Surgery for Pituitary Tumor Apoplexy Is Associated with Rapid Headache and Cranial Nerve Improvement
Abstract
:1. Introduction
2. Methods
2.1. Record Collection
2.2. Statistical Analyses
3. Results
3.1. Baseline Characteristics
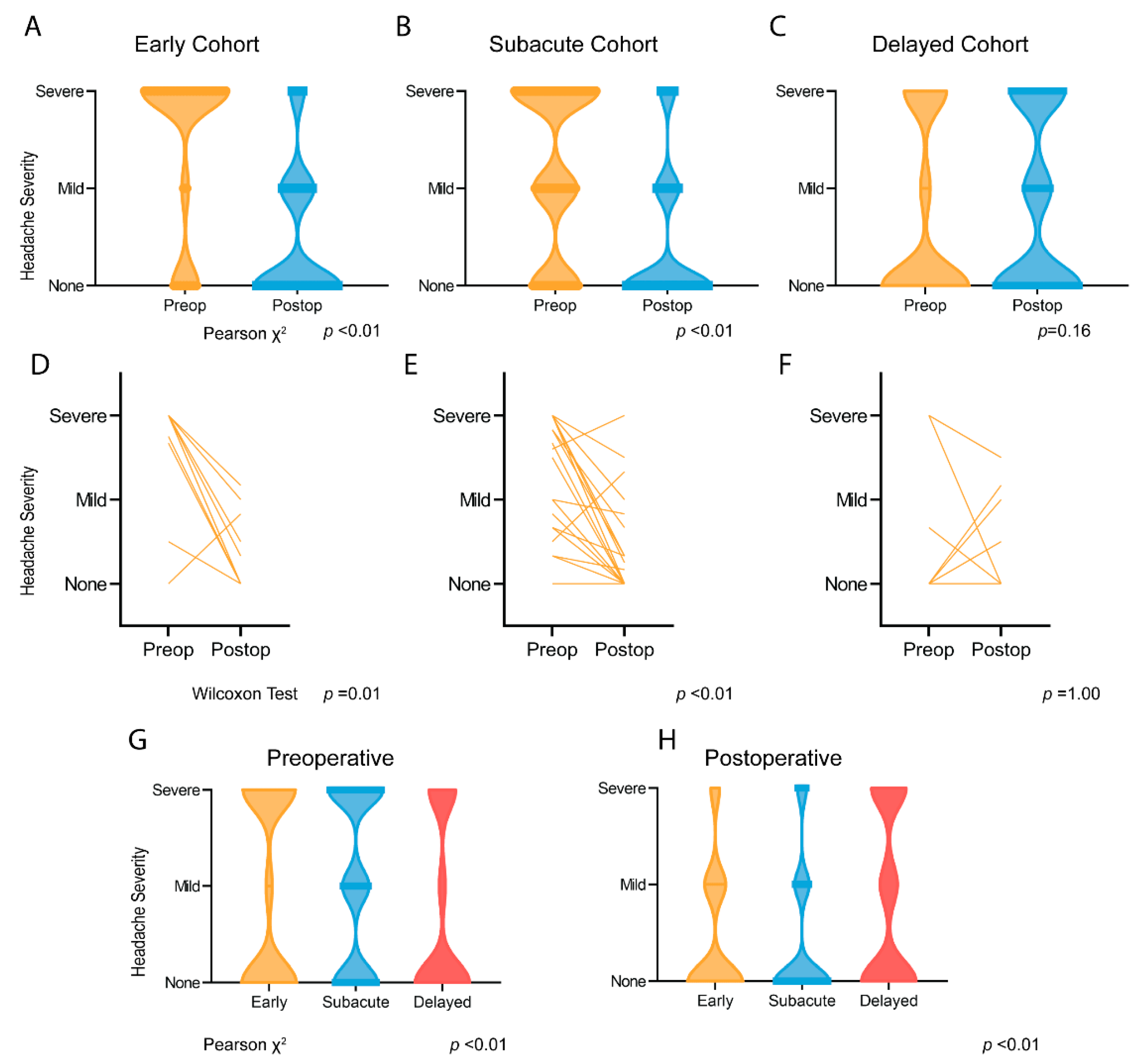
3.2. Headache Presentation and Resolution
3.3. Clinical Presentation of Cranial Nerve Deficits
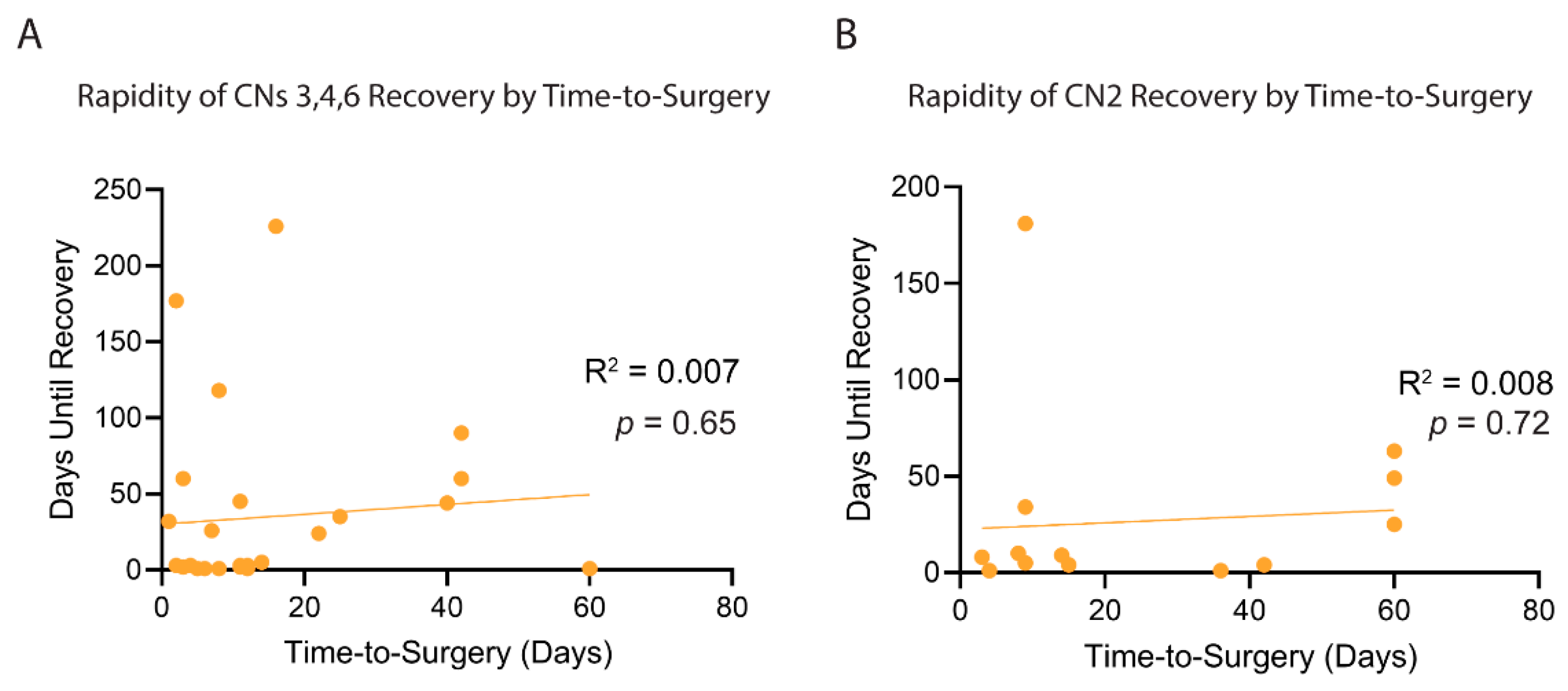
3.4. Resolution and Improvement in Cranial Nerve Impairments
3.5. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Briet, C.; Salenave, S.; Bonneville, J.-F.; Laws, E.R.; Chanson, P. Pituitary apoplexy. Endocr. Rev. 2015, 36, 622–645. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Dunn, I.F.; Laws, E.R. Pituitary apoplexy. Endocrine 2015, 48, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.C.; Hamrahian, A.H.; Weil, R.J.; Kennedy, L. Pituitary tumor apoplexy. J. Clin. Neurosci. 2015, 22, 939–944. [Google Scholar] [CrossRef]
- Arafah, B.M.; Prunty, D.; Ybarra, J.; Hlavin, M.L.; Selman, W.R. The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas. J. Clin. Endocrinol. Metab. 2000, 85, 1789–1793. [Google Scholar] [PubMed] [Green Version]
- Levy, M.J.; Matharu, M.S.; Meeran, K.; Powell, M.; Goadsby, P.J. The clinical characteristics of headache in patients with pituitary tumours. Brain 2005, 128, 1921–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, Y.; Sasagawa, Y.; Oishi, M.; Kita, D.; Misaki, K.; Fukui, I.; Tachibana, O.; Nakada, M. Contribution of intrasellar pressure elevation to headache manifestation in pituitary adenoma evaluated with intraoperative pressure measurement. Neurosurgery 2019, 84, 599–606. [Google Scholar] [CrossRef]
- Zayour, D.H.; Selman, W.R.; Arafah, B.M. Extreme elevation of intrasellar pressure in patients with pituitary tumor apoplexy: Relation to pituitary function. J. Clin. Endocrinol. Metab. 2004, 89, 5649–5654. [Google Scholar] [CrossRef] [Green Version]
- Brougham, M.; Heusner, A.P.; Adams, R.D. Acute degenerative changes in adenomas of the pituitary body--with special reference to pituitary apoplexy. J. Neurosurg. 1950, 7, 421–439. [Google Scholar] [CrossRef]
- Epstein, S.; Pimstone, B.L.; de Villiers, J.C.; Jackson, W.P. Pituitary apoplexy in five patients with pituitary tumours. Br. Med. J. 1971, 2, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Dawson, B.H.; Kothandaram, P. Acute massive infarction of pituitary adenomas: A study of five patients. J. Neurosurg. 1972, 37, 275–279. [Google Scholar] [CrossRef]
