Association between Vascular Calcification and Esophagojejunal Anastomotic Complications after Total Gastrectomy for Gastric Cancer: A Propensity-Matched Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Definitions of Complications
2.2. Patients & Matching
2.3. Anastomosis Technique
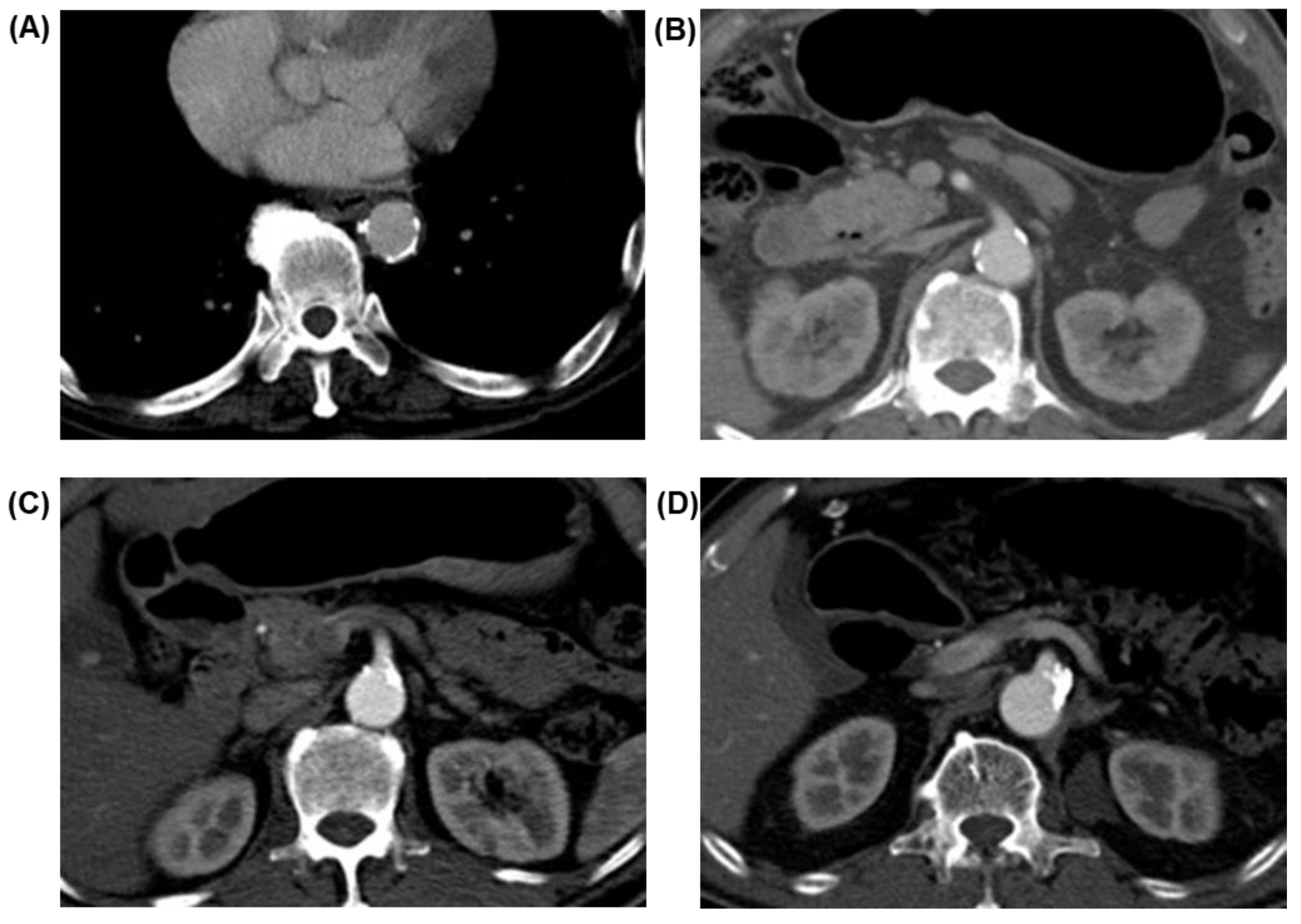
2.4. Image Acquisition and Evaluation
2.5. Statistical Analysis
3. Results
Baseline Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aslaner, R.; Pekcevik, Y.; Sahin, H.; Toka, O. Variations in the origin of inferior phrenic arteries and their relationship to celiac axis variations on CT angiography. Kor. J. Radiol. 2017, 18, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Information Committee of the Korean Gastric Cancer Association. Korean Gastric Cancer Association-Led Nationwide Survey on Surgically Treated Gastric Cancers in J. Gastr. Cancer 2021, 21, 221–235.
