Effect of Lenalidomide Maintenance in Chronic Lymphocytic Leukemia: A Meta-Analysis and Trial-Sequential Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Eligibility Criteria
2.4. Outcome Measurement
2.5. Data Extraction and Management
2.6. Statistical Analysis
2.7. Trial Sequential Analysis (TSA)
3. Results
3.1. Search Results
3.2. Characteristics of Included Studies
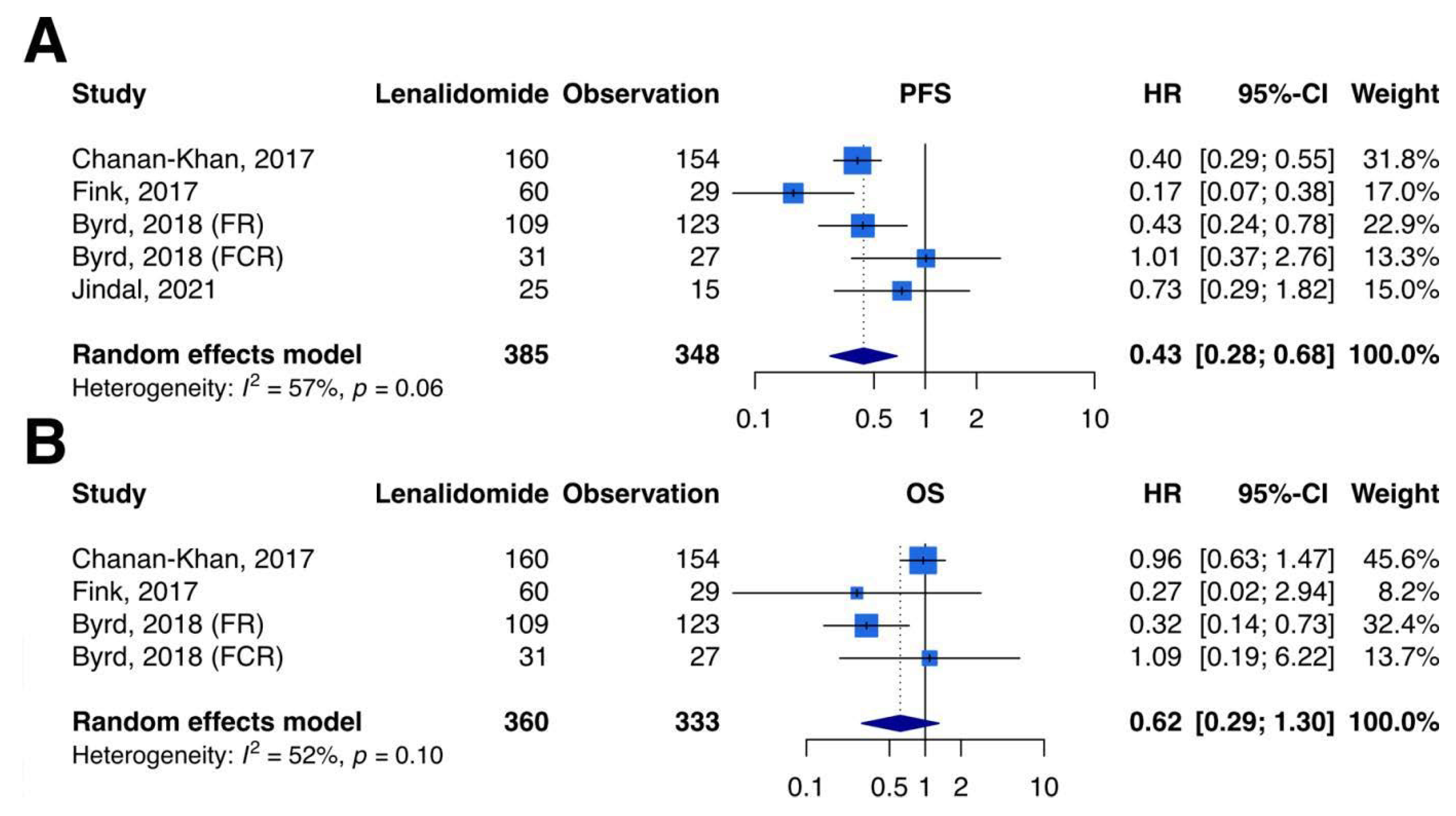
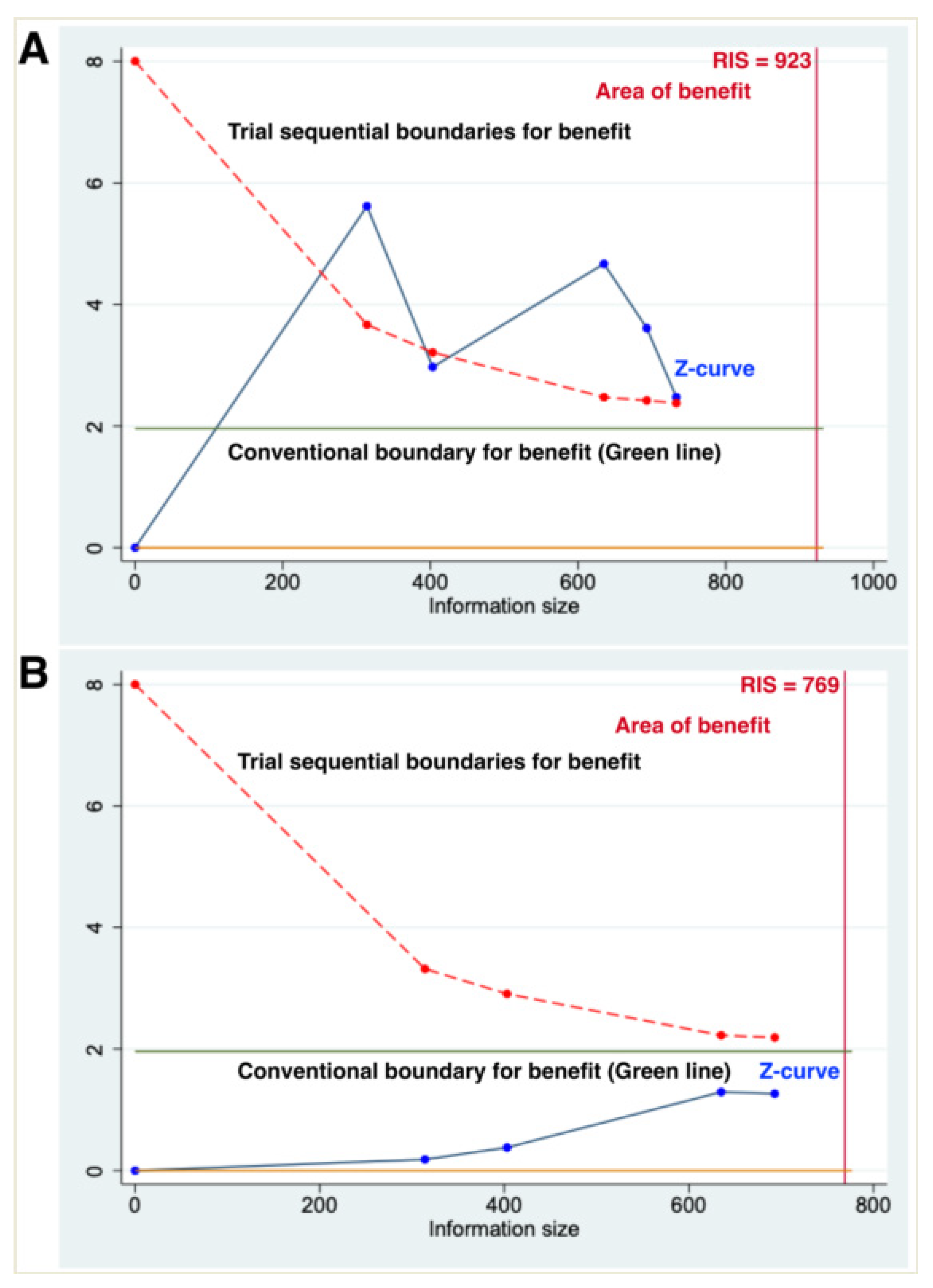
3.3. Primary Outcomes–PFS
3.4. Primary Outcomes–OS
3.5. Secondary Outcomes
3.6. Subgroup Analysis of PFS in the High-MRD Group
3.7. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCL-2 | B-cell lymphoma-2 protein |
| BR | Bendamustine and Rituximab |
| BTK | Bruton’s tyrosine kinase |
| CIs | Confidence intervals |
| CLL | Chronic lymphocytic leukemia |
| CR | Complete response |
| DB | Double blind |
| FAE | Fatal adverse events |
| FCR | Fludarabine, cyclophosphamide, and rituximab |
| FCRB | Fludarabine, cyclophosphamide, rituximab, and bendamustine |
| FDA | Food and Drug Administration |
| FISH | Fluorescence in situ hybridization |
| FR | Fludarabine and rituximab |
| TD | treatment discontinuation |
| HR | Hazard ratio |
| IGHV | Immunoglobulin heavy chain variable region |
| ITT | Intention to treat |
| MC | Multiple centers |
| MRD | Minimal residual disease |
| OP | Open label |
| ORs | Odds ratios |
| OS | Overall survival |
| PFS | Progression-free survival |
| PI3K | Phosphoinositide 3-kinase |
| PR | Partial response |
| R-Chlorambucil | Rituximab and chlorambucil |
| RCTs | Randomized controlled trials |
| SAE | Serious adverse events |
| SC | Single center |
| TSA | Trial sequential analysis |
References
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Hillmen, P.; Hallek, M.; Buske, C. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v78–v84. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am. J. Hematol. 2019, 94, 1266–1287. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.R.; Sawitsky, A.; Cronkite, E.P.; Chanana, A.D.; Levy, R.N.; Pasternack, B.S. Clinical staging of chronic lymphocytic leukemia. Blood 1975, 46, 219–234. [Google Scholar] [CrossRef]
- Binet, J.L.; Auquier, A.; Dighiero, G.; Chastang, C.; Piguet, H.; Goasguen, J.; Vaugier, G.; Potron, G.; Colona, P.; Oberling, F.; et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 1981, 48, 198–206. [Google Scholar] [CrossRef]