- Ebersold, M.J.; Laws, E.R., Jr.; Scheithauer, B.W.; Randall, R.V. Pituitary apoplexy treated by transsphenoidal surgery: A clinicopathological and immunocytochemical study. J. Neurosurg. 1983, 58, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Findling, J.W.; Tyrrell, J.B.; Aron, D.C.; Fitzgerald, P.A.; Wilson, C.B.; Forsham, P.H. Silent pituitary apoplexy: Subclinical infarction of an adrenocorticotropin-producing pituitary adenoma. J. Clin. Endocrinol. Metab. 1981, 52, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Onesti, S.T.; Wisniewski, T.; Post, K.D. Clinical versus subclinical pituitary apoplexy: Presentation, surgical management, and outcome in 21 patients. Neurosurgery 1990, 26, 980–986. [Google Scholar] [CrossRef]
- Glick, R.P.; Tiesi, J.A. Subacute pituitary apoplexy: Clinical and magnetic resonance imaging characteristics. Neurosurgery 1990, 27, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Park, Y.-S.; Kwon, J.-T.; Nam, T.-K.; Lee, T.-J.; Kim, J.-K. Radiological apoplexy and its correlation with acute clinical presentation, angiogenesis and tumor microvascular density in pituitary adenomas. J. Korean Neurosurg. Soc. 2011, 50, 281. [Google Scholar] [CrossRef]
- Ranabir, S.; Baruah, M.P. Pituitary apoplexy: Its incidence and clinical significance. J. Neurosurg. 1981, 55, 187–193. [Google Scholar]
- Rajasekaran, S.; Vanderpump, M.; Baldeweg, S.; Drake, W.; Reddy, N.; Lanyon, M.; Markey, A.; Plant, G.; Powell, M.; Sinha, S.; et al. UK guidelines for the management of pituitary apoplexy. Clin. Endocrinol. 2011, 74, 9–20. [Google Scholar] [CrossRef]
- Reddy, N.L.; Rajasekaran, S.; Han, T.S.; Theodoraki, A.; Drake, W.; Vanderpump, M.; Baldeweg, S.; Wass, J.A.H. An objective scoring tool in the management of patients with pituitary apoplexy. Clin. Endocrinol. 2011, 75, 723. [Google Scholar] [CrossRef]
- Almeida, J.P.; Sanchez, M.M.; Karekezi, C.; Warsi, N.; Fernández-Gajardo, R.; Panwar, J.; Mansouri, A.; Suppiah, S.; Nassiri, F.; Nejad, R.; et al. Pituitary apoplexy: Results of surgical and conservative management clinical series and review of the literature. World Neurosurg. 2019, 130, e988–e999. [Google Scholar] [CrossRef]