- Yoo, H.M.; Lee, H.H.; Shim, J.H.; Jeon, H.M.; Park, C.H.; Song, K.Y. Negative impact of leakage on survival of patients undergoing curative resection for advanced gastric cancer. J. Surg. Oncol. 2011, 104, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Biebl, M.; Dadras, M.; Struecker, B.; Sauer, I.M.; Thuss-Patience, P.C.; Chopra, S.; Fikatas, P.; Bahra, M.; Seehofer, D.; et al. Anastomotic leak predicts diminished long-term survival after resection for gastric and esophageal cancer. Surgery 2016, 160, 191–203. [Google Scholar] [CrossRef]
- Inokuchi, M.; Otsuki, S.; Fujimori, Y.; Sato, Y.; Nakagawa, M.; Kojima, K. Systematic review of anastomotic complications of esophagojejunostomy after laparoscopic total gastrectomy. World J. Gastroenterol. 2015, 21, 9656–9665. [Google Scholar] [CrossRef]
- Kim, S.H.; Son, S.Y.; Park, Y.S.; Ahn, S.H.; Park, D.J.; Kim, H.H. Risk factors for anastomotic leakage: A retrospective cohort study in a single gastric surgical unit. J. Gastric Cancer 2015, 15, 167–175. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, W.; Lee, H.; Lee, K.H.; Park, J.C.; Shin, S.K.; Lee, S.K.; Lee, Y.C.; Noh, S.H. Endoscopy-guided balloon dilation of benign anastomotic strictures after radical gastrectomy for gastric cancer. Gut Liver 2014, 8, 394–399. [Google Scholar] [CrossRef][Green Version]
- Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean practice guideline for gastric cancer 2018: An evidence-based, multi-disciplinary approach. J. Gastric Cancer 2019, 19, 1–48. [Google Scholar] [CrossRef]
- Van Rossum, P.S.N.; Haverkamp, L.; Verkooijen, H.M.; Van Leeuwen, M.S.; Van Hillegersberg, R.; Ruurda, J.P. Calcification of arteries supplying the gastric tube: A new risk factor for anastomotic leakage after esophageal surgery. Radiology 2015, 274, 124–132. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, G.; Li, J.; Qu, B.; Shi, S.; Feng, X.; Feng, H.; Jiang, J.; Xue, Q.; He, J. Calcification of arteries supplying the gastric tube increases the risk of anastomotic leakage after esophagectomy with cervical anastomosis. J. Thorac. Dis. 2016, 8, 3551–3562. [Google Scholar] [CrossRef]
- Goense, L.; van Rossum, P.S.N.; Weijs, T.J.; van Det, M.J.; Nieuwenhuijzen, G.A.; Luyer, M.D.; van Leeuwen, M.S.; van Hillegersberg, R.; Ruurda, J.P.; Kouwenhoven, E.A. Aortic Calcification Increases the Risk of Anastomotic Leakage after Ivor-Lewis Esophagectomy. Ann. Thorac. Surg. 2016, 102, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, B.J.; Evans, E.; Bundred, J.; Hodson, J.; Whiting, J.L.; Forde, C.; Griffiths, E.A. Vascular calcification does not predict anastomotic leak or conduit necrosis following oesophagectomy. World J. Gastrointest. Surg. 2019, 11, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Hohenwalter, E.J. Chronic mesenteric ischemia: Diagnosis and treatment. Semin. Interv. Radiol. 