- Siddon, A.J.; Rinder, H.M. Pathology consultation on evaluating prognosis in incidental monoclonal lymphocytosis and chronic lymphocytic leukemia. Am. J. Clin. Pathol. 2013, 139, 708–712. [Google Scholar] [CrossRef]
- Fischer, K.; Bahlo, J.; Fink, A.M.; Goede, V.; Herling, C.D.; Cramer, P.; Langerbeins, P.; von Tresckow, J.; Engelke, A.; Maurer, C.; et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: Updated results of the CLL8 trial. Blood 2016, 127, 208–215. [Google Scholar] [CrossRef]
- Thompson, P.A.; Tam, C.S.; O’Brien, S.M.; Wierda, W.G.; Stingo, F.; Plunkett, W.; Smith, S.C.; Kantarjian, H.M.; Freireich, E.J.; Keating, M.J. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood 2016, 127, 303–309. [Google Scholar] [CrossRef]
- Eichhorst, B.; Fink, A.M.; Bahlo, J.; Busch, R.; Kovacs, G.; Maurer, C.; Lange, E.; Köppler, H.; Kiehl, M.; Sökler, M.; et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): An international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016, 17, 928–942. [Google Scholar] [CrossRef]
- Rossi, D.; Rasi, S.; Spina, V.; Bruscaggin, A.; Monti, S.; Ciardullo, C.; Deambrogi, C.; Khiabanian, H.; Serra, R.; Bertoni, F.; et al. Integrated mutational and cytogenetic analysis identifies new prognostic subgroups in chronic lymphocytic leukemia. Blood 2013, 121, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Stilgenbauer, S.; Benner, A.; Leupolt, E.; Kröber, A.; Bullinger, L.; Döhner, K.; Bentz, M.; Lichter, P. Genomic aberrations and survival in chronic lymphocytic leukemia. N. Engl. J. Med. 2000, 343, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Keating, M.; Do, K.A.; O’Brien, S.; Huh, Y.O.; Jilani, I.; Lerner, S.; Kantarjian, H.M.; Albitar, M. CD38 expression as an important prognostic factor in B-cell chronic lymphocytic leukemia. Blood 2001, 98, 181–186. [Google Scholar] [CrossRef]
- Gentile, M.; Mauro, F.R.; Calabrese, E.; De Propris, M.S.; Giammartini, E.; Mancini, F.; Milani, M.L.; Guarini, A.; Foà, R. The prognostic value of CD38 expression in chronic lymphocytic leukaemia patients studied prospectively at diagnosis: A single institute experience. Br. J. Haematol. 2005, 130, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Rassenti, L.Z.; Huynh, L.; Toy, T.L.; Chen, L.; Keating, M.J.; Gribben, J.G.; Neuberg, D.S.; Flinn, I.W.; Rai, K.R.; Byrd, J.C.; et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N. Engl. J. Med. 2004, 351, 893–901. [Google Scholar] [CrossRef]