- Goshtasbi, K.; Abiri, A.; Sahyouni, R.; Mahboubi, H.; Raefsky, S.; Kuan, E.C.; Hsu, F.P.K.; Cadena, G. Visual and endocrine recovery following conservative and surgical treatment of pituitary apoplexy: A meta-analysis. World Neurosurg. 2019, 132, 33–40. [Google Scholar] [CrossRef]
- Hage, R.; Eshraghi, S.R.; Oyesiku, N.M.; Ioachimescu, A.G.; Newman, N.J.; Biousse, V.; Bruce, B.B. Third, fourth, and sixth cranial nerve palsies in pituitary apoplexy. World Neurosurg. 2016, 94, 447–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabuk, B.; Kaya, N.S.; Polat, C.; Geyik, A.M.; Icli, D.; Anik, I.; Ceylan, S. Outcome in pituitary apoplexy patients, stratified by delay between symptom appearance and surgery: A single center retrospective analysis. Clin. Neurol. Neurosurg. 2021, 210, 106991. [Google Scholar] [CrossRef] [PubMed]
- Bujawansa, S.; Thondam, S.K.; Steele, C.; Cuthbertson, D.J.; Gilkes, C.E.; Noonan, C.; Bleaney, C.W.; Macfarlane, I.A.; Javadpour, M.; Daousi, C. Presentation, management and outcomes in acute pituitary apoplexy: A large single-centre experience from the United Kingdom. Clin. Endocrinol. 2014, 80, 419–424. [Google Scholar] [CrossRef]
- Semple, P.L.; Webb, M.K.; de Villiers, J.C.; Laws, E.R., Jr. Pituitary apoplexy. Neurosurgery 2005, 56, 65–73. [Google Scholar] [CrossRef]
- Pangal, D.J.; Chesney, K.; Memel, Z.; Bonney, P.A.; Strickland, B.A.; Carmichael, J.; Shiroishi, M.; Liu, C.-S.J.; Zada, G. Pituitary apoplexy case series: Outcomes after endoscopic endonasal transsphenoidal surgery at a single tertiary center. World Neurosurg. 2020, 137, e366–e372. [Google Scholar] [CrossRef]
- Bills, D.C.; Meyer, F.B.; Laws, E.R., Jr.; Davis, D.H.; Ebersold, M.J.; Scheithauer, B.W.; Ilstrup, D.M.; Abboud, C.F. A retrospective analysis of pituitary apoplexy. Neurosurgery 1993, 33, 602–609. [Google Scholar] [PubMed]
- Randeva, H.S.; Schoebel, J.; Byrne, J.; Esiri, M.; Adams, C.B.; Wass, J.A. Classical pituitary apoplexy: Clinical features, management and outcome. Clin. Endocrinol. 1999, 51, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Budohoski, K.P.; Khawari, S.; Cavalli, A.; Quah, B.L.; Kolias, A.; Waqar, M.; Krishnan, P.G.; Lawes, I.; Cains, F.; Arwyn-Jones, J.; et al. Long-term oncological outcomes after haemorrhagic apoplexy in pituitary adenoma managed operatively and non-operatively. Acta Neurochirurgica 2022, 164, 1–9. [Google Scholar] [CrossRef]