2009, 26, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Wright, C.M.; Criqui, M.H.; Allison, M.A. Superior mesenteric artery calcification is associated with cardiovascular risk factors, systemic calcified atherosclerosis, and increased mortality. J. Vasc. Surg. 2018, 67, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.S.; Hitch, D.C.; Armstrong, D.N. The role of tissue ischemia in the pathogenesis of anastomotic stricture. Surgery 1988, 104, 824–829. [Google Scholar] [PubMed]
- Roobottom, C.A.; Dubbins, P.A. Significant disease of the celiac and superior mesenteric arteries in asymptomatic patients: Predictive value of Doppler sonography. AJR 1993, 161, 985–988. [Google Scholar] [CrossRef]
- Fawcett, A.; Shembekar, M.; Church, J.S.; Vashisht, R.; Springall, R.G.; Nott, D.M. Smoking, hypertension, and colonic anastomotic healing; A combined clinical and histopathological study. Gut 1996, 38, 714–718. [Google Scholar] [CrossRef]
- Schietroma, M.; Cecilia, E.M.; Carlei, F.; Sista, F.; De Santis, G.; Piccione, F.; Amicucci, G. Prevention of anastomotic leakage after total gastrectomy with perioperative supplemental oxygen administration: A prospective randomized, double-blind, controlled, single-center trial. Ann. Surg. Oncol. 2013, 20, 1584–1590. [Google Scholar] [CrossRef]
- Campbell, C.; Reames, M.K.; Robinson, M.; Symanowski, J.; Salo, J.C. Conduit Vascular Evaluation is Associated with Reduction in Anastomotic Leak After Esophagectomy. J. Gastrointest. Surg. 2015, 19, 806–812. [Google Scholar] [CrossRef]
- Slooter, M.D.; Eshuis, W.J.; Cuesta, M.A.; Gisbertz, S.S.; van Berge Henegouwen, M.I. Fluorescent imaging using indocyanine green during esophagectomy to prevent surgical morbidity: A systematic review and meta-analysis. J. Thorac. Dis. 2019, 11 (Suppl 5), S755–S765. [Google Scholar] [CrossRef]
- Huh, Y.J.; Lee, H.J.; Kim, T.H.; Choi, Y.S.; Park, J.H.; Son, Y.G.; Suh, Y.S.; Kong, S.H.; Yang, H.K. Efficacy of Assessing Intraoperative Bowel Perfusion with Near-Infrared Camera in Laparoscopic Gastric Cancer Surgery. J. Laparoendosc Adv. Surg. Tech. 2019, 29, 476–483. [Google Scholar] [CrossRef] [PubMed]
| Artery | Arteria Calcification Score | ||
|---|---|---|---|
| Score 0 | Score 1 | Score 2 | |
| Aorta | Absent | Minor calcifications: Nine or fewer foci and three or fewer foci extending over three or more sections | Major calcifications: More than nine foci or more than three foci extending over three or more sections |
| SMA | Absent | Minor calcifications: Extending over fewer than three sections or maximum cross-sectional diameter of single focus 10 mm or smaller | Major calcifications: Extending over three or more sections and maximum cross-sectional diameter of single focus, larger than 10 mm |
| Jejunal | Absent | One or more calcifications | Not applicable |
| LIPA | Absent | One or more calcifications | Not applicable |