- Orchard, J.A.; Ibbotson, R.E.; Davis, Z.; Wiestner, A.; Rosenwald, A.; Thomas, P.W.; Hamblin, T.J.; Staudt, L.M.; Oscier, D.G. ZAP-70 expression and prognosis in chronic lymphocytic leukaemia. Lancet 2004, 363, 105–111. [Google Scholar] [CrossRef]
- Evans, J.; Ziebland, S.; Pettitt, A.R. Incurable, invisible and inconclusive: Watchful waiting for chronic lymphocytic leukaemia and implications for doctor-patient communication. Eur. J. Cancer Care 2012, 21, 67–77. [Google Scholar] [CrossRef]
- Messori, A.; Fadda, V.; Maratea, D.; Trippoli, S. First-line treatments for chronic lymphocytic leukaemia: Interpreting efficacy data by network meta-analysis. Ann. Hematol. 2015, 94, 1003–1009. [Google Scholar] [CrossRef]
- Bauer, K.; Rancea, M.; Roloff, V.; Elter, T.; Hallek, M.; Engert, A.; Skoetz, N. Rituximab, ofatumumab and other monoclonal anti-CD20 antibodies for chronic lymphocytic leukaemia. Cochrane Database Syst. Rev. 2012, 11, Cd008079. [Google Scholar] [CrossRef]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef]
- Jain, N.; Keating, M.; Thompson, P.; Ferrajoli, A.; Burger, J.; Borthakur, G.; Takahashi, K.; Estrov, Z.; Fowler, N.; Kadia, T.; et al. Ibrutinib and Venetoclax for First-Line Treatment of CLL. N. Engl. J. Med. 2019, 380, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Wierda, W.; O’Brien, S.; Wen, S.; Faderl, S.; Garcia-Manero, G.; Thomas, D.; Do, K.A.; Cortes, J.; Koller, C.; Beran, M.; et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J. Clin. Oncol. 2005, 23, 4070–4078. [Google Scholar] [CrossRef] [PubMed]
- Shindiapina, P.; Awan, F.T. Management of patients with relapsed chronic lymphocytic leukemia. Am. J. Hematol. Oncol. 2016, 12, 25–30. [Google Scholar]
- Cramer, P.; Fink, A.M.; Busch, R.; Eichhorst, B.; Wendtner, C.M.; Pflug, N.; Langerbeins, P.; Bahlo, J.; Goede, V.; Schubert, F.; et al. Second-line therapies of patients initially treated with fludarabine and cyclophosphamide or fludarabine, cyclophosphamide and rituximab for chronic lymphocytic leukemia within the CLL8 protocol of the German CLL Study Group. Leuk Lymphoma 2013, 54, 1821–1822. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): A randomised, controlled, phase 3 trial. Lancet 2020, 395, 1278–1291. [Google Scholar] [CrossRef]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Kay, N.E.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N. Engl. J. Med. 2014, 371, 213–223. [Google Scholar] [CrossRef]