- Zaidi, H.A.; Cote, D.J.; Burke, W.T.; Castlen, J.P.; Bi, W.L.; Laws, E.R., Jr.; Dunn, I.F. Time Course of Symptomatic Recovery After Endoscopic Transsphenoidal Surgery for Pituitary Adenoma Apoplexy in the Modern Era. World Neurosurg. 2016, 96, 434–439. [Google Scholar] [CrossRef]
- Loder, E.; Burch, R. Measuring pain intensity in headache trials: Which scale to use? Cephalalgia 2012, 32, 179–182. [Google Scholar] [CrossRef]
- Leyer, C.; Castinetti, F.; Morange, I.; Gueydan, M.; Oliver, C.; Conte-Devolx, B.; Dufour, H.; Brue, T. A conservative management is preferable in milder forms of pituitary tumor apoplexy. J. Endocrinol. Investig. 2011, 34, 502–509. [Google Scholar] [CrossRef]
- Maccagnan, P.; Macedo, C.L.; Kayath, M.J.; Nogueira, R.G.; Abucham, J. Conservative management of pituitary apoplexy: A prospective study. J. Clin. Endocrinol. Metab. 1995, 80, 2190–2197. [Google Scholar] [CrossRef] [PubMed]
- Ayuk, J.; McGregor, E.J.; Mitchell, R.D.; Gittoes, N.J. Acute management of pituitary apoplexy--surgery or conservative management? Clin. Endocrinol. 2004, 61, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sibal, L.; Ball, S.G.; Connolly, V.; James, R.A.; Kane, P.; Kelly, W.F.; Kendall-Taylor, P.; Mathias, D.; Perros, P.; Quinton, R.; et al. Pituitary apoplexy: A review of clinical presentation, management and outcome in 45 cases. Pituitary 2004, 7, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Giritharan, S.; Gnanalingham, K.; Kearney, T. Pituitary apoplexy–bespoke patient management allows good clinical outcome. Clin. Endocrinol. 2016, 85, 415–422. [Google Scholar] [CrossRef] [PubMed]

| Total | Operative Timeframe Cohort | p Value | |||
|---|---|---|---|---|---|
| Early (<=72 h) | Subacute (4 d–14 d) | Delayed (>14d) | |||
| Number of Patients (%) | 59 (100) | 13 (22) | 27 (45) | 19 (32) | - |
| Headaches | 59 (100) | 13 (100) | 27 (100) | 19 (100) | - |
| Radiologic Hemorrhage | 59 (100) | 13 (100) | 27 (100) | 19 (100) | - |
| Hemorrhage or Necrosis | 59 (100) | 13 (100) | 27 (100) | 19 (100) | - |
| Mean Time to Surgery (d) | 17.6 | 1.9 | 9.0 | 40.4 | <0.01 |
| Mean Tumor Diameter (mm) | 24.9 | 32.3 | 22.9 | 22.7 | <0.01 |
| Cranial Nerve Deficits | 46 (78) | 11 (85) | 22 (81) | 13 (68) | 0.46 |