| Variable | Complication Group (N = 30) | Non-Complication Group (N = 30) | p |
|---|---|---|---|
| Age group (years) <60 60–70 >70 | 7 (23.3) 16 (53.3) 7 (23.3) | 12 (40.0) 9 (30.0) 9 (30.0) | 0.172 |
| Sex Male Female | 23 (76.7)7 (23.3) | 22 (73.3)8 (26.7) | 0.766 |
| BMI (kg/m2) Underweight (<18.5) Normal (18.5–22.99) Overweight (23.00–29.00) Obese (≥30) | 0 (0.0) 15 (50.0) 15 (50.0) 0 (0.0) | 1 (3.3) 14 (46.7) 14 (46.7) 1 (3.3) | 0.558 |
| CVD/Smoking Yes No | 17 (56.7) 13 (43.3) | 16 (53.3) 14 (46.7) | 0.795 |
| Approach Open Laparoscopic | 22 (73.3) 8 (26.7) | 22 (73.3) 8 (26.7) | 1.000 |
| Remnant stomach Yes No | 5 (16.7) 25 (83.3) | 5 (16.7) 25 (83.3) | 1.000 |
| Pathological stage I II III IV | 17 (56.7) 5 (16.7) 7 (23.3) 1 (3.3) | 19 (63.3) 1 (3.3) 8 (26.7) 2 (6.7) | 0.365 |
| Arterial Calcification Score | Anastomotic Complications (N = 30) | No Anastomotic Complications (N = 30) | p |
|---|---|---|---|
| Aorta | |||
| 0–1 (No/Minor) 2 (Major) | 25 (83.3) 5 (16.7%) | 27 (90.0) 3 (10.0) | 0.440 |
| SMA | |||
| 0–1 (No/Minor) 2 (Major) | 25 (83.3) 5 (16.7) | 30 (100) 0 (0.0) | 0.020 |
| Jejunal artery | |||
| 0 (Absent) 1 (Present) | 30 (100) 0 (0.0) | 30 (100) 0 (0.0) | N/A |
| LIPA | |||
| 0 (Absent) 1 (Present) | 30 (100) 0 (0.0) | 30 (100) 0 (0.0) | N/A |
| Anastomotic Complications | SMA Calcification Score (N = 60) | p | |
| 0–1 (No/Minor) | 2 (Major) | 0.028 | |
| Leak | |||
| 23 (38.3%) | 20 (87.0%) | 3 (13.0%) | |
| Stricture | |||
| 7 (11.7%) | 5 (71.4%) | 2 (28.6%) | |
| None | |||
| 30 (50.0%) | 30 (100%) | 0 (0.0%) | |
| Total | |||
| 60 (100%) | 55 (91.7%) | 5 (8.3%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-L.; Jeon, C.-H.; Park, K.-B.; Seo, H.-S.; Lee, H.-H. Association between Vascular Calcification and Esophagojejunal Anastomotic Complications after Total Gastrectomy for Gastric Cancer: A Propensity-Matched Study. Curr. Oncol. 2022, 29, 3224-3231. https://doi.org/10.3390/curroncol29050262
Lee S-L, Jeon C-H, Park K-B, Seo H-S, Lee H-H. Association between Vascular Calcification and Esophagojejunal Anastomotic Complications after Total Gastrectomy for Gastric Cancer: A Propensity-Matched Study. Current Oncology. 2022; 29(5):3224-3231. https://doi.org/10.3390/curroncol29050262
Chicago/Turabian StyleLee, Su-Lim, Chul-Hyo Jeon, Ki-Bum Park, Ho-Seok Seo, and Han-Hong Lee. 2022. "Association between Vascular Calcification and Esophagojejunal Anastomotic Complications after Total Gastrectomy for Gastric Cancer: A Propensity-Matched Study" Current Oncology 29, no. 5: 3224-3231. https://doi.org/10.3390/curroncol29050262
APA StyleLee, S.-L., Jeon, C.-H., Park, K.-B., Seo, H.-S., & Lee, H.-H. (2022). Association between Vascular Calcification and Esophagojejunal Anastomotic Complications after Total Gastrectomy for Gastric Cancer: A Propensity-Matched Study. Current Oncology, 29(5), 3224-3231. https://doi.org/10.3390/curroncol29050262