- Furman, R.R.; Sharman, J.P.; Coutre, S.E.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.; et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2014, 370, 997–1007. [Google Scholar] [CrossRef]
- Facon, T. Maintenance therapy for multiple myeloma in the era of novel agents. Hematol. Am. Soc. Hematol. Educ. Program 2015, 2015, 279–285. [Google Scholar] [CrossRef]
- O’Brien, S.; Kay, N.E. Maintenance therapy for B-chronic lymphocytic leukemia. Clin. Adv. Hematol. Oncol. 2011, 9, 22–31. [Google Scholar]
- Lee, C.H.; Chen, P.H.; Lin, C.; Wang, C.Y.; Ho, C.L. A network meta-analysis of maintenance therapy in chronic lymphocytic leukemia. PLoS ONE 2020, 15, e0226879. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; McCarthy, P.L. Immunomodulatory Drugs in Multiple Myeloma: Mechanisms of Action and Clinical Experience. Drugs 2017, 77, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Fowler, N.H.; Davis, R.E.; Rawal, S.; Nastoupil, L.; Hagemeister, F.B.; McLaughlin, P.; Kwak, L.W.; Romaguera, J.E.; Fanale, M.A.; Fayad, L.E.; et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: An open-label, phase 2 trial. Lancet Oncol. 2014, 15, 1311–1318. [Google Scholar] [CrossRef]
- Itchaki, G.; Brown, J.R. Lenalidomide in the treatment of chronic lymphocytic leukemia. Expert Opin. Investig. Drugs 2017, 26, 633–650. [Google Scholar] [CrossRef] [PubMed]
- Riches, J.C.; Gribben, J.G. Mechanistic and Clinical Aspects of Lenalidomide Treatment for Chronic Lymphocytic Leukemia. Curr. Cancer Drug Targets 2016, 16, 689–700. [Google Scholar] [CrossRef]
- González-Rodríguez, A.P.; Payer, A.R.; Acebes-Huerta, A.; Huergo-Zapico, L.; Villa-Alvarez, M.; Gonzalez-García, E.; Gonzalez, S. Lenalidomide and chronic lymphocytic leukemia. Biomed. Res. Int. 2013, 2013, 932010. [Google Scholar] [CrossRef][Green Version]
- Takahashi, K.; Hu, B.; Wang, F.; Yan, Y.; Kim, E.; Vitale, C.; Patel, K.P.; Strati, P.; Gumbs, C.; Little, L.; et al. Clinical implications of cancer gene mutations in patients with chronic lymphocytic leukemia treated with lenalidomide. Blood 2018, 131, 1820–1832. [Google Scholar] [CrossRef]
- Kotla, V.; Goel, S.; Nischal, S.; Heuck, C.; Vivek, K.; Das, B.; Verma, A. Mechanism of action of lenalidomide in hematological malignancies. J. Hematol. Oncol. 2009, 2, 36. [Google Scholar] [CrossRef]