| Blindness, Uni or Bilateral | 5 (8) | 3 (23) | 2 (7) | 0 (0) | 0.06 |
| Endoscopic Endonasal | 56 (95) | 12 (92) | 25 (93) | 19 (100) | 0.47 |
| Sublabial | 3 (5) | 1 (8) | 2 (7) | 0 (0) | |
| Cranial Nerve | Unilateral | Bilateral | p Value |
|---|---|---|---|
| II | 7 | 15 | |
| III | 26 | 0 | <0.01 |
| VI | 13 | 1 |
| Total | Operative Timeframe Cohort | p Value | |||
|---|---|---|---|---|---|
| Early (<=72 h) | Subacute (4 d–14 d) | Delayed (>14 d) | |||
| Deep Vein Thrombosis | 2 (3) | 0 (0) | 2 (7) | 0 (0) | 0.29 |
| Diabetes Insipidus | 5 (8) | 1 (7) | 3 (11) | 1 (5) | 0.78 |
| Heparin Induced Thrombocytopenia | 1 (2) | 1 (7) | 0 (0) | 0 (0) | 0.17 |
| Pneumonia | 1 (2) | 0 (0) | 1 (3) | 0 (0) | 0.55 |
| Cerebrospinal Fluid Leak | 10 (17) | 2 (15) | 5 (19) | 3 (16) | 0.96 |
| Death During Hospitalization | 1 (2) | 1 (7) | 0 (0) | 0 (0) | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cross, K.A.; Desai, R.; Vellimana, A.; Liu, Y.; Rich, K.; Zipfel, G.; Dacey, R.; Chicoine, M.; Klatt-Cromwell, C.; McJunkin, J.; et al. Surgery for Pituitary Tumor Apoplexy Is Associated with Rapid Headache and Cranial Nerve Improvement. Curr. Oncol. 2022, 29, 4914-4922. https://doi.org/10.3390/curroncol29070390
Cross KA, Desai R, Vellimana A, Liu Y, Rich K, Zipfel G, Dacey R, Chicoine M, Klatt-Cromwell C, McJunkin J, et al. Surgery for Pituitary Tumor Apoplexy Is Associated with Rapid Headache and Cranial Nerve Improvement. Current Oncology. 2022; 29(7):4914-4922. https://doi.org/10.3390/curroncol29070390
Chicago/Turabian StyleCross, Kevin A., Rupen Desai, Ananth Vellimana, Yupeng Liu, Keith Rich, Gregory Zipfel, Ralph Dacey, Michael Chicoine, Cristine Klatt-Cromwell, Jonathan McJunkin, and et al. 2022. "Surgery for Pituitary Tumor Apoplexy Is Associated with Rapid Headache and Cranial Nerve Improvement" Current Oncology 29, no. 7: 4914-4922. https://doi.org/10.3390/curroncol29070390
APA StyleCross, K. A., Desai, R., Vellimana, A., Liu, Y., Rich, K., Zipfel, G., Dacey, R., Chicoine, M., Klatt-Cromwell, C., McJunkin, J., Pipkorn, P., Schneider, J. S., Silverstein, J., & Kim, A. H. (2022). Surgery for Pituitary Tumor Apoplexy Is Associated with Rapid Headache and Cranial Nerve Improvement. Current Oncology, 29(7), 4914-4922. https://doi.org/10.3390/curroncol29070390





