: Successful Management of a Refractory Plasmablastic Lymphoma Patient with Tislelizumab and Lenalidomide. Front. Immunol. 2021, 12, 702593. [Google Scholar] [CrossRef]
- Marrero, W.D.; Cruz-Chacón, A.; Castillo, C.; Cabanillas, F. Successful Use of Bortezomib-Lenalidomide Combination as Treatment for a Patient with Plasmablastic Lymphoma. Clin. Lymphoma Myeloma Leuk. 2018, 18, e275–e277. [Google Scholar] [CrossRef] [PubMed]
- Holderness, B.M.; Malhotra, S.; Levy, N.B.; Danilov, A.V. Brentuximab vedotin demonstrates activity in a patient with plasmablastic lymphoma arising from a background of chronic lymphocytic leukemia. J. Clin. Oncol. 2013, 31, e197–e199. [Google Scholar] [CrossRef] [PubMed]
- Dittus, C.; Miller, J.A.; Wehbie, R.; Castillo, J.J. Daratumumab with ifosfamide, carboplatin and etoposide for the treatment of relapsed plasmablastic lymphoma. Br. J. Haematol. 2022; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kathrotiya, M.; Radhakrishnan, V.S.; Bhave, S.J.; Kumar, J.; Roychowdhury, M.; Arun, I.; Das, J.; Chandy, M.; Nair, R. Relapsed plasmablastic lymphoma in a HIV-negative patient: Pushing the envelope. Clin. Case Rep. 2020, 9, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Ricker, E.C.; Ryu, Y.K.; Amengual, J.E. Daratumumab plus chemotherapy induces complete responses in a consecutive series of four patients with plasmablastic lymphoma. Blood 2021, 138, 4573. [Google Scholar] [CrossRef]
- Principal Investigator: Ferreri, A. Study to Evaluate Combined Treatment of Daratumumab, Bortezomib and Dexamethasone in PBL Patient’s. Available online: https://clinicaltrials.gov/ct2/show/NCT04915248 (accessed on 7 June 2021).
- Pileri, S.A.; Mazzara, S.; Derenzini, E. Plasmablastic lymphoma: One or more tumors? Haematologica 2021, 106, 2542–2543. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabry, W.; Wu, Y.; Kodad, S.G. Bortezomib, Lenalidomide and Dexamethasone Combination Induced Complete Remission in Relapsed/Refractory Plasmablastic Lymphoma: Case Report of a Potential Novel Treatment Approach. Curr. Oncol. 2022, 29, 5042-5053. https://doi.org/10.3390/curroncol29070399
Sabry W, Wu Y, Kodad SG. Bortezomib, Lenalidomide and Dexamethasone Combination Induced Complete Remission in Relapsed/Refractory Plasmablastic Lymphoma: Case Report of a Potential Novel Treatment Approach. Current Oncology. 2022; 29(7):5042-5053. https://doi.org/10.3390/curroncol29070399
Chicago/Turabian StyleSabry, Waleed, Yue Wu, and Shruthi Ganeshappa Kodad. 2022. "Bortezomib, Lenalidomide and Dexamethasone Combination Induced Complete Remission in Relapsed/Refractory Plasmablastic Lymphoma: Case Report of a Potential Novel Treatment Approach" Current Oncology 29, no. 7: 5042-5053. https://doi.org/10.3390/curroncol29070399
APA StyleSabry, W., Wu, Y., & Kodad, S. G. (2022). Bortezomib, Lenalidomide and Dexamethasone Combination Induced Complete Remission in Relapsed/Refractory Plasmablastic Lymphoma: Case Report of a Potential Novel Treatment Approach. Current Oncology, 29(7), 5042-5053. https://doi.org/10.3390/curroncol29070399