- Ramsay, A.G.; Johnson, A.J.; Lee, A.M.; Gorgün, G.; Le Dieu, R.; Blum, W.; Byrd, J.C.; Gribben, J.G. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J. Clin. Investig. 2008, 118, 2427–2437. [Google Scholar] [CrossRef]
- Wu, L.; Adams, M.; Carter, T.; Chen, R.; Muller, G.; Stirling, D.; Schafer, P.; Bartlett, J.B. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin. Cancer Res. 2008, 14, 4650–4657. [Google Scholar] [CrossRef]
- Acebes-Huerta, A.; Huergo-Zapico, L.; Gonzalez-Rodriguez, A.P.; Fernandez-Guizan, A.; Payer, A.R.; López-Soto, A.; Gonzalez, S. Lenalidomide induces immunomodulation in chronic lymphocytic leukemia and enhances antitumor immune responses mediated by NK and CD4 T cells. Biomed. Res. Int. 2014, 2014, 265840. [Google Scholar] [CrossRef] [PubMed]
- Chanan-Khan, A.; Miller, K.C.; Musial, L.; Lawrence, D.; Padmanabhan, S.; Takeshita, K.; Porter, C.W.; Goodrich, D.W.; Bernstein, Z.P.; Wallace, P.; et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: Results of a phase II study. J. Clin. Oncol. 2006, 24, 5343–5349. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.I.; Bergsagel, P.L.; Paul, H.; Xu, W.; Lau, A.; Dave, N.; Kukreti, V.; Wei, E.; Leung-Hagesteijn, C.; Li, Z.H.; et al. Single-agent lenalidomide in the treatment of previously untreated chronic lymphocytic leukemia. J. Clin. Oncol. 2011, 29, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.J.; Montserrat, E.; Rai, K.R.; et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008, 111, 5446–5456. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common Terminology Criteria for Adverse Events: (CTCAE); National Cancer Institute, National Institutes of Health, US Department of Health and Human Services: Frederick, MD, USA, 2010. [Google Scholar]
- Tripepi, G.; Chesnaye, N.C.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Intention to treat and per protocol analysis in clinical trials. Nephrology (Carlton) 2020, 25, 513–517. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Pereira, T.V.; Patsopoulos, N.A.; Salanti, G.; Ioannidis, J.P. Critical interpretation of Cochran’s Q test depends on power and prior assumptions about heterogeneity. Res. Synth. Methods 2010, 1, 149–161. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Smith, A.F. Trial sequential analysis: Adding a new dimension to meta-analysis. Anaesthesia 2020, 75, 15–20. [Google Scholar] [CrossRef]
- Wetterslev, J.; Jakobsen, J.C.; Gluud, C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med. Res. Methodol. 2017, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Miladinovic, B.; Hozo, I.; Djulbegovic, B. Trial Sequential Boundaries for Cumulative Meta-Analyses. Stata J. 2013, 13, 77–91. [Google Scholar] [CrossRef]
- Fink, A.M.; Bahlo, J.; Robrecht, S.; Al-Sawaf, O.; Aldaoud, A.; Hebart, H.; Jentsch-Ullrich, K.; Dörfel, S.; Fischer, K.; Wendtner, C.M.; et al. Lenalidomide maintenance after first-line therapy for high-risk chronic lymphocytic leukaemia (CLLM1): Final results from a randomised, double-blind, phase 3 study. Lancet Haematol. 2017, 4, e475–e486. [Google Scholar] [CrossRef]
- Byrd, J.C.; Ruppert, A.S.; Heerema, N.A.; Halvorson, A.E.; Hoke, E.; Smith, M.R.; Godwin, J.E.; Couban, S.; Fehniger, T.A.; Thirman, M.J.; et al. Lenalidomide consolidation benefits patients with CLL receiving chemoimmunotherapy: Results for CALGB 10404 (Alliance). Blood Adv. 2018, 2, 1705–1718. [Google Scholar] [CrossRef]
- Jindal, N.; Lad, D.P.; Malhotra, P.; Prakash, G.; Khadwal, A.; Jain, A.; Sachdeva, M.S.; Sreedharanunni, S.; Naseem, S.; Varma, N.; et al. Randomized controlled trial of individualized, low dose, fixed duration lenalidomide maintenance versus observation after frontline chemo-immunotherapy in CLL. Leuk Lymphoma 2021, 62, 1674–1681. [Google Scholar] [CrossRef]
- Chanan-Khan, A.A.; Zaritskey, A.; Egyed, M.; Vokurka, S.; Semochkin, S.; Schuh, A.; Kassis, J.; Simpson, D.; Zhang, J.; Purse, B.; et al. Lenalidomide maintenance therapy in previously treated chronic lymphocytic leukaemia (CONTINUUM): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Haematol. 2017, 4, e534–e543. [Google Scholar] [CrossRef]
- Debray, T.P.A.; Moons, K.G.M.; Riley, R.D. Detecting small-study effects and funnel plot asymmetry in meta-analysis of survival data: A comparison of new and existing tests. Res. Synth. Methods 2018, 9, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.; Aurran, T.; Tam, C.S.; Sartor, M.; Letestu, R.; Carney, D.; Cull, G.; Levy, V.; Leblond, V.; Bene, M.C.; et al. Interim Analysis of Lenalidomide Consolidation on Minimal Residual Disease in Patients with Chronic Lymphocytic Leukemia Following Initial FCR Chemotherapy—CLL6 Residuum Study of the Australian Leukaemia and Lymphoma Group (ALLG) and the French Innovative Leukemia Organization (FILO). Blood 2016, 128, 2053. [Google Scholar] [CrossRef]
- Beauchemin, C.; Johnston, J.B.; Lapierre, M.; Aissa, F.; Lachaine, J. Relationship between progression-free survival and overall survival in chronic lymphocytic leukemia: A literature-based analysis. Curr. Oncol. 2015, 22, e148–e156. [Google Scholar] [CrossRef] [PubMed]
- Freidlin, B.; Little, R.F.; Korn, E.L. Design Issues in Randomized Clinical Trials of Maintenance Therapies. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Petrucci, M.T.; Foà, R.; Catalano, J.; Kropff, M.; Terpos, E.; Zhang, J.; Grote, L.; Jacques, C.; Palumbo, A. Impact of maintenance therapy on subsequent treatment in patients with newly diagnosed multiple myeloma: Use of “progression-free survival 2” as a clinical trial end-point. Haematologica 2015, 100, e328–e330. [Google Scholar] [CrossRef][Green Version]
- Mbanya, Z.; Chadda, S. Time to Second Objective Disease Progression (PFS2): An Emerging Clinical Trial Endpoint with Regulatory and Reimbursement Implications. Blood 2014, 124, 6005. [Google Scholar] [CrossRef]
- Fürstenau, M.; De Silva, N.; Eichhorst, B.; Hallek, M. Minimal Residual Disease Assessment in CLL: Ready for Use in Clinical Routine? Hemasphere 2019, 3, e287. [Google Scholar] [CrossRef]
- García-Marco, J.A.; Jiménez, J.L.; Recasens, V.; Zarzoso, M.F.; González-Barca, E.; De Marcos, N.S.; Ramírez, M.J.; Parraga, F.J.P.; Yañez, L.; De La Serna Torroba, J.; et al. High prognostic value of measurable residual disease detection by flow cytometry in chronic lymphocytic leukemia patients treated with front-line fludarabine, cyclophosphamide, and rituximab, followed by three years of rituximab maintenance. Haematologica 2019, 104, 2249–2257. [Google Scholar] [CrossRef]
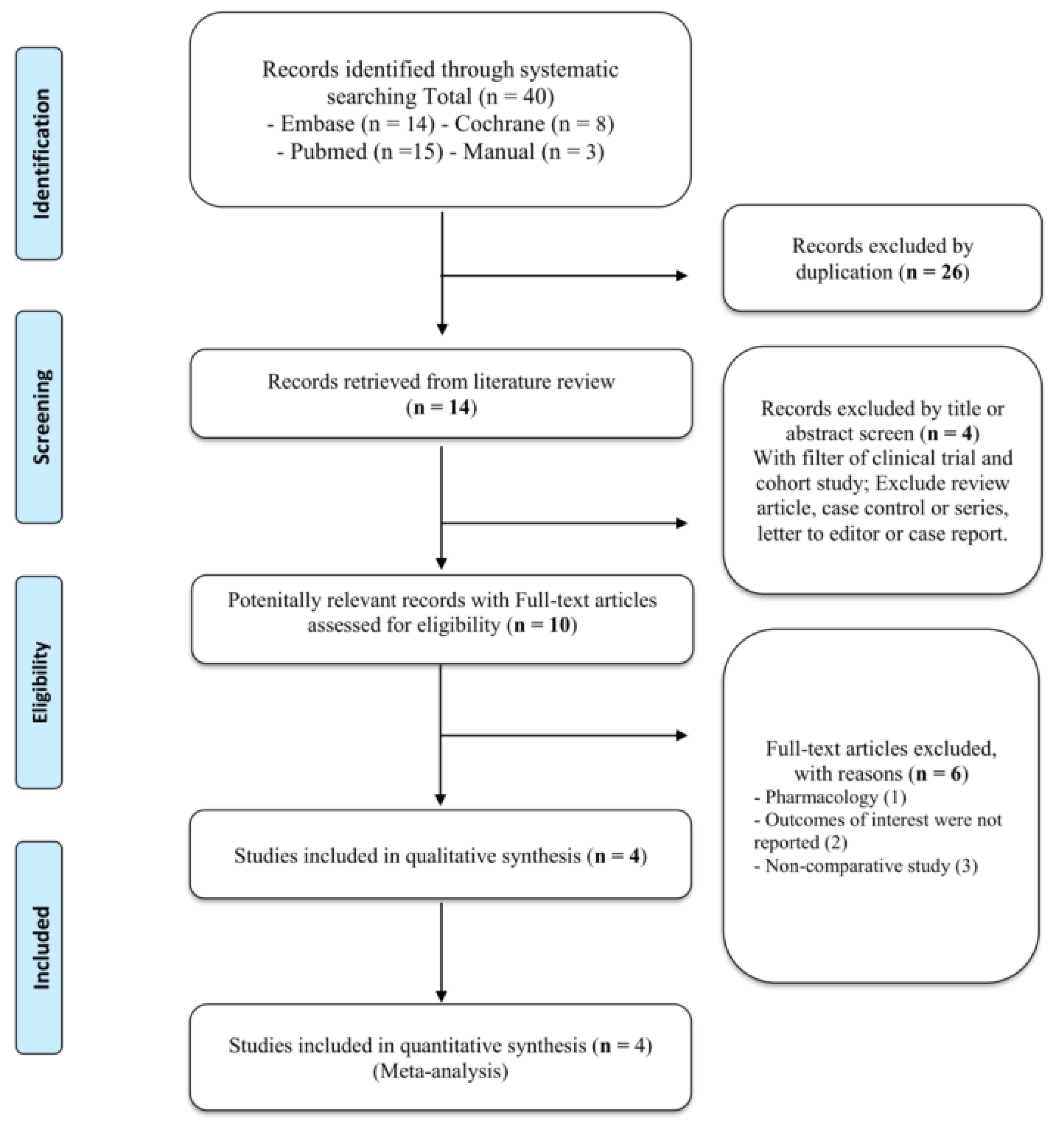
| Trial Name/Registration Code/PublicationYear | Study Design | Treatment Comparison/Experimental Regimen | Cases | Mean Age | Follow Up (Range)/Analysis | Frontline Cases (%)/FCR Regimen(%) | CR/PR (%) | Frontline Regiments | |
|---|---|---|---|---|---|---|---|---|---|
| CONTINUUM (NCT00774345) Chanan-Khan, 2017 [61] | Phase III, DB, MC, RCT | Lenalidomide vs. placebo | Oral 2.5 mg/daily (maximal 5 mg/daily) | 160 vs. 154 | 63 vs. 63 | 31.5 months (18.9–50.8)/ITT | 28%/98.9% | 23.9%/76.1% | FCR, chlorambucil, alemtuzumab |
| CLLM1 (NCT01556776) Fink, 2017 [58] | Phase III, DB, MC, RCT | Lenalidomide vs. placebo | Oral 5 mg/daily (maximal 15 mg/daily) | 60 vs. 29 | 64 vs. 64 | 17.9 months (9.1–28.1)/ITT | 100%/22.1% | 39.3%/60.7% | FCRB |
| CALGB 10404 (NCT00602459) Byrd, 2018 [59] | Phase II, OP, MC, RCT | Lenalidomide vs. observation (FR group) | Oral 5 mg/daily (maximal 10 mg/daily) | 109 vs. 123 | 62 vs. 61 | 73.0 months (2.0–112.0)/ITT | 100%/0% | 32.0%/37.0% | FR |
| Lenalidomide vs. observation (FCR group) | 31 vs. 27 | 59 vs. 60 | 73.0 months (2.0–112.0)/ITT | 100%/100% | 35.0%/39.0% | FCR | |||
| Jindal et al. (CTRI/2018/07/014716) Jindal, 2021 [60] | Phase II, OP, SC, RCT | Lenalidomide vs. observation | Oral 5 mg/daily (maximal 10 mg/daily) | 25 vs. 15 | 60 vs. 62 | 22.0 months (4.0–30.0)/ITT | 100%/20% | 56.0%/66.7% | BR, FCR, R-Chlorambucil, Chlorambucil |
| Outcome | Comparison Trial Number (N) | Patients Number (N) | Measurement (95% CIs) | Cochran Q p-Value for Heterogeneity | I2 (%) |
|---|---|---|---|---|---|
| Grade 3–4 neutropenia | 4 * | 733 | Random-effects, OR, 2.30 (0.84 to 6.28) | <0.01 | 81% |
| Treatment discontinuation | 4 * | 733 | Random-effects, OR, 0.76 (0.29 to 1.99) | <0.01 | 84% |
| Serious adverse events | 2 | 400 | Fixed-effect, OR, 4.64 (2.96 to 7.26) | 0.34 | 0% |
| Fatal adverse events | 4 * | 733 | Fixed-effect, OR, 0.86 (0.28 to 2.63) | 0.66 | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.-Y.; Jhou, H.-J.; Chen, P.-H.; Lee, C.-H. Effect of Lenalidomide Maintenance in Chronic Lymphocytic Leukemia: A Meta-Analysis and Trial-Sequential Analysis. Curr. Oncol. 2022, 29, 4245-4259. https://doi.org/10.3390/curroncol29060339
Yu T-Y, Jhou H-J, Chen P-H, Lee C-H. Effect of Lenalidomide Maintenance in Chronic Lymphocytic Leukemia: A Meta-Analysis and Trial-Sequential Analysis. Current Oncology. 2022; 29(6):4245-4259. https://doi.org/10.3390/curroncol29060339
Chicago/Turabian StyleYu, Tsung-Ying, Hong-Jie Jhou, Po-Huang Chen, and Cho-Hao Lee. 2022. "Effect of Lenalidomide Maintenance in Chronic Lymphocytic Leukemia: A Meta-Analysis and Trial-Sequential Analysis" Current Oncology 29, no. 6: 4245-4259. https://doi.org/10.3390/curroncol29060339
APA StyleYu, T.-Y., Jhou, H.-J., Chen, P.-H., & Lee, C.-H. (2022). Effect of Lenalidomide Maintenance in Chronic Lymphocytic Leukemia: A Meta-Analysis and Trial-Sequential Analysis. Current Oncology, 29(6), 4245-4259. https://doi.org/10.3390/curroncol29060339





